

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin B (Homo sapiens (Human)) | BDBM50107639 (3-{2-[2-(4-Chloro-2-fluoro-benzoylamino)-3-m-tolyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50107633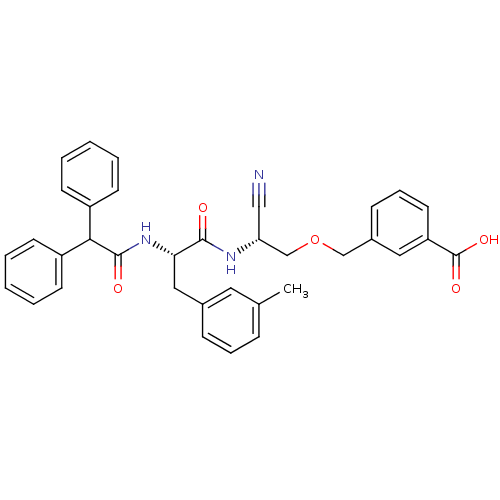 (3-[2-Cyano-2-(2-diphenylacetylamino-3-m-tolyl-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50107633 (3-[2-Cyano-2-(2-diphenylacetylamino-3-m-tolyl-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50107646 (3-[4-Cyano-4-(2-diphenylacetylamino-3-m-tolyl-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50107623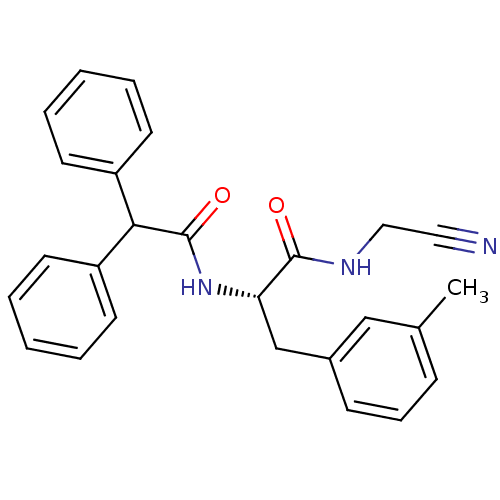 (CHEMBL140756 | N-Cyanomethyl-2-diphenylacetylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity of the compound against recombinant human cathepsin L (cat L) expressed in baculovirus | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50107626 (3-{(R)-2-Cyano-2-[(S)-2-(2,4-difluoro-benzoylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50107620 (3-[2-(2-Benzoylamino-3-m-tolyl-propionylamino)-2-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50107638 (CHEMBL336436 | N-(Benzyloxymethyl-cyano-methyl)-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50107650 (CHEMBL138661 | N-Cyanomethyl-3-(3,5-dimethyl-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50107647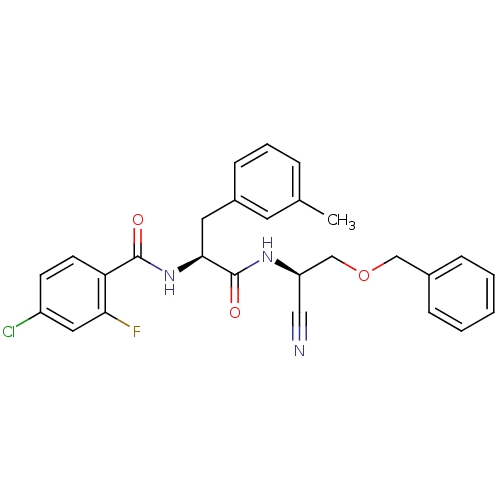 (CHEMBL140506 | N-{1-[(Benzyloxymethyl-cyano-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50107630 (3-[4-Cyano-4-(2-diphenylacetylamino-3-m-tolyl-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50107633 (3-[2-Cyano-2-(2-diphenylacetylamino-3-m-tolyl-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity of the compound against recombinant human cathepsin L (cat L) expressed in baculovirus | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50107628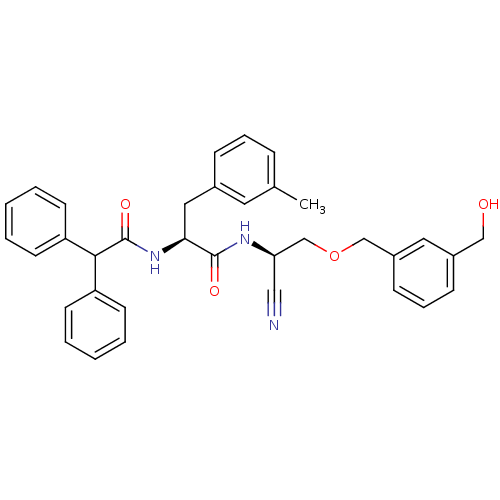 (CHEMBL343423 | N-[Cyano-(3-hydroxymethyl-benzyloxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50107621 (4-[2-Cyano-2-(2-diphenylacetylamino-3-m-tolyl-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50107622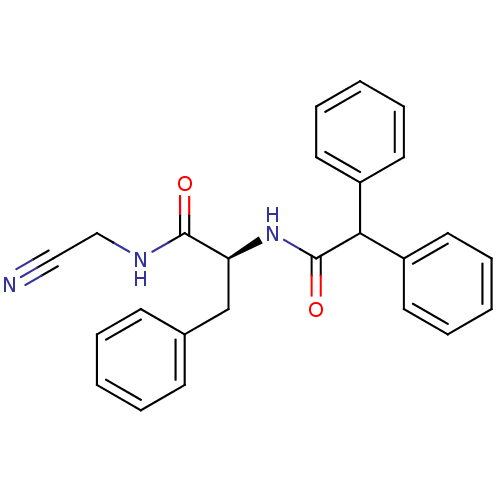 (CHEMBL342145 | N-Cyanomethyl-2-diphenylacetylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity of the compound against recombinant human cathepsin L (cat L) expressed in baculovirus | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50107625 (3,4-Dichloro-N-[1-(cyanomethyl-carbamoyl)-2-m-toly...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 31.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50107629 (3-[2-Cyano-2-(2-pentanoylamino-3-m-tolyl-propionyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 35.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50107634 (CHEMBL140606 | N-{1-[(Benzyloxymethyl-cyano-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50107633 (3-[2-Cyano-2-(2-diphenylacetylamino-3-m-tolyl-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity of the compound against recombinant human cathepsin S (cat S) expressed in baculovirus | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50107631 (3-[2-Cyano-2-(2-diphenylacetylamino-3-m-tolyl-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50107622 (CHEMBL342145 | N-Cyanomethyl-2-diphenylacetylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity of the compound against recombinant human cathepsin S (cat S) expressed in baculovirus | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50107641 (4-Chloro-N-[1-(cyanomethyl-carbamoyl)-2-m-tolyl-et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50107623 (CHEMBL140756 | N-Cyanomethyl-2-diphenylacetylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50107623 (CHEMBL140756 | N-Cyanomethyl-2-diphenylacetylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50107623 (CHEMBL140756 | N-Cyanomethyl-2-diphenylacetylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity of the compound against recombinant human cathepsin S (cat S) expressed in baculovirus | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50107644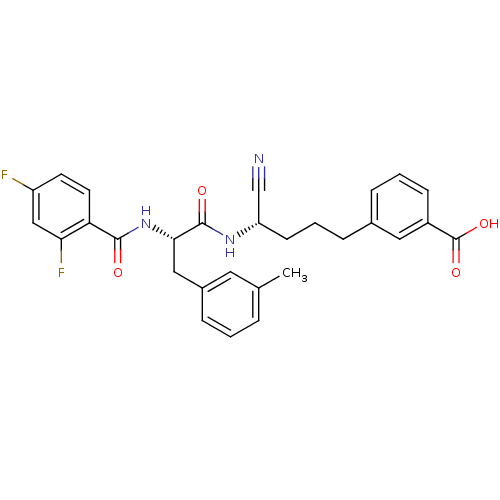 (3-{4-Cyano-4-[2-(2,4-difluoro-benzoylamino)-3-m-to...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50107627 (CHEMBL139132 | N-Cyanomethyl-2-diphenylacetylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50107636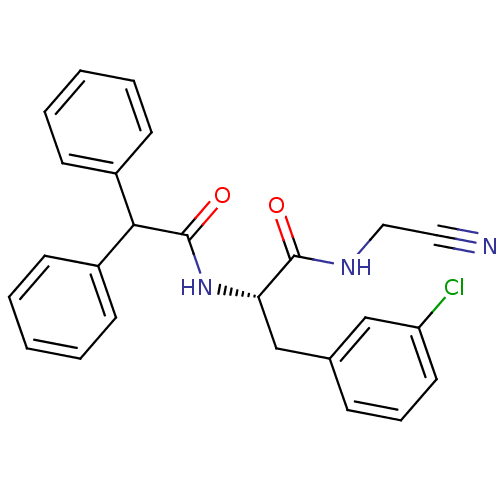 (3-(3-Chloro-phenyl)-N-cyanomethyl-2-diphenylacetyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50107632 (CHEMBL423923 | N-Cyanomethyl-2-diphenylacetylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 136 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50107649 (3-[3-Cyano-3-(2-diphenylacetylamino-3-m-tolyl-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 138 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50107635 (CHEMBL140794 | N-Cyanomethyl-3-phenyl-2-phenylacet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity of the compound against recombinant human cathepsin L (cat L) expressed in baculovirus | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50107635 (CHEMBL140794 | N-Cyanomethyl-3-phenyl-2-phenylacet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 226 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity of the compound against recombinant human cathepsin S (cat S) expressed in baculovirus | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50107637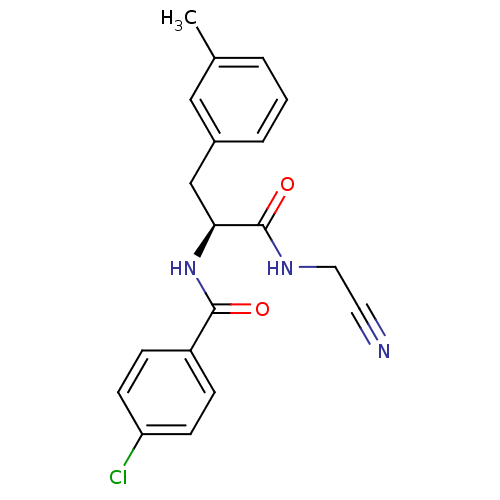 (4-Chloro-N-[1-(cyanomethyl-carbamoyl)-2-m-tolyl-et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 308 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50107648 (3-(3-Aminomethyl-5-methyl-phenyl)-N-cyanomethyl-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 358 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50107622 (CHEMBL342145 | N-Cyanomethyl-2-diphenylacetylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 496 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50107626 (3-{(R)-2-Cyano-2-[(S)-2-(2,4-difluoro-benzoylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 554 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity of the compound against recombinant human cathepsin L (cat L) expressed in baculovirus | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50107643 (CHEMBL140824 | N-[1-(Cyanomethyl-carbamoyl)-2-m-to...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 591 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50107640 (CHEMBL345081 | N-Cyanomethyl-2-diphenylacetylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 652 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50107626 (3-{(R)-2-Cyano-2-[(S)-2-(2,4-difluoro-benzoylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 937 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity of the compound against recombinant human cathepsin S (cat S) expressed in baculovirus | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50107624 (3-(3-Carbamimidoyl-phenyl)-N-cyanomethyl-2-dipheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50107619 (CHEMBL337111 | N-Cyanomethyl-2-diphenylacetylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50107645 (CHEMBL138586 | N-[1-(Cyanomethyl-carbamoyl)-2-m-to...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50107642 (CHEMBL440347 | Pentanoic acid [1-(cyanomethyl-carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50107635 (CHEMBL140794 | N-Cyanomethyl-3-phenyl-2-phenylacet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM20096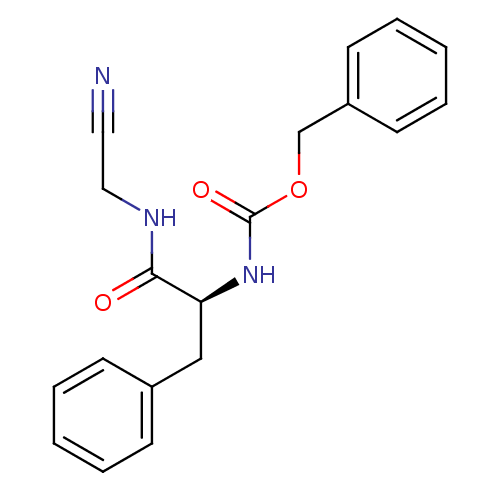 (CHEMBL344174 | benzyl N-[(1S)-1-[(cyanomethyl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. | J Med Chem 44: 4524-34 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||