

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Enoyl-[acyl-carrier-protein] reductase [NADH] (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50425950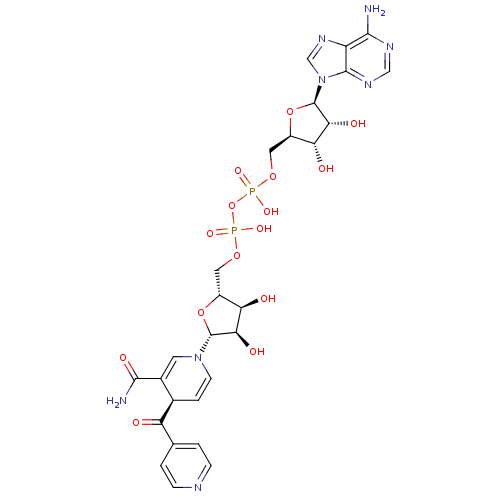 (CHEMBL2311561 | Isoniazid-NAD) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu Institute of Technology Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis InhA | Eur J Med Chem 60: 333-9 (2013) Article DOI: 10.1016/j.ejmech.2012.12.012 BindingDB Entry DOI: 10.7270/Q2DZ09MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM85786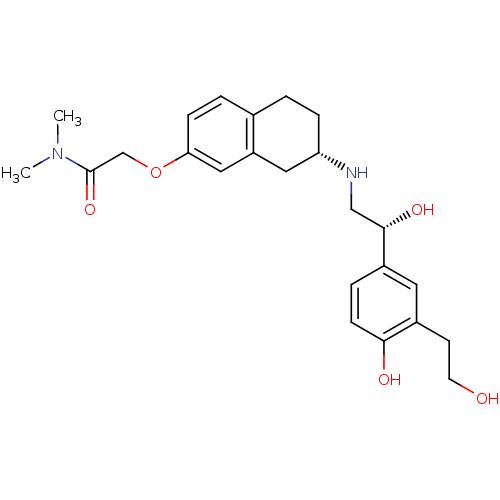 (KUR-1246) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PubMed | 25.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 297: 666-71 (2001) BindingDB Entry DOI: 10.7270/Q2D798Z1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dioxygenase (Pseudomonas paucimobilis) | BDBM50111620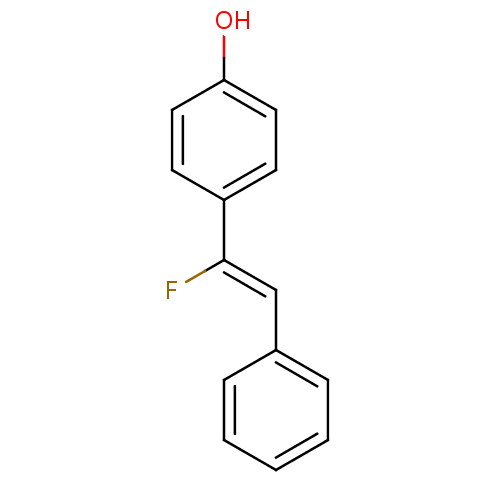 (4-((Z)-1-Fluoro-2-phenyl-vinyl)-phenol | CHEMBL147...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Curated by ChEMBL | Assay Description Tested for the inhibition of Lignostilbene-alpha, beta-dioxygenase (LSD) | Bioorg Med Chem Lett 12: 1139-42 (2002) BindingDB Entry DOI: 10.7270/Q2KS6QV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate kinase (Mycobacterium tuberculosis) | BDBM50131916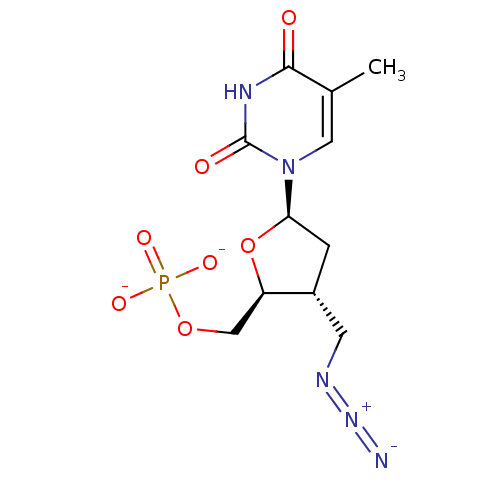 (Phosphoric acid mono-[3-azidomethyl-5-(5-methyl-2,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu Institute of Technology Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis thymidine monophosphate kinase | Eur J Med Chem 60: 333-9 (2013) Article DOI: 10.1016/j.ejmech.2012.12.012 BindingDB Entry DOI: 10.7270/Q2DZ09MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM85786 (KUR-1246) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 297: 666-71 (2001) BindingDB Entry DOI: 10.7270/Q2D798Z1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate kinase (Mycobacterium tuberculosis) | BDBM50131914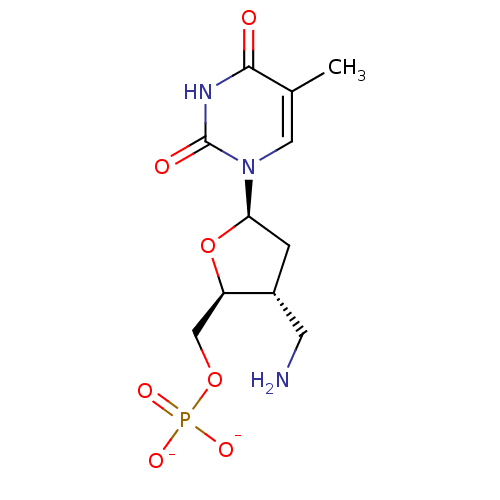 (Phosphoric acid mono-[3-aminomethyl-5-(5-methyl-2,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu Institute of Technology Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis thymidine monophosphate kinase | Eur J Med Chem 60: 333-9 (2013) Article DOI: 10.1016/j.ejmech.2012.12.012 BindingDB Entry DOI: 10.7270/Q2DZ09MS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50472624 (CHEMBL62439) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. | J Med Chem 43: 2040-8 (2000) Article DOI: 10.1021/jm9906015 BindingDB Entry DOI: 10.7270/Q2H134R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50472616 (CHEMBL65325) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. | J Med Chem 43: 2040-8 (2000) Article DOI: 10.1021/jm9906015 BindingDB Entry DOI: 10.7270/Q2H134R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50472627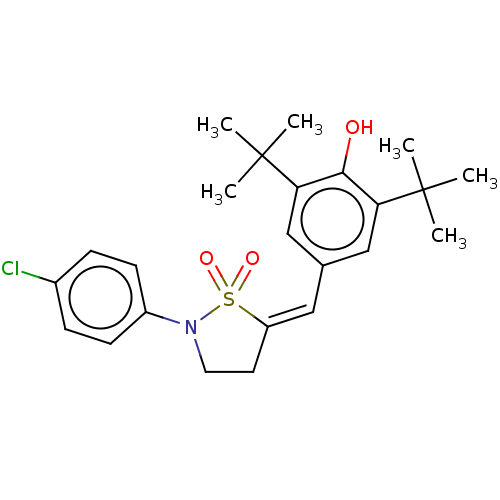 (CHEMBL304489) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. | J Med Chem 43: 2040-8 (2000) Article DOI: 10.1021/jm9906015 BindingDB Entry DOI: 10.7270/Q2H134R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50472632 (CHEMBL65140) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. | J Med Chem 43: 2040-8 (2000) Article DOI: 10.1021/jm9906015 BindingDB Entry DOI: 10.7270/Q2H134R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50472618 (CHEMBL303092) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. | J Med Chem 43: 2040-8 (2000) Article DOI: 10.1021/jm9906015 BindingDB Entry DOI: 10.7270/Q2H134R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50472623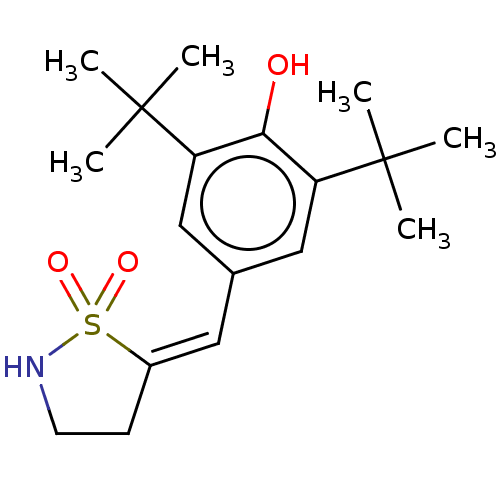 (CHEMBL294100) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. | J Med Chem 43: 2040-8 (2000) Article DOI: 10.1021/jm9906015 BindingDB Entry DOI: 10.7270/Q2H134R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50472613 (CHEMBL305076) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. | J Med Chem 43: 2040-8 (2000) Article DOI: 10.1021/jm9906015 BindingDB Entry DOI: 10.7270/Q2H134R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50472612 (CHEMBL60496) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. | J Med Chem 43: 2040-8 (2000) Article DOI: 10.1021/jm9906015 BindingDB Entry DOI: 10.7270/Q2H134R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50472605 (CHEMBL64507) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. | J Med Chem 43: 2040-8 (2000) Article DOI: 10.1021/jm9906015 BindingDB Entry DOI: 10.7270/Q2H134R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50483553 (CHEBI:76007 | Dolutegravir Sodium | GSK1349572 | G...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of Human immunodeficiency virus 1 Integrase using [3H]labeled target DNA as substrate after 45 mins by scintil... | Antimicrob Agents Chemother 55: 813-21 (2011) Article DOI: 10.1128/AAC.01209-10 BindingDB Entry DOI: 10.7270/Q2RX9FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50062551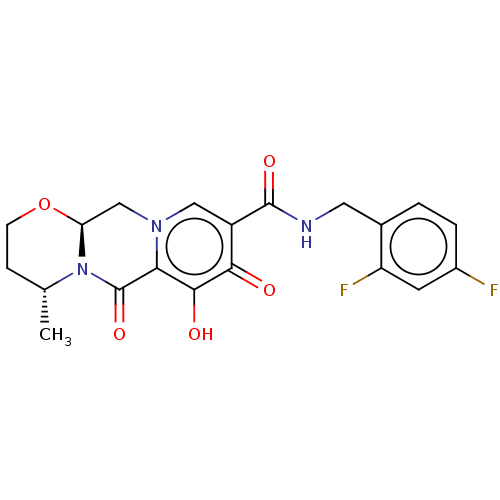 (CHEBI:76010 | Dolutegravir | GSK1349572 | S-349572) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development Curated by ChEMBL | Assay Description Inhibition of HIV-1 integrase strand transfer activity using [3H]-DNA as substrate preincubated for 60 mins prior to substrate addition measured afte... | J Med Chem 56: 5901-16 (2013) Article DOI: 10.1021/jm400645w BindingDB Entry DOI: 10.7270/Q21G0Q64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. | J Med Chem 43: 2040-8 (2000) Article DOI: 10.1021/jm9906015 BindingDB Entry DOI: 10.7270/Q2H134R8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50492496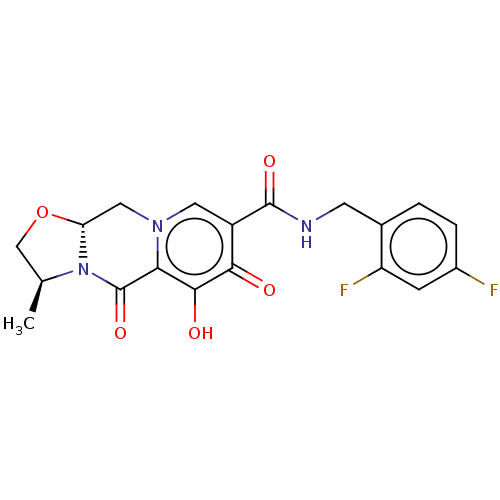 (Cabotegravir | GSK-1265744A | GSK1265744 | GSK1265...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development Curated by ChEMBL | Assay Description Inhibition of HIV-1 integrase strand transfer activity using [3H]-DNA as substrate preincubated for 60 mins prior to substrate addition measured afte... | J Med Chem 56: 5901-16 (2013) Article DOI: 10.1021/jm400645w BindingDB Entry DOI: 10.7270/Q21G0Q64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM579099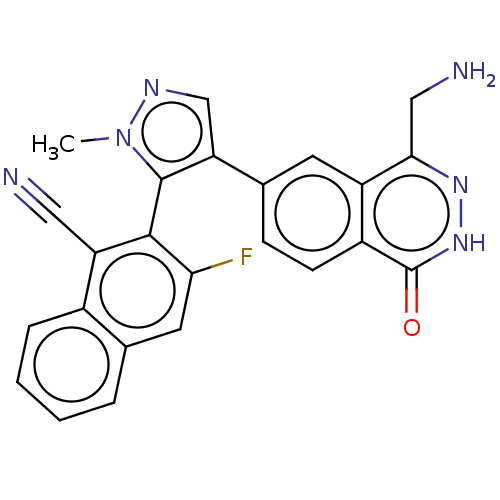 (& 16-2 | US11479551, Example 16-1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PRMT5 methyltransferase activity in MTAP knockout human HCT-116 cells assessed as inhibition of PRMT5- mediated SDMA modification level... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01900 BindingDB Entry DOI: 10.7270/Q2DB85RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM579099 (& 16-2 | US11479551, Example 16-1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PRMT5 methyltransferase activity in MTAP knockout human HCT-116 cells assessed as inhibition of PRMT5- mediated SDMA modification level... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01900 BindingDB Entry DOI: 10.7270/Q2DB85RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM25351 (N-[2-(4-{[(4-fluorophenyl)methyl]carbamoyl}-5-hydr...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus 1 integrase by strand transfer scintillation proximity assay | Antimicrob Agents Chemother 52: 901-8 (2008) Article DOI: 10.1128/aac.01218-07 BindingDB Entry DOI: 10.7270/Q2JQ13VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50480673 (Isentress | Isentress hd | MK-0518 | MK-0518 POTAS...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of Human immunodeficiency virus 1 Integrase using [3H]labeled target DNA as substrate after 45 mins by scintil... | Antimicrob Agents Chemother 55: 813-21 (2011) Article DOI: 10.1128/AAC.01209-10 BindingDB Entry DOI: 10.7270/Q2RX9FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50585596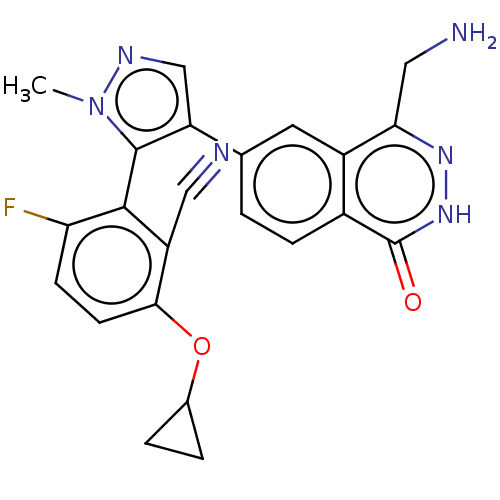 (CHEMBL5076766) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PRMT5 methyltransferase activity in MTAP knockout human HCT-116 cells assessed as inhibition of PRMT5- mediated SDMA modification level... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01900 BindingDB Entry DOI: 10.7270/Q2DB85RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50492497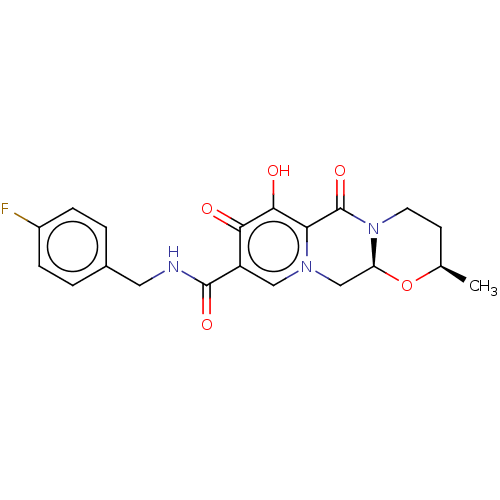 (CHEMBL2403118) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development Curated by ChEMBL | Assay Description Inhibition of HIV-1 integrase strand transfer activity using [3H]-DNA as substrate preincubated for 60 mins prior to substrate addition measured afte... | J Med Chem 56: 5901-16 (2013) Article DOI: 10.1021/jm400645w BindingDB Entry DOI: 10.7270/Q21G0Q64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM579030 (2-(4-(4-(aminomethyl)-1-oxo-1,2- dihydrophthalazin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PRMT5 methyltransferase activity in MTAP knockout human HCT-116 cells assessed as inhibition of PRMT5- mediated SDMA modification level... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01900 BindingDB Entry DOI: 10.7270/Q2DB85RR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50362167 (CHEMBL1941129) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGD2 from human CRTH2 receptor expressed in HEK293 cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 22: 1194-7 (2012) Article DOI: 10.1016/j.bmcl.2011.11.079 BindingDB Entry DOI: 10.7270/Q2765FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50362160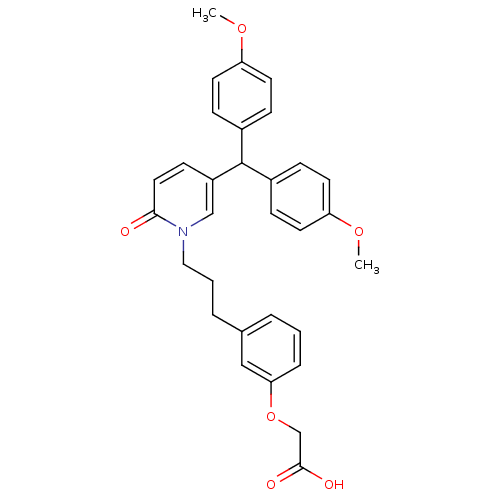 (CHEMBL1941121) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGD2 from human CRTH2 receptor expressed in HEK293 cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 22: 1194-7 (2012) Article DOI: 10.1016/j.bmcl.2011.11.079 BindingDB Entry DOI: 10.7270/Q2765FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM579105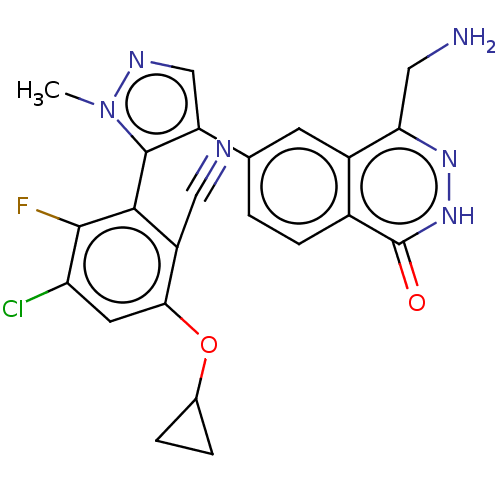 (US11479551, Example 16-7 | US11479551, Example 16-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PRMT5 methyltransferase activity in MTAP knockout human HCT-116 cells assessed as inhibition of PRMT5- mediated SDMA modification level... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01900 BindingDB Entry DOI: 10.7270/Q2DB85RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM579105 (US11479551, Example 16-7 | US11479551, Example 16-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PRMT5 methyltransferase activity in MTAP knockout human HCT-116 cells assessed as inhibition of PRMT5- mediated SDMA modification level... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01900 BindingDB Entry DOI: 10.7270/Q2DB85RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50183273 ((S)-6-(3-chloro-2-fluorobenzyl)-1-(1-hydroxy-3-met...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of Human immunodeficiency virus 1 Integrase using [3H]labeled target DNA as substrate after 45 mins by scintil... | Antimicrob Agents Chemother 55: 813-21 (2011) Article DOI: 10.1128/AAC.01209-10 BindingDB Entry DOI: 10.7270/Q2RX9FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50183273 ((S)-6-(3-chloro-2-fluorobenzyl)-1-(1-hydroxy-3-met...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus 1 integrase by strand transfer scintillation proximity assay | Antimicrob Agents Chemother 52: 901-8 (2008) Article DOI: 10.1128/aac.01218-07 BindingDB Entry DOI: 10.7270/Q2JQ13VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50472615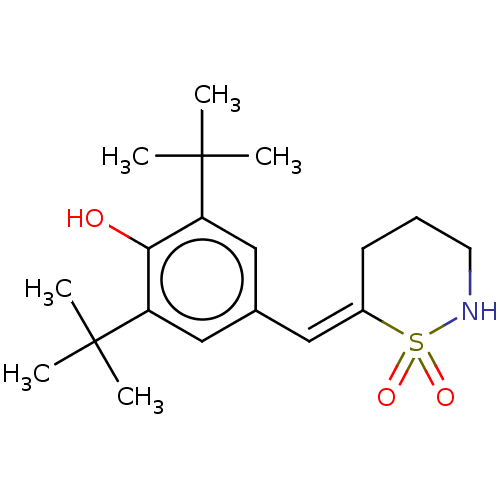 (CHEMBL64813) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. | J Med Chem 43: 2040-8 (2000) Article DOI: 10.1021/jm9906015 BindingDB Entry DOI: 10.7270/Q2H134R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50362159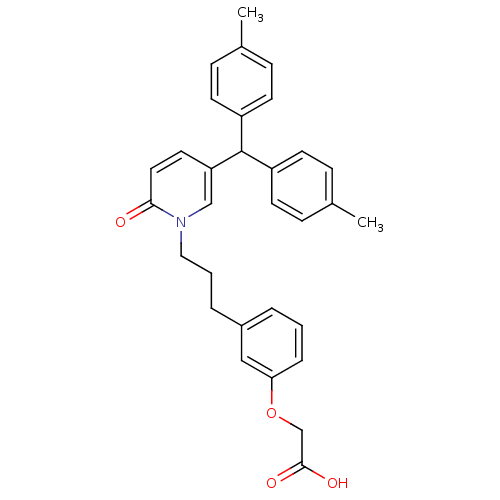 (CHEMBL1941120) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGD2 from human CRTH2 receptor expressed in HEK293 cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 22: 1194-7 (2012) Article DOI: 10.1016/j.bmcl.2011.11.079 BindingDB Entry DOI: 10.7270/Q2765FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50045107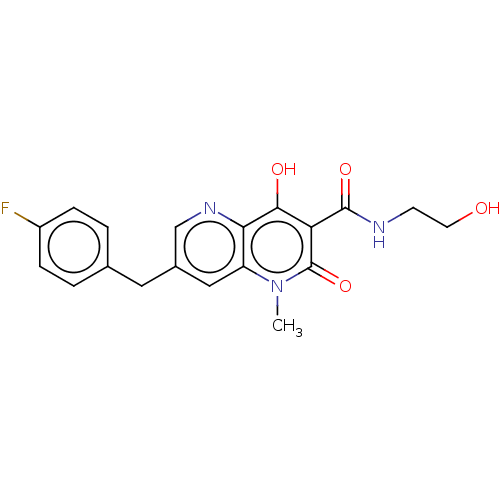 (CHEMBL1256978 | GSK364735) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus 1 integrase by strand transfer scintillation proximity assay | Antimicrob Agents Chemother 52: 901-8 (2008) Article DOI: 10.1128/aac.01218-07 BindingDB Entry DOI: 10.7270/Q2JQ13VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM579105 (US11479551, Example 16-7 | US11479551, Example 16-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PRMT5 methyltransferase activity in MTAP knockout human HCT-116 cells assessed as inhibition of PRMT5- mediated SDMA modification level... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01900 BindingDB Entry DOI: 10.7270/Q2DB85RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM578996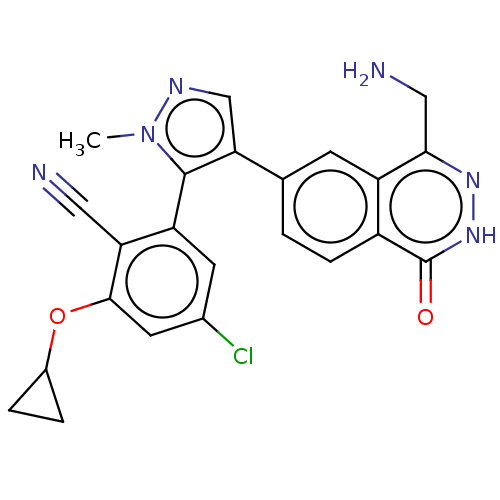 (2-(4-(4-(aminomethyl)-1-oxo-1,2- dihydrophthalazin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PRMT5 methyltransferase activity in MTAP knockout human HCT-116 cells assessed as inhibition of PRMT5- mediated SDMA modification level... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01900 BindingDB Entry DOI: 10.7270/Q2DB85RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50585595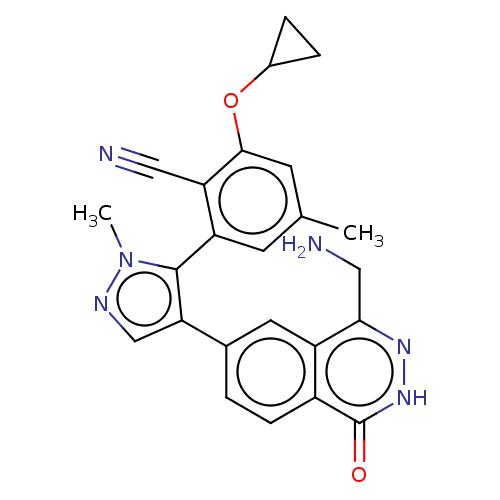 (CHEMBL5076613) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PRMT5 methyltransferase activity in MTAP knockout human HCT-116 cells assessed as inhibition of PRMT5- mediated SDMA modification level... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01900 BindingDB Entry DOI: 10.7270/Q2DB85RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50472610 (CHEMBL60725) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. | J Med Chem 43: 2040-8 (2000) Article DOI: 10.1021/jm9906015 BindingDB Entry DOI: 10.7270/Q2H134R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50362158 (CHEMBL1941119) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGD2 from human CRTH2 receptor expressed in HEK293 cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 22: 1194-7 (2012) Article DOI: 10.1016/j.bmcl.2011.11.079 BindingDB Entry DOI: 10.7270/Q2765FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50472622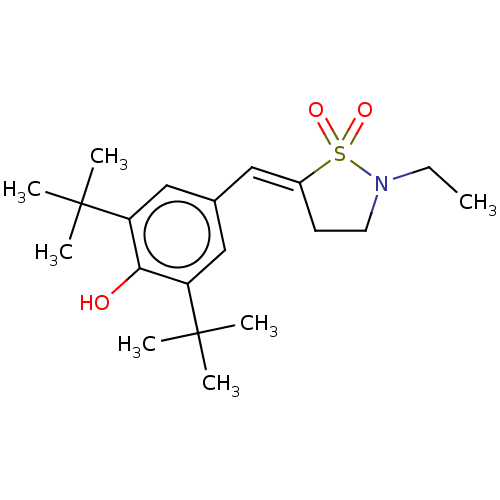 (CHEMBL291543) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. | J Med Chem 43: 2040-8 (2000) Article DOI: 10.1021/jm9906015 BindingDB Entry DOI: 10.7270/Q2H134R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50362166 (CHEMBL1941128) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGD2 from human CRTH2 receptor expressed in HEK293 cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 22: 1194-7 (2012) Article DOI: 10.1016/j.bmcl.2011.11.079 BindingDB Entry DOI: 10.7270/Q2765FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50021581 (CHEMBL414850 | L-870810) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus 1 integrase by strand transfer scintillation proximity assay | Antimicrob Agents Chemother 52: 901-8 (2008) Article DOI: 10.1128/aac.01218-07 BindingDB Entry DOI: 10.7270/Q2JQ13VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50472631 (CHEMBL60738) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. | J Med Chem 43: 2040-8 (2000) Article DOI: 10.1021/jm9906015 BindingDB Entry DOI: 10.7270/Q2H134R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50485874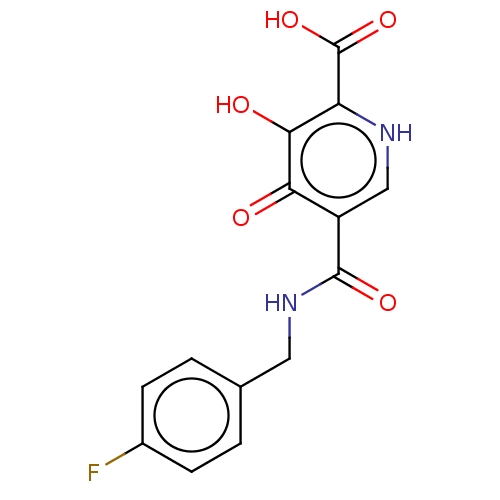 (CHEMBL2171671) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity using pre-incubation and wash with Mg2+ cofactor by ELISA based microtiter plate integration as... | J Med Chem 55: 8735-44 (2012) Article DOI: 10.1021/jm3010459 BindingDB Entry DOI: 10.7270/Q27M0BTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50183273 ((S)-6-(3-chloro-2-fluorobenzyl)-1-(1-hydroxy-3-met...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of 20 nM [3H]GSK304649 from Human immunodeficiency virus 1 integrase by scintillation proximity assay | Antimicrob Agents Chemother 52: 901-8 (2008) Article DOI: 10.1128/aac.01218-07 BindingDB Entry DOI: 10.7270/Q2JQ13VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50362157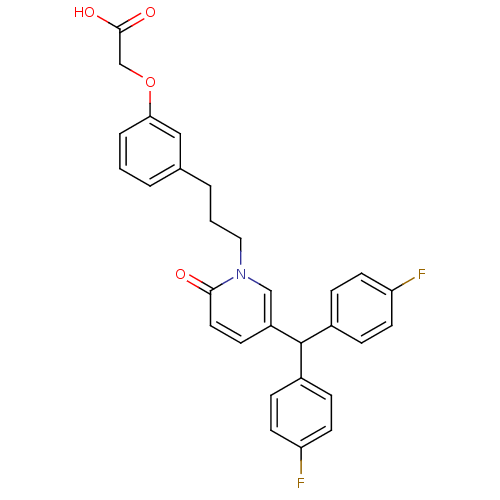 (CHEMBL1941118) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGD2 from human CRTH2 receptor expressed in HEK293 cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 22: 1194-7 (2012) Article DOI: 10.1016/j.bmcl.2011.11.079 BindingDB Entry DOI: 10.7270/Q2765FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM579047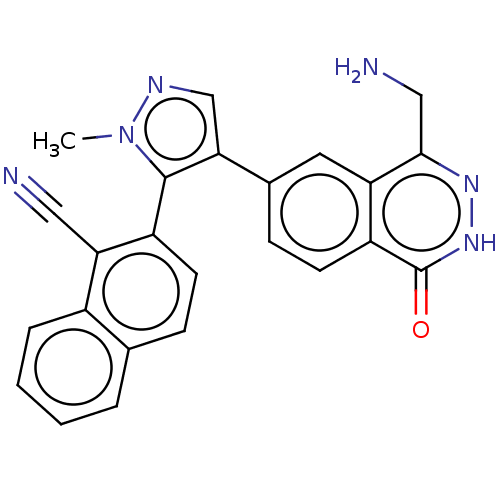 (2-(4-(4-(aminomethyl)-1-oxo-1,2- dihydrophthalazin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PRMT5 methyltransferase activity in MTAP knockout human HCT-116 cells assessed as inhibition of PRMT5- mediated SDMA modification level... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01900 BindingDB Entry DOI: 10.7270/Q2DB85RR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50362164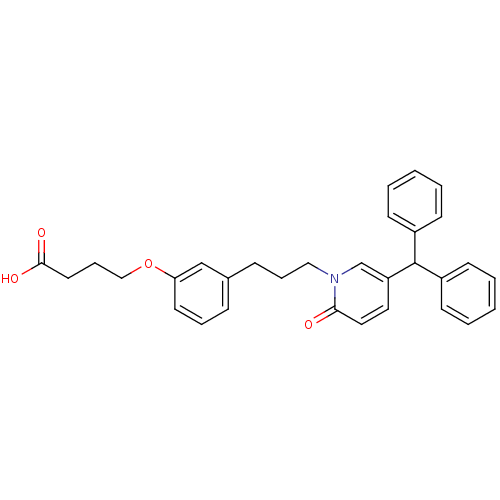 (CHEMBL1941126) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGD2 from human CRTH2 receptor expressed in HEK293 cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 22: 1194-7 (2012) Article DOI: 10.1016/j.bmcl.2011.11.079 BindingDB Entry DOI: 10.7270/Q2765FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50585597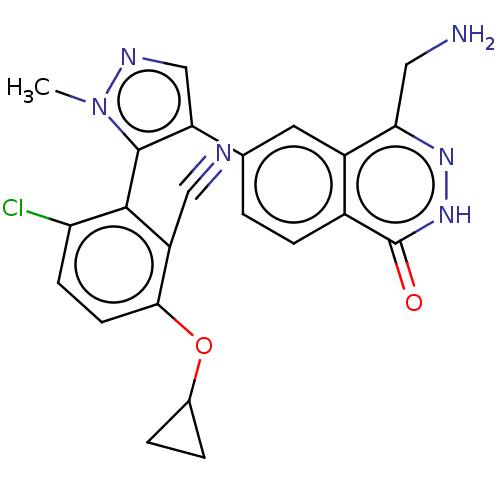 (CHEMBL5083379) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PRMT5 methyltransferase activity in MTAP knockout human HCT-116 cells assessed as inhibition of PRMT5- mediated SDMA modification level... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01900 BindingDB Entry DOI: 10.7270/Q2DB85RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 231 total ) | Next | Last >> |