

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50079482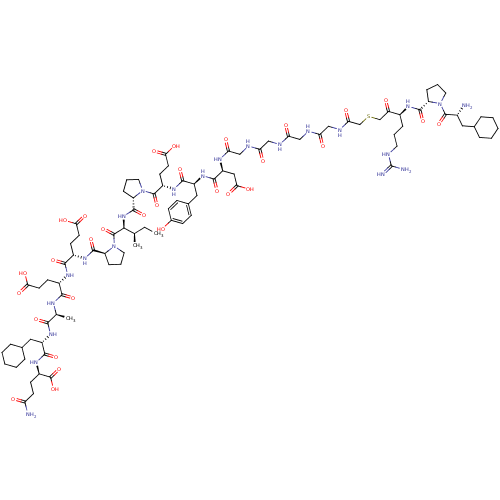 (Arginyl Ketomethylene analogue | CHEMBL410589) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.000350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council Canada Curated by ChEMBL | Assay Description Inhibitory constant against thrombin | J Med Chem 42: 3109-15 (1999) Article DOI: 10.1021/jm9807297 BindingDB Entry DOI: 10.7270/Q2VQ31WT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50079489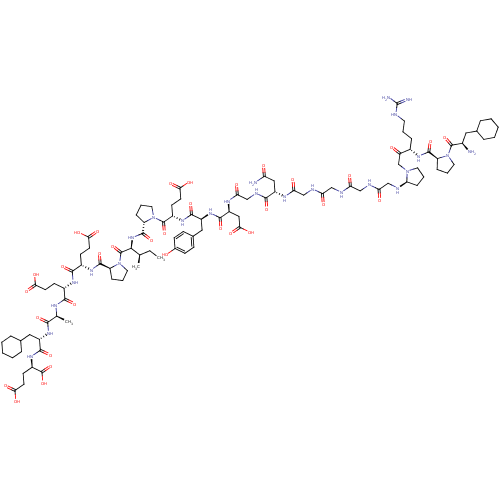 (Arginyl Ketomethylene analogue | CHEMBL428116) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.000570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council Canada Curated by ChEMBL | Assay Description Inhibitory constant against thrombin | J Med Chem 42: 3109-15 (1999) Article DOI: 10.1021/jm9807297 BindingDB Entry DOI: 10.7270/Q2VQ31WT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50079476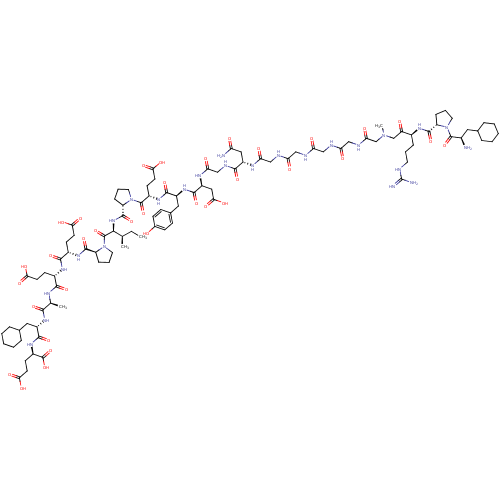 (Arginyl Ketomethylene analogue | CHEMBL437873) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.000870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council Canada Curated by ChEMBL | Assay Description Inhibitory constant against thrombin | J Med Chem 42: 3109-15 (1999) Article DOI: 10.1021/jm9807297 BindingDB Entry DOI: 10.7270/Q2VQ31WT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50079479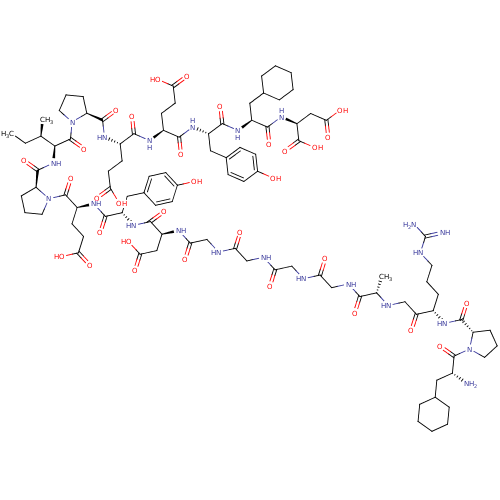 (Arginyl Ketomethylene analogue | CHEMBL407043) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.00150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council Canada Curated by ChEMBL | Assay Description Inhibitory constant against thrombin | J Med Chem 42: 3109-15 (1999) Article DOI: 10.1021/jm9807297 BindingDB Entry DOI: 10.7270/Q2VQ31WT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50079485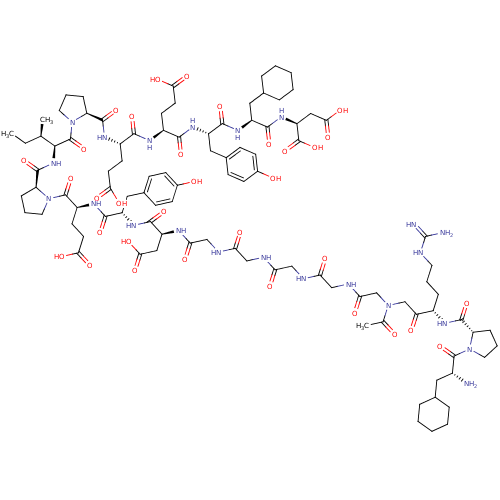 (Arginyl Ketomethylene analogue | CHEMBL414489) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.00170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council Canada Curated by ChEMBL | Assay Description Inhibitory constant against thrombin | J Med Chem 42: 3109-15 (1999) Article DOI: 10.1021/jm9807297 BindingDB Entry DOI: 10.7270/Q2VQ31WT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50079478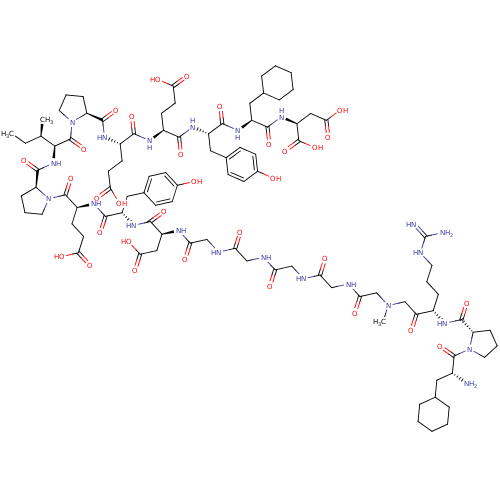 (Arginyl Ketomethylene analogue | CHEMBL414760) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council Canada Curated by ChEMBL | Assay Description Inhibitory constant against thrombin | J Med Chem 42: 3109-15 (1999) Article DOI: 10.1021/jm9807297 BindingDB Entry DOI: 10.7270/Q2VQ31WT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50483336 (CHEMBL1651153 | GRL-0476) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.00270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 54: 622-34 (2011) Article DOI: 10.1021/jm1012787 BindingDB Entry DOI: 10.7270/Q2BV7KG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM13925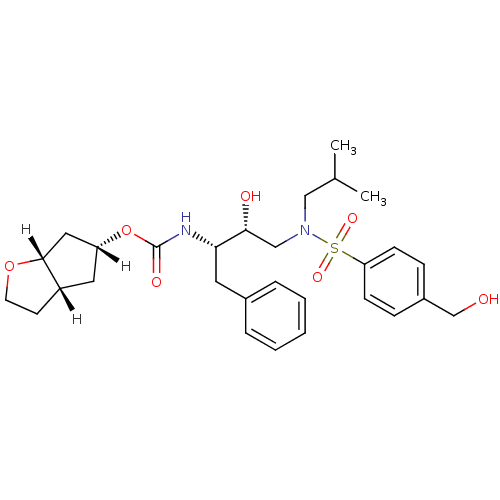 ((3aS,5R,6aR)-hexahydro-2H-cyclopenta[b]furan-5-yl ...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Graduate School of Medical and Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease dimerization in MT2 cells | J Biol Chem 282: 28709-20 (2007) Article DOI: 10.1074/jbc.M703938200 BindingDB Entry DOI: 10.7270/Q2JS9T6J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM13925 ((3aS,5R,6aR)-hexahydro-2H-cyclopenta[b]furan-5-yl ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00450 | -64.8 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 49: 5252-61 (2006) Article DOI: 10.1021/jm060561m BindingDB Entry DOI: 10.7270/Q23R0R41 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM13925 ((3aS,5R,6aR)-hexahydro-2H-cyclopenta[b]furan-5-yl ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00450 | -64.8 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 49: 5252-61 (2006) Article DOI: 10.1021/jm060561m BindingDB Entry DOI: 10.7270/Q23R0R41 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50483338 (CHEMBL1651155) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 54: 622-34 (2011) Article DOI: 10.1021/jm1012787 BindingDB Entry DOI: 10.7270/Q2BV7KG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50481584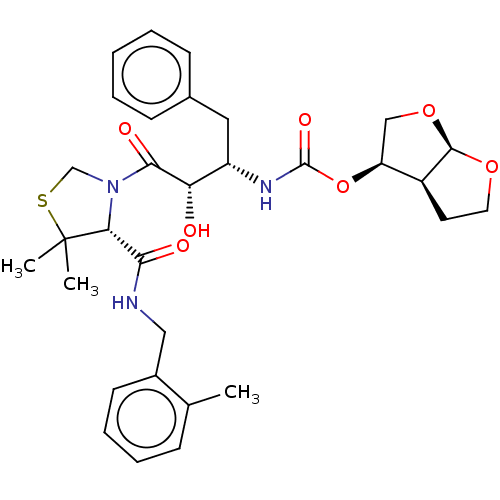 (CHEMBL589988 | GRL-0355) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem Lett 20: 1241-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.123 BindingDB Entry DOI: 10.7270/Q2CJ8HB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343599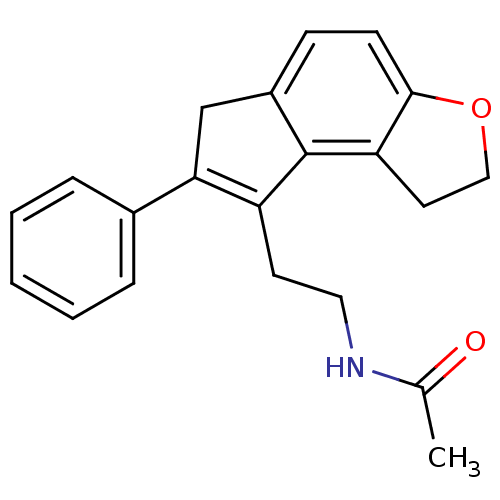 (CHEMBL1774522 | N-[2-(7-Phenyl-1,6-dihydro-2H-inde...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of 2-[125I]iodomelatonin from human MT2 receptor expressed in CHO cells | J Med Chem 54: 4207-18 (2011) Article DOI: 10.1021/jm200385u BindingDB Entry DOI: 10.7270/Q2HT2PN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM4685 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Graduate School of Medical and Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease dimerization in MT2 cells | J Biol Chem 282: 28709-20 (2007) Article DOI: 10.1074/jbc.M703938200 BindingDB Entry DOI: 10.7270/Q2JS9T6J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50343599 (CHEMBL1774522 | N-[2-(7-Phenyl-1,6-dihydro-2H-inde...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of 2-[125I]iodomelatonin from human MT1 receptor expressed in CHO cells | J Med Chem 54: 4207-18 (2011) Article DOI: 10.1021/jm200385u BindingDB Entry DOI: 10.7270/Q2HT2PN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM86812 (CAS_45263784 | NSC_45263784 | rac-2-(6-fluoro-5-(4...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by PDSP Ki Database | Bioorg Med Chem 16: 746-54 (2008) Article DOI: 10.1016/j.bmc.2007.10.027 BindingDB Entry DOI: 10.7270/Q2H130KC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50079491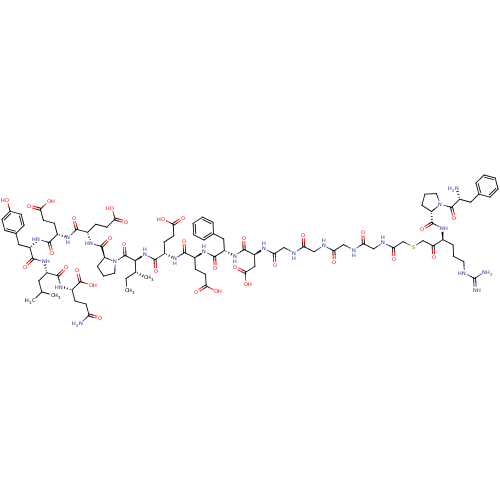 (Arginyl Ketomethylene analogue | CHEMBL412457) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.00940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council Canada Curated by ChEMBL | Assay Description Inhibitory constant against thrombin | J Med Chem 42: 3109-15 (1999) Article DOI: 10.1021/jm9807297 BindingDB Entry DOI: 10.7270/Q2VQ31WT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM9236 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Graduate School of Medical and Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease dimerization in MT2 cells | J Biol Chem 282: 28709-20 (2007) Article DOI: 10.1074/jbc.M703938200 BindingDB Entry DOI: 10.7270/Q2JS9T6J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50483334 (CHEMBL1651160) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 54: 622-34 (2011) Article DOI: 10.1021/jm1012787 BindingDB Entry DOI: 10.7270/Q2BV7KG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM35968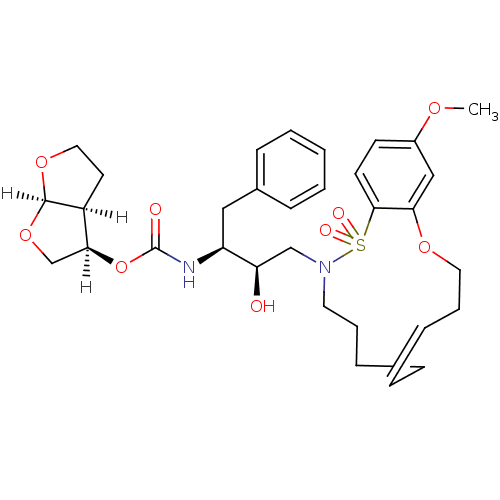 (cyclic compound, 14c | cyclic compound, 14c-Z) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0120 | -62.3 | n/a | n/a | 4.60 | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 52: 7689-705 (2009) Article DOI: 10.1021/jm900695w BindingDB Entry DOI: 10.7270/Q2G44NN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM35968 (cyclic compound, 14c | cyclic compound, 14c-Z) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0120 | -62.3 | n/a | n/a | 4.60 | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 52: 7689-705 (2009) Article DOI: 10.1021/jm900695w BindingDB Entry DOI: 10.7270/Q2G44NN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0140 | -62.0 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 49: 5252-61 (2006) Article DOI: 10.1021/jm060561m BindingDB Entry DOI: 10.7270/Q23R0R41 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0140 | -62.0 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 49: 5252-61 (2006) Article DOI: 10.1021/jm060561m BindingDB Entry DOI: 10.7270/Q23R0R41 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9236 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0140 | -62.0 | n/a | n/a | 1.20 | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 52: 7689-705 (2009) Article DOI: 10.1021/jm900695w BindingDB Entry DOI: 10.7270/Q2G44NN7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9236 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0140 | -62.0 | n/a | n/a | 1.20 | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 52: 7689-705 (2009) Article DOI: 10.1021/jm900695w BindingDB Entry DOI: 10.7270/Q2G44NN7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50118470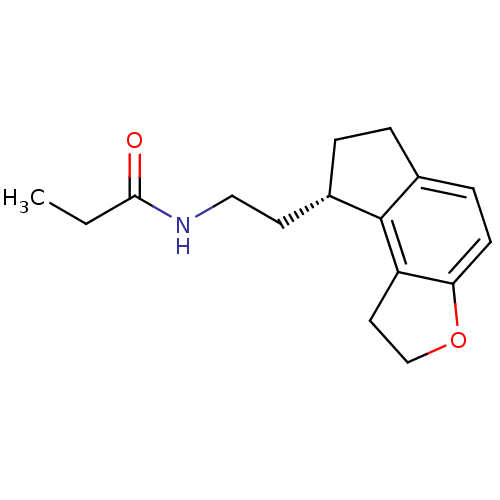 (CHEMBL1218 | N-[2-(1,6,7,8-Tetrahydro-2H-3-oxa-as-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human MT1 expressed in CHO cells | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01836 BindingDB Entry DOI: 10.7270/Q25H7M2B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50476647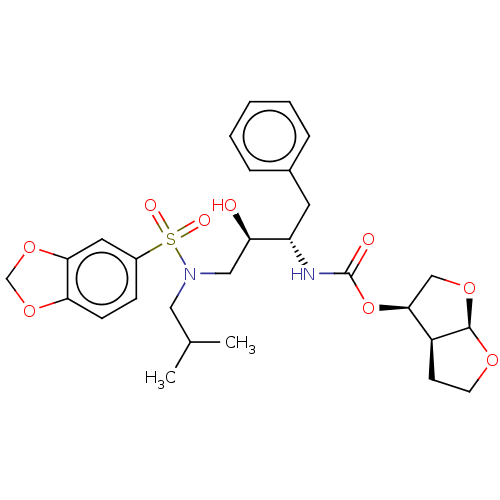 (CHEMBL178593 | GRL-98065) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Graduate School of Medical and Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease dimerization in MT2 cells | J Biol Chem 282: 28709-20 (2007) Article DOI: 10.1074/jbc.M703938200 BindingDB Entry DOI: 10.7270/Q2JS9T6J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem Lett 20: 1241-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.123 BindingDB Entry DOI: 10.7270/Q2CJ8HB8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0160 | -61.6 | n/a | n/a | 1.60 | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 52: 7689-705 (2009) Article DOI: 10.1021/jm900695w BindingDB Entry DOI: 10.7270/Q2G44NN7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Graduate School of Medical and Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease dimerization in MT2 cells | J Biol Chem 282: 28709-20 (2007) Article DOI: 10.1074/jbc.M703938200 BindingDB Entry DOI: 10.7270/Q2JS9T6J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0160 | -61.6 | n/a | n/a | 1.60 | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 52: 7689-705 (2009) Article DOI: 10.1021/jm900695w BindingDB Entry DOI: 10.7270/Q2G44NN7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM35984 (cyclic compound, 15d) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0170 | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 52: 7689-705 (2009) Article DOI: 10.1021/jm900695w BindingDB Entry DOI: 10.7270/Q2G44NN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM35984 (cyclic compound, 15d) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0170 | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 52: 7689-705 (2009) Article DOI: 10.1021/jm900695w BindingDB Entry DOI: 10.7270/Q2G44NN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM50100717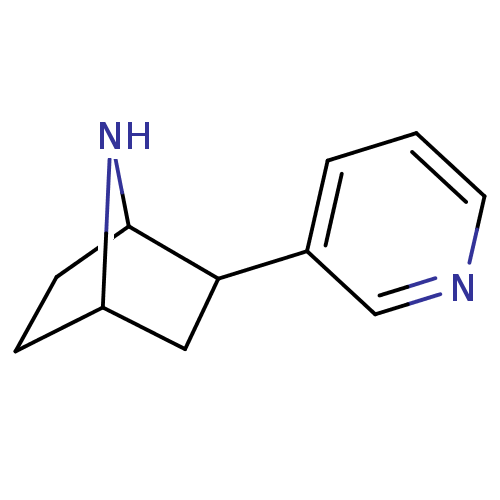 (2-(pyridin-3-yl)-7-aza-bicyclo[2.2.1]heptane | 2-P...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by PDSP Ki Database | Bioorg Med Chem 16: 746-54 (2008) Article DOI: 10.1016/j.bmc.2007.10.027 BindingDB Entry DOI: 10.7270/Q2H130KC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50162061 (2-(5-Ethynyl-pyridin-3-yl)-7-aza-bicyclo[2.2.1]hep...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]epibatidine binding to Nicotinic acetylcholine receptor alpha4-beta2 in rat cerebral cortex | J Med Chem 48: 1221-8 (2005) Article DOI: 10.1021/jm040160b BindingDB Entry DOI: 10.7270/Q26W9BV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM25367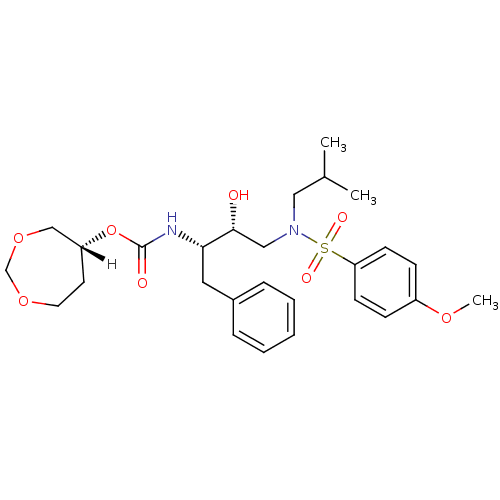 ((5R)-1,3-dioxepan-5-yl N-[(2S,3R)-3-hydroxy-4-[(4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB Article PubMed | 0.0260 | -60.4 | 4.90 | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 51: 6021-33 (2008) Article DOI: 10.1021/jm8004543 BindingDB Entry DOI: 10.7270/Q2G15Z51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM25367 ((5R)-1,3-dioxepan-5-yl N-[(2S,3R)-3-hydroxy-4-[(4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB Article PubMed | 0.0260 | -60.4 | 4.90 | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 51: 6021-33 (2008) Article DOI: 10.1021/jm8004543 BindingDB Entry DOI: 10.7270/Q2G15Z51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by PDSP Ki Database | Bioorg Med Chem 16: 746-54 (2008) Article DOI: 10.1016/j.bmc.2007.10.027 BindingDB Entry DOI: 10.7270/Q2H130KC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]epibatidine binding to Nicotinic acetylcholine receptor alpha4-beta2 in rat cerebral cortex | J Med Chem 48: 1221-8 (2005) Article DOI: 10.1021/jm040160b BindingDB Entry DOI: 10.7270/Q26W9BV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50347590 (CHEMBL1802028) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of 2-[125I]iodomelatonin from human MT1 receptor expressed in CHO cells | J Med Chem 54: 4207-18 (2011) Article DOI: 10.1021/jm200385u BindingDB Entry DOI: 10.7270/Q2HT2PN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50575683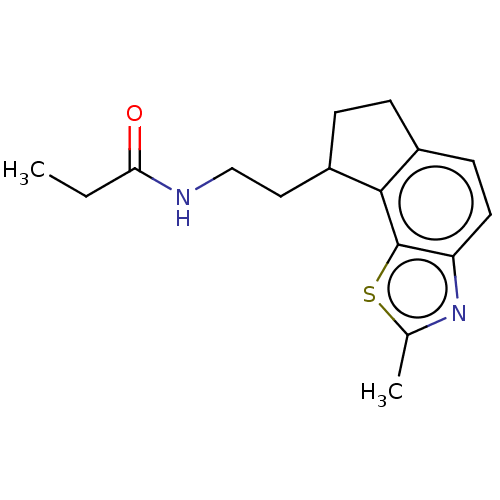 (CHEMBL4868554) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of 2-[125l]-lodomelatonin from human MT1 expressed in CHO cell membrane incubated for 150 mins by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01836 BindingDB Entry DOI: 10.7270/Q25H7M2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50478337 (CHEMBL403306 | GRL-0036A) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Graduate School of Medical and Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease dimerization in MT2 cells | J Biol Chem 282: 28709-20 (2007) Article DOI: 10.1074/jbc.M703938200 BindingDB Entry DOI: 10.7270/Q2JS9T6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM86815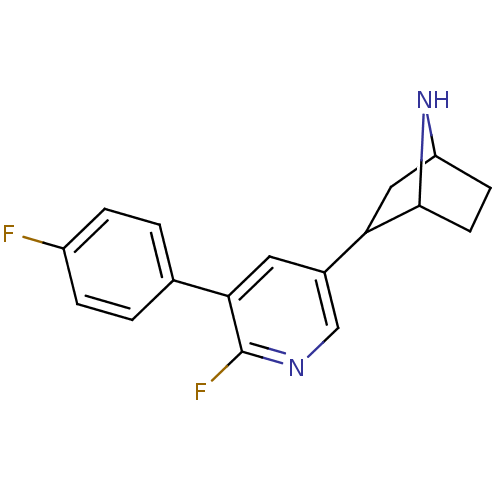 (CAS_45266019 | NSC_45266019 | rac-2-(6-fluoro-5-(4...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by PDSP Ki Database | Bioorg Med Chem 16: 746-54 (2008) Article DOI: 10.1016/j.bmc.2007.10.027 BindingDB Entry DOI: 10.7270/Q2H130KC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50162062 (2-(5-Iodo-pyridin-3-yl)-7-methyl-7-aza-bicyclo[2.2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]epibatidine binding to Nicotinic acetylcholine receptor alpha4-beta2 in rat cerebral cortex | J Med Chem 48: 1221-8 (2005) Article DOI: 10.1021/jm040160b BindingDB Entry DOI: 10.7270/Q26W9BV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A,L591M] (Human immunodeficiency virus type 1) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University | Assay Description The inhibition assays were performed in microtiter plate wells by mixing enzyme and fluorescent peptide substrate in the presence of inhibitor compou... | J Med Chem 49: 1379-87 (2006) Article DOI: 10.1021/jm050943c BindingDB Entry DOI: 10.7270/Q20C4T0V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM103443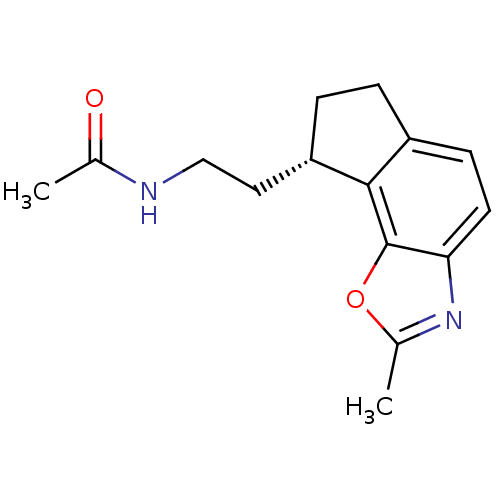 (US8552037, 90) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of 2-[125l]-lodomelatonin from human MT1 expressed in CHO cell membrane incubated for 150 mins by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01836 BindingDB Entry DOI: 10.7270/Q25H7M2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM50442965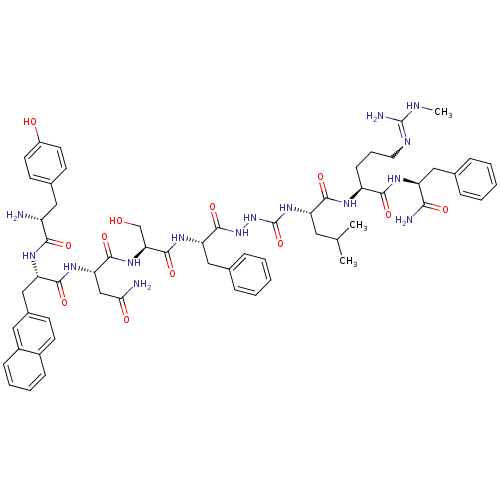 (CHEMBL3086282) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Binding affinity to human KISS1R expressed in CHO cell membranes | J Med Chem 56: 8298-307 (2013) Article DOI: 10.1021/jm401056w BindingDB Entry DOI: 10.7270/Q25M675S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM50442966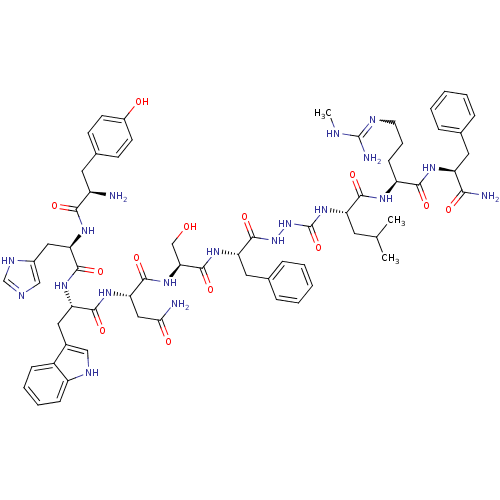 (CHEMBL3087927) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Binding affinity to human KISS1R expressed in CHO cell membranes | J Med Chem 56: 8298-307 (2013) Article DOI: 10.1021/jm401056w BindingDB Entry DOI: 10.7270/Q25M675S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50079480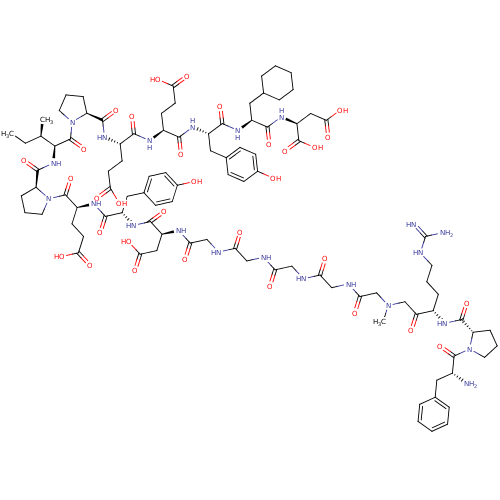 (Arginyl Ketomethylene analogue | CHEMBL415375) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council Canada Curated by ChEMBL | Assay Description Inhibitory constant against thrombin | J Med Chem 42: 3109-15 (1999) Article DOI: 10.1021/jm9807297 BindingDB Entry DOI: 10.7270/Q2VQ31WT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM31822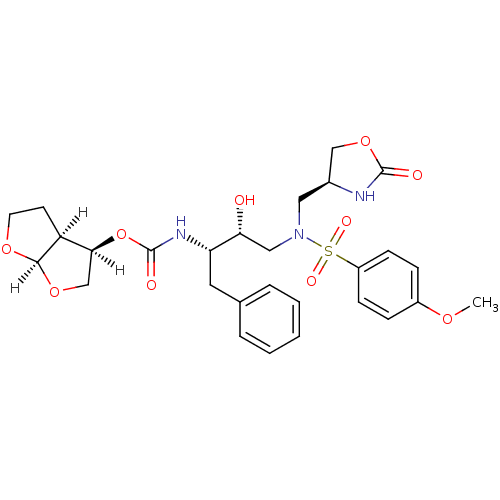 (oxazolidinone, 31) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0350 | -59.7 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 52: 3902-14 (2009) Article DOI: 10.1021/jm900303m BindingDB Entry DOI: 10.7270/Q20G3HHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 16388 total ) | Next | Last >> |