 Found 19372 hits with Last Name = 'ko' and Initial = 'h'
Found 19372 hits with Last Name = 'ko' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Somatostatin receptor type 4
(RAT) | BDBM82253
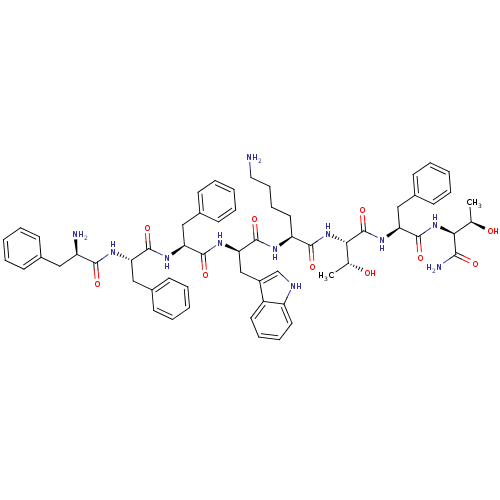
(BIM 23052 | CAS_133073-82-2)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](N)Cc1ccccc1)[C@@H](C)O)C(N)=O |r| Show InChI InChI=1S/C61H75N11O10/c1-37(73)52(54(64)75)71-60(81)50(34-42-25-13-6-14-26-42)70-61(82)53(38(2)74)72-56(77)47(29-17-18-30-62)66-59(80)51(35-43-36-65-46-28-16-15-27-44(43)46)69-58(79)49(33-41-23-11-5-12-24-41)68-57(78)48(32-40-21-9-4-10-22-40)67-55(76)45(63)31-39-19-7-3-8-20-39/h3-16,19-28,36-38,45,47-53,65,73-74H,17-18,29-35,62-63H2,1-2H3,(H2,64,75)(H,66,80)(H,67,76)(H,68,78)(H,69,79)(H,70,82)(H,71,81)(H,72,77)/t37-,38-,45-,47+,48+,49+,50+,51-,52+,53+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 44: 385-92 (1993)
BindingDB Entry DOI: 10.7270/Q2X065KZ |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(RAT) | BDBM50097783
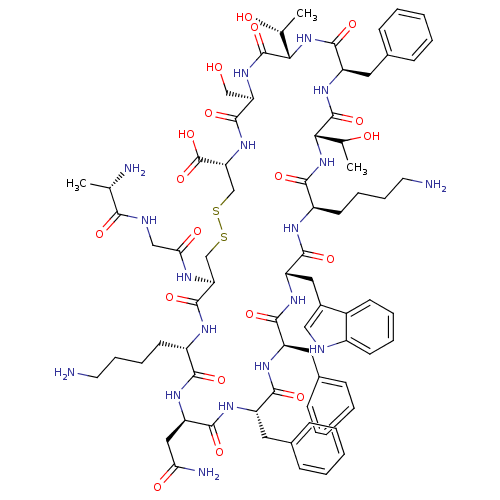
(CHEMBL438401 | D-Trp8 SST-14 | SOMATOSTATIN | SRIF...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@@H](NC(=O)[C@@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)C(C)O Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42?,43+,50-,51+,52-,53+,54+,55-,56+,57+,58-,59+,62-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 44: 385-92 (1993)
BindingDB Entry DOI: 10.7270/Q2X065KZ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50125999

((R)-2-[(S)-2-Amino-3-(4-hydroxy-2,6-dimethyl-pheny...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCCC(N)=O Show InChI InChI=1S/C29H42N8O5/c1-17-13-20(38)14-18(2)21(17)16-22(30)26(40)36-23(9-6-11-35-29(32)33)28(42)37-24(15-19-7-4-3-5-8-19)27(41)34-12-10-25(31)39/h3-5,7-8,13-14,22-24,38H,6,9-12,15-16,30H2,1-2H3,(H2,31,39)(H,34,41)(H,36,40)(H,37,42)(H4,32,33,35)/t22-,23+,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00205 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University
Curated by ChEMBL
| Assay Description
Ability of the compound to displace [3H]-DAMGO from mu opioid receptor |
Bioorg Med Chem Lett 13: 1269-72 (2003)
BindingDB Entry DOI: 10.7270/Q2XS5TQ5 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(RAT) | BDBM82463
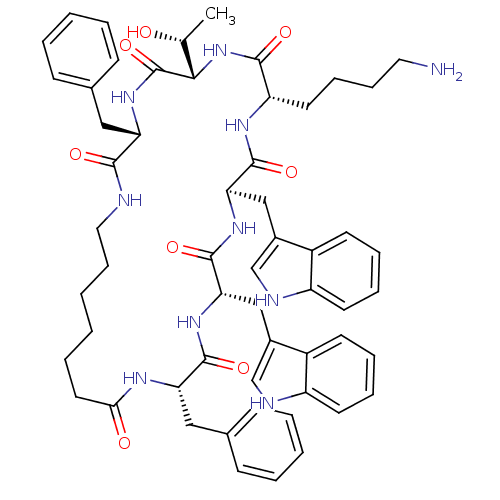
(Cyclo[L-Trp-D-Trp-L-Lys-L-Thr-L-Phe-(7-amino*hepta...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)CCCCCCNC(=O)[C@H](Cc2ccccc2)NC1=O Show InChI InChI=1S/C57H70N10O8/c1-36(68)51-57(75)66-46(30-37-18-6-4-7-19-37)52(70)59-29-17-3-2-10-27-50(69)62-47(31-38-20-8-5-9-21-38)54(72)64-49(33-40-35-61-44-25-14-12-23-42(40)44)56(74)65-48(32-39-34-60-43-24-13-11-22-41(39)43)55(73)63-45(53(71)67-51)26-15-16-28-58/h4-9,11-14,18-25,34-36,45-49,51,60-61,68H,2-3,10,15-17,26-33,58H2,1H3,(H,59,70)(H,62,69)(H,63,73)(H,64,72)(H,65,74)(H,66,75)(H,67,71)/t36-,45+,46+,47+,48-,49+,51+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 44: 385-92 (1993)
BindingDB Entry DOI: 10.7270/Q2X065KZ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50039022
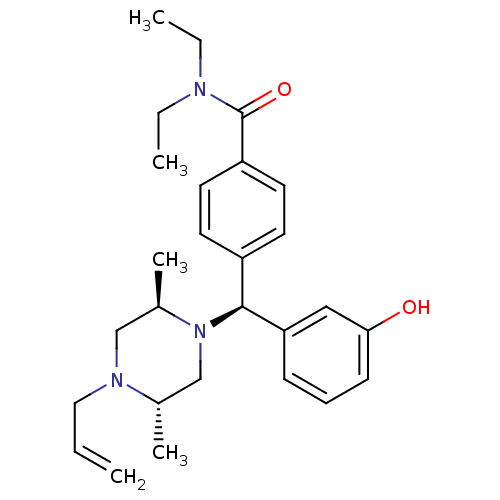
(4-[(S)-((2R,5S)-4-Allyl-2,5-dimethyl-piperazin-1-y...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)[C@H](N1C[C@H](C)N(CC=C)C[C@H]1C)c1cccc(O)c1 Show InChI InChI=1S/C27H37N3O2/c1-6-16-29-18-21(5)30(19-20(29)4)26(24-10-9-11-25(31)17-24)22-12-14-23(15-13-22)27(32)28(7-2)8-3/h6,9-15,17,20-21,26,31H,1,7-8,16,18-19H2,2-5H3/t20-,21+,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 45: 330-4 (1994)
Article DOI: 10.1016/j.bioorg.2015.02.008
BindingDB Entry DOI: 10.7270/Q21Z42X1 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM82552
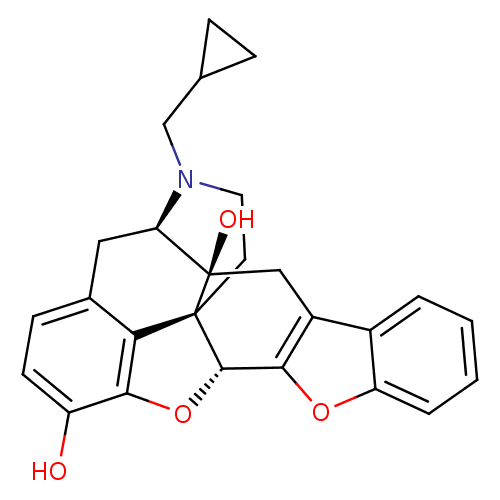
(CAS_111555-58-9 | NTB | naltrindolebenzofuran)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)c1oc2ccccc2c1C[C@@]35O |r| Show InChI InChI=1S/C26H25NO4/c28-18-8-7-15-11-20-26(29)12-17-16-3-1-2-4-19(16)30-22(17)24-25(26,21(15)23(18)31-24)9-10-27(20)13-14-5-6-14/h1-4,7-8,14,20,24,28-29H,5-6,9-13H2/t20-,24+,25+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 45: 330-4 (1994)
Article DOI: 10.1016/j.bioorg.2015.02.008
BindingDB Entry DOI: 10.7270/Q21Z42X1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50001714
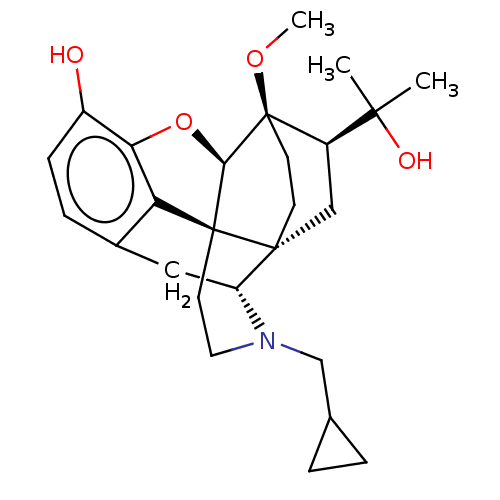
(2-[3-cyclopropylmethyl-11-hydroxy-15-methoxy-(1S,2...)Show SMILES CO[C@]12CC[C@@]3(C[C@@H]1C(C)(C)O)[C@H]1Cc4ccc(O)c5O[C@@H]2[C@]3(CCN1CC1CC1)c45 |THB:8:7:22.21:4.3| Show InChI InChI=1S/C26H35NO4/c1-23(2,29)18-13-24-8-9-26(18,30-3)22-25(24)10-11-27(14-15-4-5-15)19(24)12-16-6-7-17(28)21(31-22)20(16)25/h6-7,15,18-19,22,28-29H,4-5,8-14H2,1-3H3/t18-,19-,22-,24-,25+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 45: 330-4 (1994)
Article DOI: 10.1016/j.bioorg.2015.02.008
BindingDB Entry DOI: 10.7270/Q21Z42X1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM82551
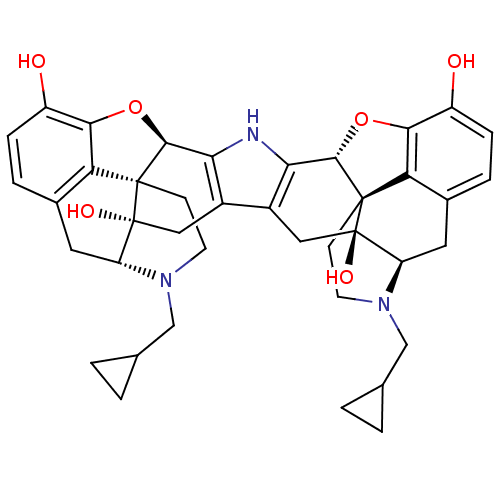
(C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)c1[nH]c2[C@@H]4Oc6c7c(C[C@H]8N(CC9CC9)CC[C@@]47[C@@]8(O)Cc2c1C[C@@]35O)ccc6O |r| Show InChI InChI=1S/C40H43N3O6/c44-25-7-5-21-13-27-39(46)15-23-24-16-40(47)28-14-22-6-8-26(45)34-30(22)38(40,10-12-43(28)18-20-3-4-20)36(49-34)32(24)41-31(23)35-37(39,29(21)33(25)48-35)9-11-42(27)17-19-1-2-19/h5-8,19-20,27-28,35-36,41,44-47H,1-4,9-18H2/t27-,28-,35+,36+,37+,38+,39-,40-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 45: 330-4 (1994)
Article DOI: 10.1016/j.bioorg.2015.02.008
BindingDB Entry DOI: 10.7270/Q21Z42X1 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50194731

(Boc-Phe-psi[S-CH(OH)CH2]Phe-Gln-Phe-NH2 | CHEMBL26...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C38H49N5O7/c1-38(2,3)50-37(49)43-30(22-26-15-9-5-10-16-26)32(44)24-28(21-25-13-7-4-8-14-25)35(47)41-29(19-20-33(39)45)36(48)42-31(34(40)46)23-27-17-11-6-12-18-27/h4-18,28-32,44H,19-24H2,1-3H3,(H2,39,45)(H2,40,46)(H,41,47)(H,42,48)(H,43,49)/t28-,29+,30+,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| MMDB
Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 protease |
J Med Chem 49: 5777-84 (2006)
Article DOI: 10.1021/jm0605583
BindingDB Entry DOI: 10.7270/Q2X63MK8 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM21864
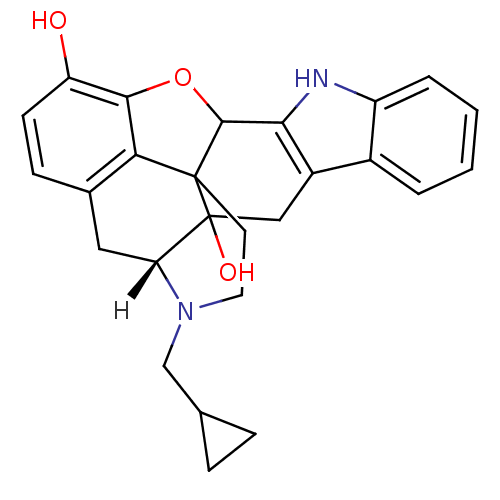
((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...)Show SMILES [H][C@@]12Cc3ccc(O)c4OC5c6[nH]c7ccccc7c6CC1(O)C5(CCN2CC1CC1)c34 |THB:27:26:21:31.2.3| Show InChI InChI=1S/C26H26N2O3/c29-19-8-7-15-11-20-26(30)12-17-16-3-1-2-4-18(16)27-22(17)24-25(26,21(15)23(19)31-24)9-10-28(20)13-14-5-6-14/h1-4,7-8,14,20,24,27,29-30H,5-6,9-13H2/t20-,24?,25?,26?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 45: 330-4 (1994)
Article DOI: 10.1016/j.bioorg.2015.02.008
BindingDB Entry DOI: 10.7270/Q21Z42X1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50126000
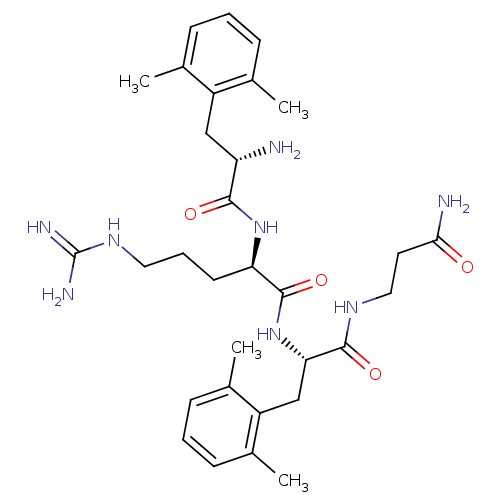
((R)-2-[(S)-2-Amino-3-(2,6-dimethyl-phenyl)-propion...)Show SMILES Cc1cccc(C)c1C[C@H](N)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c(C)cccc1C)C(=O)NCCC(N)=O Show InChI InChI=1S/C31H46N8O4/c1-18-8-5-9-19(2)22(18)16-24(32)28(41)38-25(12-7-14-37-31(34)35)30(43)39-26(29(42)36-15-13-27(33)40)17-23-20(3)10-6-11-21(23)4/h5-6,8-11,24-26H,7,12-17,32H2,1-4H3,(H2,33,40)(H,36,42)(H,38,41)(H,39,43)(H4,34,35,37)/t24-,25+,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0216 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University
Curated by ChEMBL
| Assay Description
Ability of the compound to displace [3H]-DAMGO from mu opioid receptor |
Bioorg Med Chem Lett 13: 1269-72 (2003)
BindingDB Entry DOI: 10.7270/Q2XS5TQ5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50451055
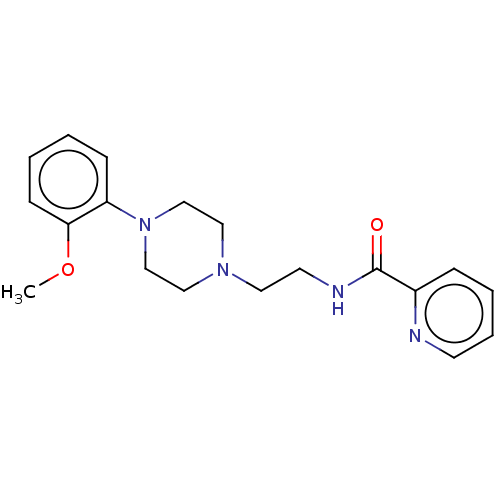
(CHEMBL4205290)Show InChI InChI=1S/C19H24N4O2/c1-25-18-8-3-2-7-17(18)23-14-12-22(13-15-23)11-10-21-19(24)16-6-4-5-9-20-16/h2-9H,10-15H2,1H3,(H,21,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Farmacia Universit£ di Napoli "Federico II" Via D. Montesano
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from serotonin 5-HT2A receptor in Sprague-Dawley rat brain cortex homogenates incubated for 15 mins by liquid scintill... |
Bioorg Med Chem 25: 5820-5837 (2017)
Article DOI: 10.1016/j.bmc.2017.09.018
BindingDB Entry DOI: 10.7270/Q2VD721F |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM83436
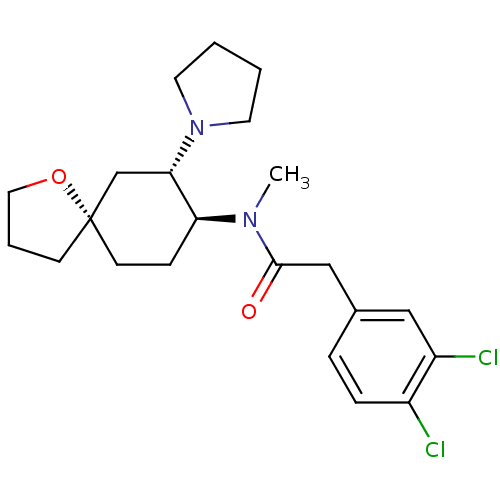
(2-(3,4-dichlorophenyl)-N-methyl-N-[(5R,7S,8S)-7-(1...)Show SMILES CN([C@H]1CC[C@@]2(CCCO2)C[C@@H]1N1CCCC1)C(=O)Cc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C22H30Cl2N2O2/c1-25(21(27)14-16-5-6-17(23)18(24)13-16)19-7-9-22(8-4-12-28-22)15-20(19)26-10-2-3-11-26/h5-6,13,19-20H,2-4,7-12,14-15H2,1H3/t19-,20-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 45: 330-4 (1994)
Article DOI: 10.1016/j.bioorg.2015.02.008
BindingDB Entry DOI: 10.7270/Q21Z42X1 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50442922
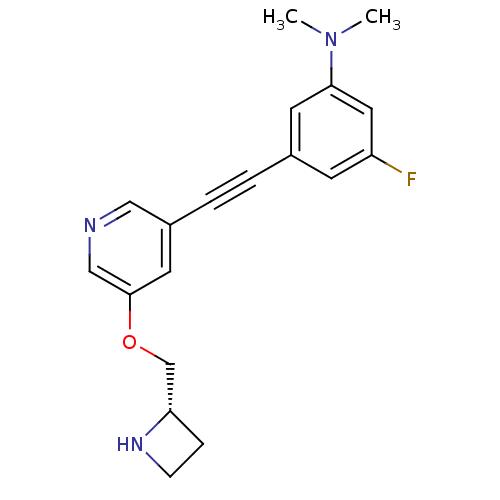
(CHEMBL3086984)Show SMILES CN(C)c1cc(F)cc(c1)C#Cc1cncc(OC[C@@H]2CCN2)c1 |r| Show InChI InChI=1S/C19H20FN3O/c1-23(2)18-8-14(7-16(20)10-18)3-4-15-9-19(12-21-11-15)24-13-17-5-6-22-17/h7-12,17,22H,5-6,13H2,1-2H3/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human alpha4beta2 nAChR after 4 hrs by cell-based assay |
J Med Chem 56: 8404-21 (2013)
Article DOI: 10.1021/jm4008455
BindingDB Entry DOI: 10.7270/Q2JW8GBV |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50442927

(CHEMBL3086994)Show InChI InChI=1S/C17H15FN2O/c18-15-3-1-2-13(8-15)4-5-14-9-17(11-19-10-14)21-12-16-6-7-20-16/h1-3,8-11,16,20H,6-7,12H2/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human alpha4beta2 nAChR after 4 hrs by cell-based assay |
J Med Chem 56: 8404-21 (2013)
Article DOI: 10.1021/jm4008455
BindingDB Entry DOI: 10.7270/Q2JW8GBV |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50126003
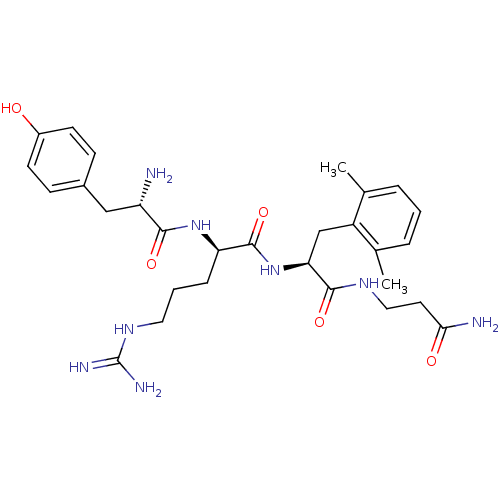
((R)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyla...)Show SMILES Cc1cccc(C)c1C[C@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCCC(N)=O Show InChI InChI=1S/C29H42N8O5/c1-17-5-3-6-18(2)21(17)16-24(27(41)34-14-12-25(31)39)37-28(42)23(7-4-13-35-29(32)33)36-26(40)22(30)15-19-8-10-20(38)11-9-19/h3,5-6,8-11,22-24,38H,4,7,12-16,30H2,1-2H3,(H2,31,39)(H,34,41)(H,36,40)(H,37,42)(H4,32,33,35)/t22-,23+,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University
Curated by ChEMBL
| Assay Description
Ability of the compound to displace [3H]-DAMGO from mu opioid receptor |
Bioorg Med Chem Lett 13: 1269-72 (2003)
BindingDB Entry DOI: 10.7270/Q2XS5TQ5 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50442921

(CHEMBL3086985)Show SMILES Cc1cc(F)cc(c1)C#Cc1cncc(OC[C@@H]2CCN2)c1 |r| Show InChI InChI=1S/C18H17FN2O/c1-13-6-14(8-16(19)7-13)2-3-15-9-18(11-20-10-15)22-12-17-4-5-21-17/h6-11,17,21H,4-5,12H2,1H3/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human alpha4beta2 nAChR after 4 hrs by cell-based assay |
J Med Chem 56: 8404-21 (2013)
Article DOI: 10.1021/jm4008455
BindingDB Entry DOI: 10.7270/Q2JW8GBV |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50442924

(CHEMBL3086982)Show InChI InChI=1S/C18H18N2O/c1-14-3-2-4-15(9-14)5-6-16-10-18(12-19-11-16)21-13-17-7-8-20-17/h2-4,9-12,17,20H,7-8,13H2,1H3/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human alpha4beta2 nAChR after 4 hrs by cell-based assay |
J Med Chem 56: 8404-21 (2013)
Article DOI: 10.1021/jm4008455
BindingDB Entry DOI: 10.7270/Q2JW8GBV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50451039
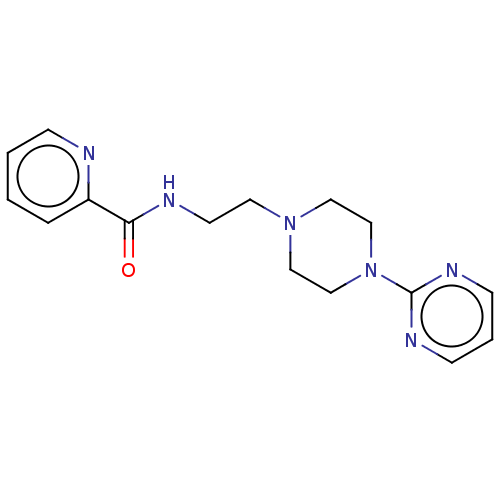
(CHEMBL4209954)Show InChI InChI=1S/C16H20N6O/c23-15(14-4-1-2-5-17-14)18-8-9-21-10-12-22(13-11-21)16-19-6-3-7-20-16/h1-7H,8-13H2,(H,18,23) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Farmacia Universit£ di Napoli "Federico II" Via D. Montesano
Curated by ChEMBL
| Assay Description
Displacement of [3H]-8-OH-DPAT from serotonin 5-HT1A receptor in Sprague-Dawley rat brain cortex homogenates incubated for 30 mins by liquid scintill... |
Bioorg Med Chem 25: 5820-5837 (2017)
Article DOI: 10.1016/j.bmc.2017.09.018
BindingDB Entry DOI: 10.7270/Q2VD721F |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50442930
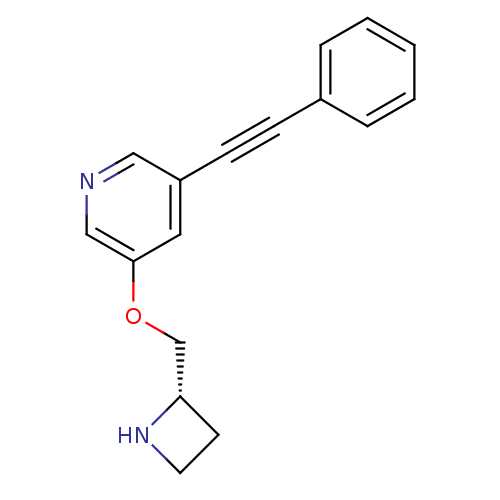
(CHEMBL3086991)Show InChI InChI=1S/C17H16N2O/c1-2-4-14(5-3-1)6-7-15-10-17(12-18-11-15)20-13-16-8-9-19-16/h1-5,10-12,16,19H,8-9,13H2/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human alpha4beta2 nAChR after 4 hrs by cell-based assay |
J Med Chem 56: 8404-21 (2013)
Article DOI: 10.1021/jm4008455
BindingDB Entry DOI: 10.7270/Q2JW8GBV |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM82555

(CAS_4424 | NSC_4424 | Naloxonazine)Show SMILES Oc1ccc2CC3N(CC=C)CCC45C(Oc1c24)C(CCC35O)=NN=C1CCC2(O)C3Cc4ccc(O)c5OC1C2(CCN3CC=C)c45 |w:24.28,18.21,TLB:27:28:43.42.41:31.32.47,22:21:5.4.17:12.11.7,20:21:5.4.17:12.11.7,THB:29:28:43.42.41:31.32.47| Show InChI InChI=1S/C38H42N4O6/c1-3-15-41-17-13-35-29-21-5-7-25(43)31(29)47-33(35)23(9-11-37(35,45)27(41)19-21)39-40-24-10-12-38(46)28-20-22-6-8-26(44)32-30(22)36(38,34(24)48-32)14-18-42(28)16-4-2/h3-8,27-28,33-34,43-46H,1-2,9-20H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 45: 330-4 (1994)
Article DOI: 10.1016/j.bioorg.2015.02.008
BindingDB Entry DOI: 10.7270/Q21Z42X1 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM123407
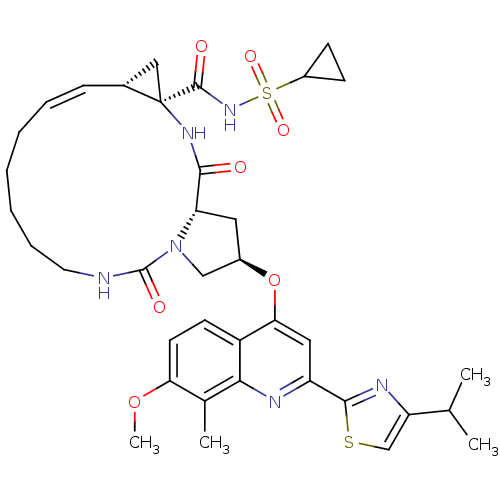
(US8741926, 91)Show SMILES COc1ccc2c(O[C@@H]3C[C@@H]4N(C3)C(=O)NCCCCC\C=C/[C@@H]3C[C@]3(NC4=O)C(=O)NS(=O)(=O)C3CC3)cc(nc2c1C)-c1nc(cs1)C(C)C |r,c:22| Show InChI InChI=1S/C37H46N6O7S2/c1-21(2)28-20-51-34(40-28)27-17-31(26-13-14-30(49-4)22(3)32(26)39-27)50-24-16-29-33(44)41-37(35(45)42-52(47,48)25-11-12-25)18-23(37)10-8-6-5-7-9-15-38-36(46)43(29)19-24/h8,10,13-14,17,20-21,23-25,29H,5-7,9,11-12,15-16,18-19H2,1-4H3,(H,38,46)(H,41,44)(H,42,45)/b10-8-/t23-,24-,29+,37-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0500 | -59.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB
US Patent
| Assay Description
The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... |
US Patent US8741926 (2014)
BindingDB Entry DOI: 10.7270/Q2Z31XBC |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM123410
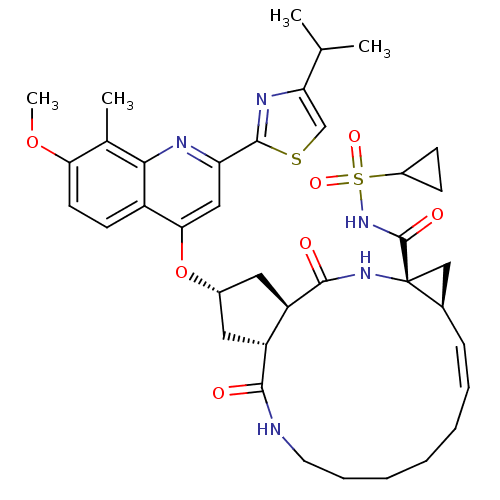
(US8741926, 82 | US8754106, 82 | US8754106, 91)Show SMILES COc1ccc2c(O[C@H]3C[C@@H]4[C@@H](C3)C(=O)N[C@@]3(C[C@H]3\C=C/CCCCCNC4=O)C(=O)NS(=O)(=O)C3CC3)cc(nc2c1C)-c1nc(cs1)C(C)C |r,c:21| Show InChI InChI=1S/C38H47N5O7S2/c1-21(2)30-20-51-36(41-30)29-18-32(26-13-14-31(49-4)22(3)33(26)40-29)50-24-16-27-28(17-24)35(45)42-38(37(46)43-52(47,48)25-11-12-25)19-23(38)10-8-6-5-7-9-15-39-34(27)44/h8,10,13-14,18,20-21,23-25,27-28H,5-7,9,11-12,15-17,19H2,1-4H3,(H,39,44)(H,42,45)(H,43,46)/b10-8-/t23-,24+,27-,28-,38-/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB
US Patent
| Assay Description
The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... |
US Patent US8754106 (2014)
BindingDB Entry DOI: 10.7270/Q2V40SWX |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50442926
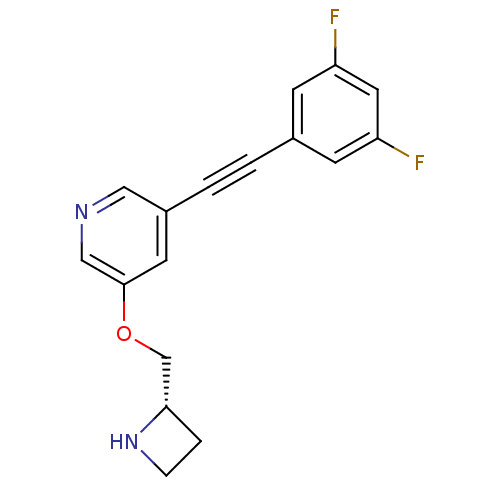
(CHEMBL3086995)Show SMILES Fc1cc(F)cc(c1)C#Cc1cncc(OC[C@@H]2CCN2)c1 |r| Show InChI InChI=1S/C17H14F2N2O/c18-14-5-12(6-15(19)8-14)1-2-13-7-17(10-20-9-13)22-11-16-3-4-21-16/h5-10,16,21H,3-4,11H2/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human alpha4beta2 nAChR after 4 hrs by cell-based assay |
J Med Chem 56: 8404-21 (2013)
Article DOI: 10.1021/jm4008455
BindingDB Entry DOI: 10.7270/Q2JW8GBV |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50131385

((2R,3R,4R,5S)-3,4,5-Trihydroxy-1-(4-phenoxy-benzen...)Show SMILES ONC(=O)[C@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1S(=O)(=O)c1ccc(Oc2ccccc2)cc1 |r| Show InChI InChI=1S/C18H20N2O8S/c21-14-10-20(15(18(24)19-25)17(23)16(14)22)29(26,27)13-8-6-12(7-9-13)28-11-4-2-1-3-5-11/h1-9,14-17,21-23,25H,10H2,(H,19,24)/t14-,15+,16+,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human matrix metalloproteinase-9 |
Bioorg Med Chem Lett 13: 2737-40 (2003)
BindingDB Entry DOI: 10.7270/Q2PC31SG |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50131385

((2R,3R,4R,5S)-3,4,5-Trihydroxy-1-(4-phenoxy-benzen...)Show SMILES ONC(=O)[C@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1S(=O)(=O)c1ccc(Oc2ccccc2)cc1 |r| Show InChI InChI=1S/C18H20N2O8S/c21-14-10-20(15(18(24)19-25)17(23)16(14)22)29(26,27)13-8-6-12(7-9-13)28-11-4-2-1-3-5-11/h1-9,14-17,21-23,25H,10H2,(H,19,24)/t14-,15+,16+,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center N-21
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant matrix metalloprotease 9 |
J Med Chem 47: 1930-8 (2004)
Article DOI: 10.1021/jm0304313
BindingDB Entry DOI: 10.7270/Q2ZS2VXF |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50125997
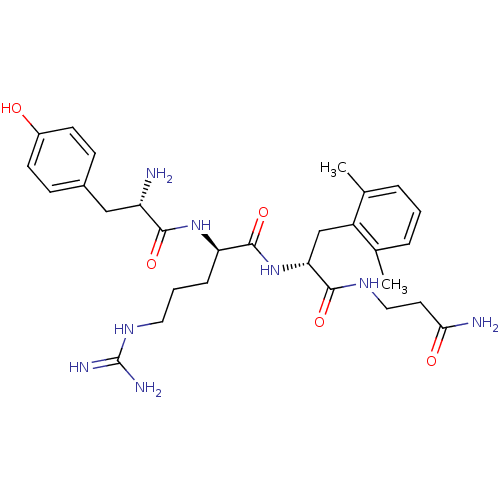
((R)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyla...)Show SMILES Cc1cccc(C)c1C[C@@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCCC(N)=O Show InChI InChI=1S/C29H42N8O5/c1-17-5-3-6-18(2)21(17)16-24(27(41)34-14-12-25(31)39)37-28(42)23(7-4-13-35-29(32)33)36-26(40)22(30)15-19-8-10-20(38)11-9-19/h3,5-6,8-11,22-24,38H,4,7,12-16,30H2,1-2H3,(H2,31,39)(H,34,41)(H,36,40)(H,37,42)(H4,32,33,35)/t22-,23+,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0618 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University
Curated by ChEMBL
| Assay Description
Ability of the compound to displace [3H]-DAMGO from mu opioid receptor |
Bioorg Med Chem Lett 13: 1269-72 (2003)
BindingDB Entry DOI: 10.7270/Q2XS5TQ5 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50382474
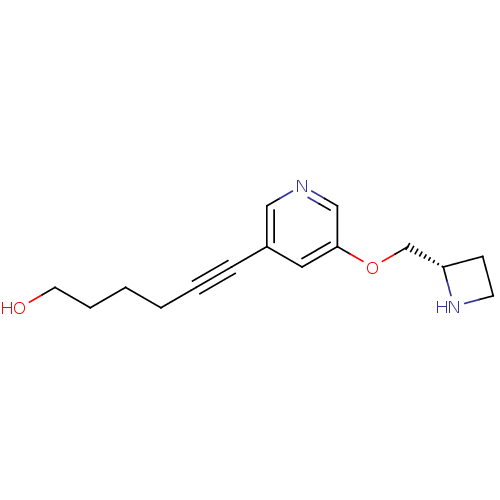
(CHEMBL2024096 | US9303017, Sazetidine-A)Show InChI InChI=1S/C15H20N2O2/c18-8-4-2-1-3-5-13-9-15(11-16-10-13)19-12-14-6-7-17-14/h9-11,14,17-18H,1-2,4,6-8,12H2/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human alpha4beta2 nAChR after 4 hrs by cell-based assay |
J Med Chem 56: 8404-21 (2013)
Article DOI: 10.1021/jm4008455
BindingDB Entry DOI: 10.7270/Q2JW8GBV |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50125996

((R)-2-[(S)-2-Amino-3-(2,6-dimethyl-phenyl)-propion...)Show SMILES Cc1cccc(C)c1C[C@H](N)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCCC(N)=O Show InChI InChI=1S/C29H42N8O4/c1-18-8-6-9-19(2)21(18)17-22(30)26(39)36-23(12-7-14-35-29(32)33)28(41)37-24(16-20-10-4-3-5-11-20)27(40)34-15-13-25(31)38/h3-6,8-11,22-24H,7,12-17,30H2,1-2H3,(H2,31,38)(H,34,40)(H,36,39)(H,37,41)(H4,32,33,35)/t22-,23+,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0623 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University
Curated by ChEMBL
| Assay Description
Ability of the compound to displace [3H]-DAMGO from mu opioid receptor |
Bioorg Med Chem Lett 13: 1269-72 (2003)
BindingDB Entry DOI: 10.7270/Q2XS5TQ5 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50001714

(2-[3-cyclopropylmethyl-11-hydroxy-15-methoxy-(1S,2...)Show SMILES CO[C@]12CC[C@@]3(C[C@@H]1C(C)(C)O)[C@H]1Cc4ccc(O)c5O[C@@H]2[C@]3(CCN1CC1CC1)c45 |THB:8:7:22.21:4.3| Show InChI InChI=1S/C26H35NO4/c1-23(2,29)18-13-24-8-9-26(18,30-3)22-25(24)10-11-27(14-15-4-5-15)19(24)12-16-6-7-17(28)21(31-22)20(16)25/h6-7,15,18-19,22,28-29H,4-5,8-14H2,1-3H3/t18-,19-,22-,24-,25+,26-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 45: 330-4 (1994)
Article DOI: 10.1016/j.bioorg.2015.02.008
BindingDB Entry DOI: 10.7270/Q21Z42X1 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM479434
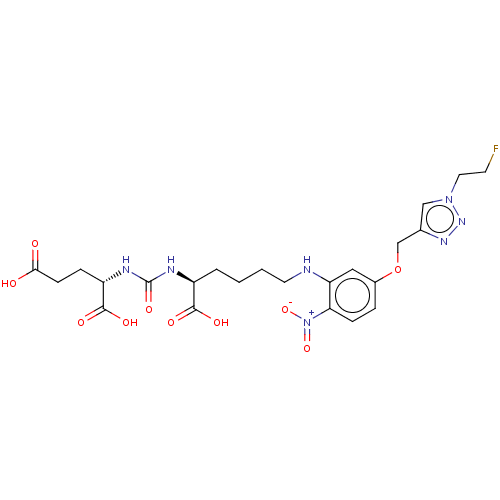
(US10894807, ID P242)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNc1cc(OCc2cn(CCF)nn2)ccc1[N+]([O-])=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C23H30FN7O10/c24-8-10-30-12-14(28-29-30)13-41-15-4-6-19(31(39)40)18(11-15)25-9-2-1-3-16(21(34)35)26-23(38)27-17(22(36)37)5-7-20(32)33/h4,6,11-12,16-17,25H,1-3,5,7-10,13H2,(H,32,33)(H,34,35)(H,36,37)(H2,26,27,38)/t16-,17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc.
US Patent
| Assay Description
The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... |
US Patent US10894807 (2021)
BindingDB Entry DOI: 10.7270/Q2SN0D25 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50442928
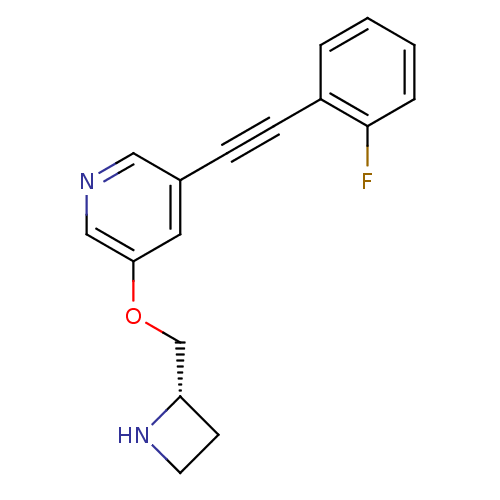
(CHEMBL3086993)Show InChI InChI=1S/C17H15FN2O/c18-17-4-2-1-3-14(17)6-5-13-9-16(11-19-10-13)21-12-15-7-8-20-15/h1-4,9-11,15,20H,7-8,12H2/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human alpha4beta2 nAChR after 4 hrs by cell-based assay |
J Med Chem 56: 8404-21 (2013)
Article DOI: 10.1021/jm4008455
BindingDB Entry DOI: 10.7270/Q2JW8GBV |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50442920

(CHEMBL3086986)Show SMILES COc1cc(F)cc(c1)C#Cc1cncc(OC[C@@H]2CCN2)c1 |r| Show InChI InChI=1S/C18H17FN2O2/c1-22-17-7-13(6-15(19)9-17)2-3-14-8-18(11-20-10-14)23-12-16-4-5-21-16/h6-11,16,21H,4-5,12H2,1H3/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human alpha4beta2 nAChR after 4 hrs by cell-based assay |
J Med Chem 56: 8404-21 (2013)
Article DOI: 10.1021/jm4008455
BindingDB Entry DOI: 10.7270/Q2JW8GBV |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(RAT) | BDBM82462
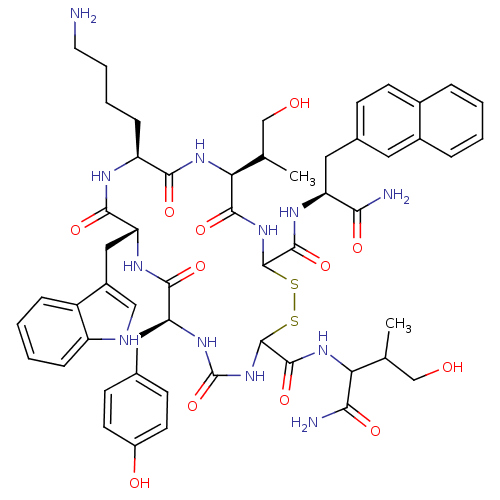
(BIM 23059 | D-Nal-c[Cys-Tyr-D-Trp-Lys-Thr-Cys]-Thr...)Show SMILES CC(CO)C(NC(=O)C1NC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)CO)C(=O)NC(SS1)C(=O)N[C@@H](Cc1ccc2ccccc2c1)C(N)=O)C(N)=O |r| Show InChI InChI=1S/C54H68N12O12S2/c1-28(26-67)42(45(57)71)63-51(77)53-66-54(78)62-40(22-30-15-18-35(69)19-16-30)47(73)61-41(24-34-25-58-37-12-6-5-11-36(34)37)48(74)59-38(13-7-8-20-55)46(72)64-43(29(2)27-68)49(75)65-52(79-80-53)50(76)60-39(44(56)70)23-31-14-17-32-9-3-4-10-33(32)21-31/h3-6,9-12,14-19,21,25,28-29,38-43,52-53,58,67-69H,7-8,13,20,22-24,26-27,55H2,1-2H3,(H2,56,70)(H2,57,71)(H,59,74)(H,60,76)(H,61,73)(H,63,77)(H,64,72)(H,65,75)(H2,62,66,78)/t28?,29?,38-,39-,40-,41+,42?,43-,52?,53?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 44: 385-92 (1993)
BindingDB Entry DOI: 10.7270/Q2X065KZ |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50109635
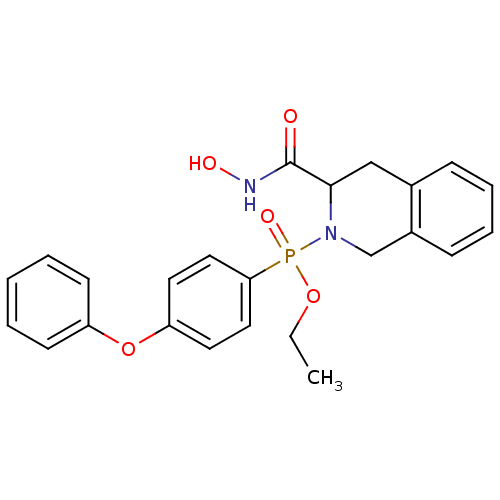
((3-Hydroxycarbamoyl-3,4-dihydro-1H-isoquinolin-2-y...)Show SMILES CCOP(=O)(N1Cc2ccccc2CC1C(=O)NO)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C24H25N2O5P/c1-2-30-32(29,22-14-12-21(13-15-22)31-20-10-4-3-5-11-20)26-17-19-9-7-6-8-18(19)16-23(26)24(27)25-28/h3-15,23,28H,2,16-17H2,1H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K.
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-9 (MMP-9)(gelatinase-B). |
J Med Chem 45: 919-29 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8G9Q |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50013388
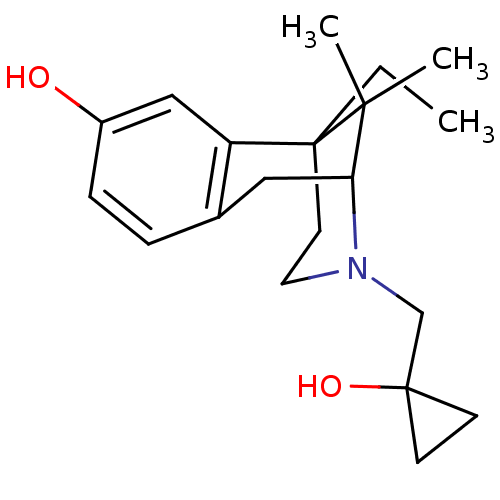
(6-Ethyl-3-(1-hydroxy-cyclopropylmethyl)-11,11-dime...)Show SMILES CCC12CCN(CC3(O)CC3)C(Cc3ccc(O)cc13)C2(C)C |TLB:6:5:20:19.13.12| Show InChI InChI=1S/C20H29NO2/c1-4-20-9-10-21(13-19(23)7-8-19)17(18(20,2)3)11-14-5-6-15(22)12-16(14)20/h5-6,12,17,22-23H,4,7-11,13H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 45: 330-4 (1994)
Article DOI: 10.1016/j.bioorg.2015.02.008
BindingDB Entry DOI: 10.7270/Q21Z42X1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM82554

(Beta C N A | CAS_115070 | NSC_115070)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)C(CCC35O)N(CCCl)CCCl |TLB:21:22:7.12.13:5.4.18,THB:23:22:7.12.13:5.4.18| Show InChI InChI=1S/C24H32Cl2N2O3/c25-8-11-27(12-9-26)17-5-6-24(30)19-13-16-3-4-18(29)21-20(16)23(24,22(17)31-21)7-10-28(19)14-15-1-2-15/h3-4,15,17,19,22,29-30H,1-2,5-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 45: 330-4 (1994)
Article DOI: 10.1016/j.bioorg.2015.02.008
BindingDB Entry DOI: 10.7270/Q21Z42X1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM82427
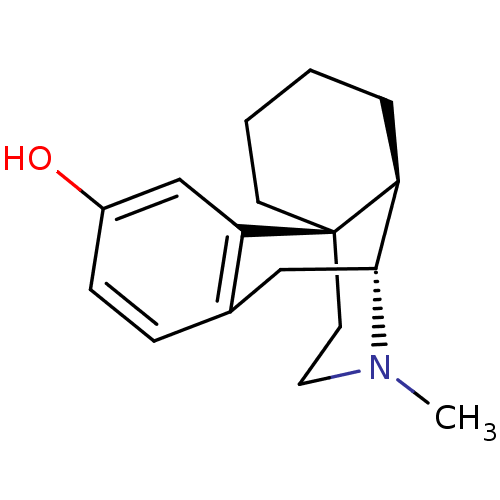
(CAS_5985-38-6 | LEVORPHANOL-tartarate)Show SMILES CN1CC[C@]23CCCC[C@H]2[C@H]1Cc1ccc(O)cc31 |r| Show InChI InChI=1S/C17H23NO/c1-18-9-8-17-7-3-2-4-14(17)16(18)10-12-5-6-13(19)11-15(12)17/h5-6,11,14,16,19H,2-4,7-10H2,1H3/t14-,16+,17+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 45: 330-4 (1994)
Article DOI: 10.1016/j.bioorg.2015.02.008
BindingDB Entry DOI: 10.7270/Q21Z42X1 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50442929
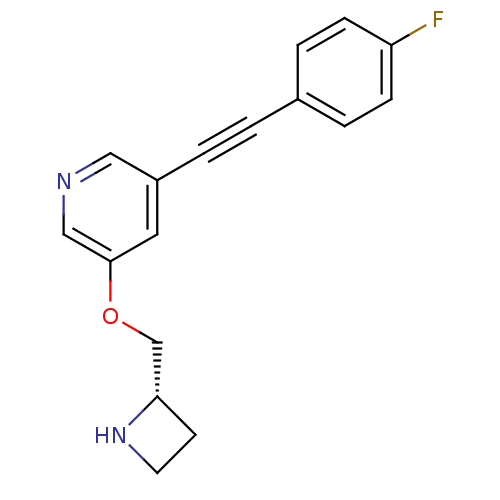
(CHEMBL3086992)Show InChI InChI=1S/C17H15FN2O/c18-15-5-3-13(4-6-15)1-2-14-9-17(11-19-10-14)21-12-16-7-8-20-16/h3-6,9-11,16,20H,7-8,12H2/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human alpha4beta2 nAChR after 4 hrs by cell-based assay |
J Med Chem 56: 8404-21 (2013)
Article DOI: 10.1021/jm4008455
BindingDB Entry DOI: 10.7270/Q2JW8GBV |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(RAT) | BDBM84630
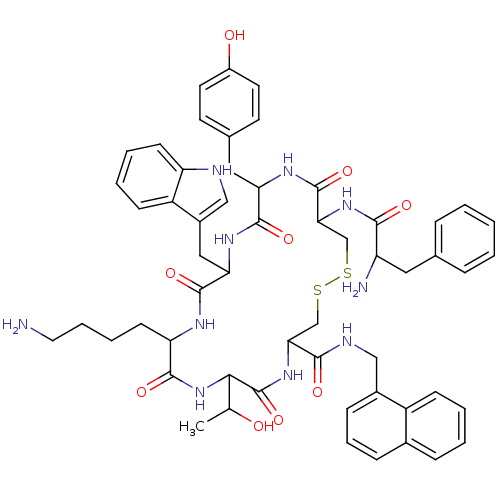
(BIM 23060)Show SMILES CC(O)C1NC(=O)C(CCCCN)NC(=O)C(Cc2c[nH]c3ccccc23)NC(=O)C(Cc2ccc(O)cc2)NC(=O)C(CSSCC(NC1=O)C(=O)NCc1cccc2ccccc12)NC(=O)C(N)Cc1ccccc1 Show InChI InChI=1S/C56H66N10O9S2/c1-33(67)49-56(75)65-47(51(70)60-29-37-16-11-15-36-14-5-6-17-40(36)37)31-76-77-32-48(64-50(69)42(58)26-34-12-3-2-4-13-34)55(74)62-45(27-35-21-23-39(68)24-22-35)53(72)63-46(28-38-30-59-43-19-8-7-18-41(38)43)54(73)61-44(52(71)66-49)20-9-10-25-57/h2-8,11-19,21-24,30,33,42,44-49,59,67-68H,9-10,20,25-29,31-32,57-58H2,1H3,(H,60,70)(H,61,73)(H,62,74)(H,63,72)(H,64,69)(H,65,75)(H,66,71) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 44: 385-92 (1993)
BindingDB Entry DOI: 10.7270/Q2X065KZ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50442923

(CHEMBL3086983)Show InChI InChI=1S/C17H15ClN2O/c18-15-3-1-2-13(8-15)4-5-14-9-17(11-19-10-14)21-12-16-6-7-20-16/h1-3,8-11,16,20H,6-7,12H2/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human alpha4beta2 nAChR after 4 hrs by cell-based assay |
J Med Chem 56: 8404-21 (2013)
Article DOI: 10.1021/jm4008455
BindingDB Entry DOI: 10.7270/Q2JW8GBV |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50143729

((2R,3R,4R,5R)-3,4,5-Trihydroxy-1-(4-phenoxy-benzen...)Show SMILES ONC(=O)[C@H]1[C@@H](O)[C@H](O)[C@H](O)CN1S(=O)(=O)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C18H20N2O8S/c21-14-10-20(15(18(24)19-25)17(23)16(14)22)29(26,27)13-8-6-12(7-9-13)28-11-4-2-1-3-5-11/h1-9,14-17,21-23,25H,10H2,(H,19,24)/t14-,15-,16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center N-21
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant matrix metalloprotease 9 |
J Med Chem 47: 1930-8 (2004)
Article DOI: 10.1021/jm0304313
BindingDB Entry DOI: 10.7270/Q2ZS2VXF |
More data for this
Ligand-Target Pair | |
Genome polyprotein [1027-1711]
(Hepatitis C virus genotype 1a (strain H77) (HCV)) | BDBM236566
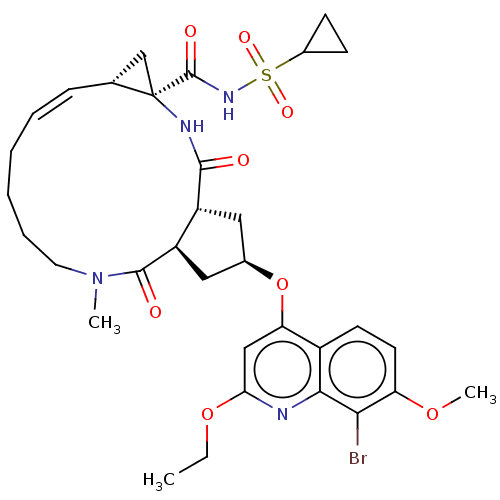
(US9365582, 7)Show SMILES CCOc1cc(O[C@@H]2C[C@@H]3[C@@H](C2)C(=O)N(C)CCCC\C=C/[C@@H]2C[C@]2(NC3=O)C(=O)NS(=O)(=O)C2CC2)c2ccc(OC)c(Br)c2n1 |r,c:21| Show InChI InChI=1S/C33H41BrN4O8S/c1-4-45-27-17-26(22-12-13-25(44-3)28(34)29(22)35-27)46-20-15-23-24(16-20)31(40)38(2)14-8-6-5-7-9-19-18-33(19,36-30(23)39)32(41)37-47(42,43)21-10-11-21/h7,9,12-13,17,19-21,23-24H,4-6,8,10-11,14-16,18H2,1-3H3,(H,36,39)(H,37,41)/b9-7-/t19-,20-,23-,24-,33-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Janssen Sciences Ireland UC
US Patent
| Assay Description
The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... |
US Patent US9365582 (2016)
BindingDB Entry DOI: 10.7270/Q2VM4B5G |
More data for this
Ligand-Target Pair | |
Genome polyprotein [1027-1711]
(Hepatitis C virus genotype 1a (strain H77) (HCV)) | BDBM236565

(US9365582, 5)Show SMILES CCOc1cc(O[C@@H]2C[C@@H]3[C@@H](C2)C(=O)N(C)CCCC\C=C/[C@@H]2C[C@]2(NC3=O)C(=O)NS(=O)(=O)C2CC2)c2ccc(OC)cc2n1 |r,c:21| Show InChI InChI=1S/C33H42N4O8S/c1-4-44-29-18-28(24-13-10-21(43-3)17-27(24)34-29)45-22-15-25-26(16-22)31(39)37(2)14-8-6-5-7-9-20-19-33(20,35-30(25)38)32(40)36-46(41,42)23-11-12-23/h7,9-10,13,17-18,20,22-23,25-26H,4-6,8,11-12,14-16,19H2,1-3H3,(H,35,38)(H,36,40)/b9-7-/t20-,22-,25-,26-,33-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Janssen Sciences Ireland UC
US Patent
| Assay Description
The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... |
US Patent US9365582 (2016)
BindingDB Entry DOI: 10.7270/Q2VM4B5G |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM123413
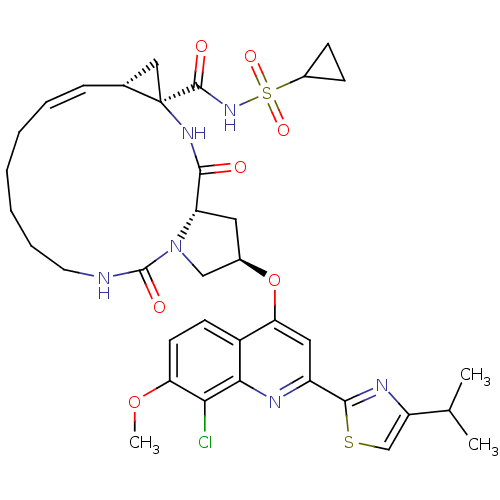
(US8741926, 94 | US8754106, 94)Show SMILES COc1ccc2c(O[C@@H]3C[C@@H]4N(C3)C(=O)NCCCCC\C=C/[C@@H]3C[C@]3(NC4=O)C(=O)NS(=O)(=O)C3CC3)cc(nc2c1Cl)-c1nc(cs1)C(C)C |r,c:22| Show InChI InChI=1S/C36H43ClN6O7S2/c1-20(2)26-19-51-33(40-26)25-16-29(24-12-13-28(49-3)30(37)31(24)39-25)50-22-15-27-32(44)41-36(34(45)42-52(47,48)23-10-11-23)17-21(36)9-7-5-4-6-8-14-38-35(46)43(27)18-22/h7,9,12-13,16,19-23,27H,4-6,8,10-11,14-15,17-18H2,1-3H3,(H,38,46)(H,41,44)(H,42,45)/b9-7-/t21-,22-,27+,36-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | -58.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB
US Patent
| Assay Description
The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... |
US Patent US8741926 (2014)
BindingDB Entry DOI: 10.7270/Q2Z31XBC |
More data for this
Ligand-Target Pair | |
Genome polyprotein [1027-1711]
(Hepatitis C virus genotype 1a (strain H77) (HCV)) | BDBM236571
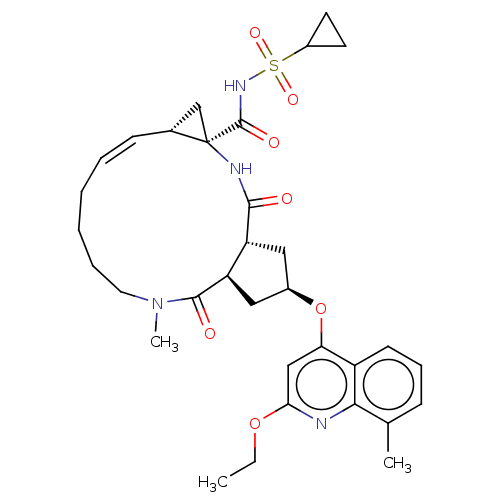
(US9365582, 13)Show SMILES CCOc1cc(O[C@@H]2C[C@@H]3[C@@H](C2)C(=O)N(C)CCCC\C=C/[C@@H]2C[C@]2(NC3=O)C(=O)NS(=O)(=O)C2CC2)c2cccc(C)c2n1 |r,c:21| Show InChI InChI=1S/C33H42N4O7S/c1-4-43-28-18-27(24-12-9-10-20(2)29(24)34-28)44-22-16-25-26(17-22)31(39)37(3)15-8-6-5-7-11-21-19-33(21,35-30(25)38)32(40)36-45(41,42)23-13-14-23/h7,9-12,18,21-23,25-26H,4-6,8,13-17,19H2,1-3H3,(H,35,38)(H,36,40)/b11-7-/t21-,22-,25-,26-,33-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Janssen Sciences Ireland UC
US Patent
| Assay Description
The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... |
US Patent US9365582 (2016)
BindingDB Entry DOI: 10.7270/Q2VM4B5G |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50109621
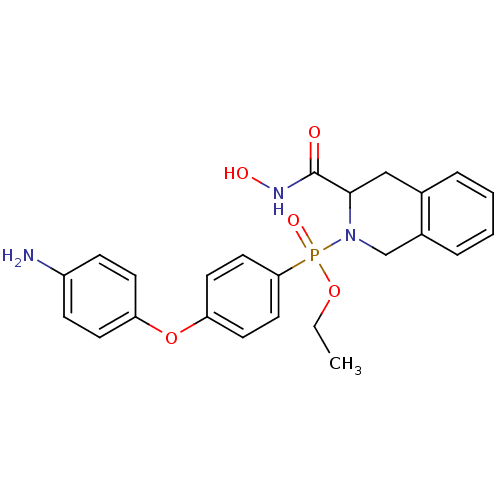
(CHEMBL425316 | [4-(4-Amino-phenoxy)-phenyl]-(3-hyd...)Show SMILES CCOP(=O)(N1Cc2ccccc2CC1C(=O)NO)c1ccc(Oc2ccc(N)cc2)cc1 Show InChI InChI=1S/C24H26N3O5P/c1-2-31-33(30,22-13-11-21(12-14-22)32-20-9-7-19(25)8-10-20)27-16-18-6-4-3-5-17(18)15-23(27)24(28)26-29/h3-14,23,29H,2,15-16,25H2,1H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K.
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-9 (MMP-9)(gelatinase-B). |
J Med Chem 45: 919-29 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8G9Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(RAT) | BDBM82470
(3-(2-Naphtyl)-D-Ala-L-Cys(1)-L-Tyr-D-Trp-L-Lys-L-V...)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CSSC[C@H](NC1=O)C(=O)N[C@@H]([C@@H](C)O)C(N)=O)NC(=O)[C@H](N)Cc1ccc2ccccc2c1 Show InChI InChI=1S/C54H69N11O10S2/c1-29(2)45-54(75)63-44(53(74)65-46(30(3)66)47(57)68)28-77-76-27-43(62-48(69)38(56)23-32-15-18-33-10-4-5-11-34(33)22-32)52(73)60-41(24-31-16-19-36(67)20-17-31)50(71)61-42(25-35-26-58-39-13-7-6-12-37(35)39)51(72)59-40(49(70)64-45)14-8-9-21-55/h4-7,10-13,15-20,22,26,29-30,38,40-46,58,66-67H,8-9,14,21,23-25,27-28,55-56H2,1-3H3,(H2,57,68)(H,59,72)(H,60,73)(H,61,71)(H,62,69)(H,63,75)(H,64,70)(H,65,74)/t30-,38-,40+,41+,42-,43+,44+,45+,46+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 44: 385-92 (1993)
BindingDB Entry DOI: 10.7270/Q2X065KZ |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM123415
(US8741926, 95 | US8754106, 95)Show SMILES COc1ccc2c(O[C@@H]3C[C@@H]4N(C3)C(=O)NCCCCC\C=C/[C@@H]3C[C@]3(NC4=O)C(=O)NS(=O)(=O)C3(C)CC3)cc(nc2c1Cl)-c1nc(cs1)C(C)C |r,c:22| Show InChI InChI=1S/C37H45ClN6O7S2/c1-21(2)26-20-52-33(41-26)25-17-29(24-11-12-28(50-4)30(38)31(24)40-25)51-23-16-27-32(45)42-37(34(46)43-53(48,49)36(3)13-14-36)18-22(37)10-8-6-5-7-9-15-39-35(47)44(27)19-23/h8,10-12,17,20-23,27H,5-7,9,13-16,18-19H2,1-4H3,(H,39,47)(H,42,45)(H,43,46)/b10-8-/t22-,23-,27+,37-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | -58.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB
US Patent
| Assay Description
The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... |
US Patent US8741926 (2014)
BindingDB Entry DOI: 10.7270/Q2Z31XBC |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM124106

(US8754106, 56)Show SMILES COc1ccc2c(O[C@@H]3C[C@@H]4[C@@H](C3)C(=O)N(C)CCCC\C=C/[C@@H]3C[C@]3(NC4=O)C(=O)NS(=O)(=O)C3CC3)cc(nc2c1Cl)-c1nc(cs1)C(C)C |r,c:22| Show InChI InChI=1S/C37H44ClN5O7S2/c1-20(2)28-19-51-34(40-28)27-17-30(24-12-13-29(49-4)31(38)32(24)39-27)50-22-15-25-26(16-22)35(45)43(3)14-8-6-5-7-9-21-18-37(21,41-33(25)44)36(46)42-52(47,48)23-10-11-23/h7,9,12-13,17,19-23,25-26H,5-6,8,10-11,14-16,18H2,1-4H3,(H,41,44)(H,42,46)/b9-7-/t21-,22-,25-,26-,37-/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB
US Patent
| Assay Description
The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... |
US Patent US8754106 (2014)
BindingDB Entry DOI: 10.7270/Q2V40SWX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data