

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM18991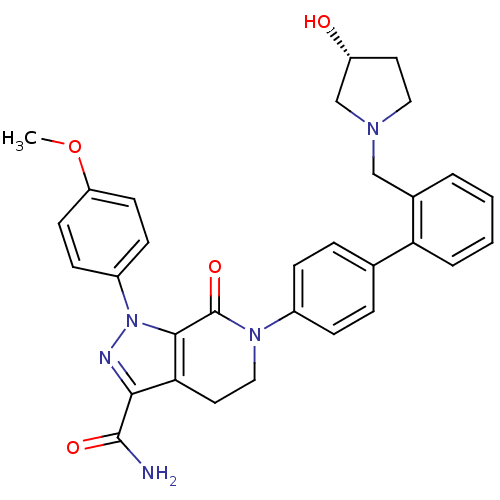 (6-[4-(2-{[(3R)-3-hydroxypyrrolidin-1-yl]methyl}phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | -57.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 50: 5339-56 (2007) Article DOI: 10.1021/jm070245n BindingDB Entry DOI: 10.7270/Q2Q23XJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM19023 (1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 50: 5339-56 (2007) Article DOI: 10.1021/jm070245n BindingDB Entry DOI: 10.7270/Q2Q23XJ9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM19010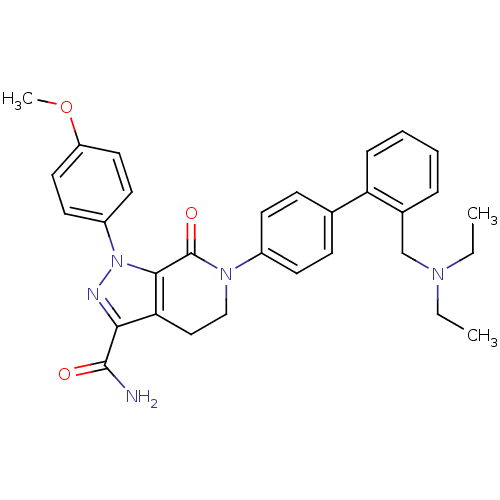 (6-(4-{2-[(diethylamino)methyl]phenyl}phenyl)-1-(4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 50: 5339-56 (2007) Article DOI: 10.1021/jm070245n BindingDB Entry DOI: 10.7270/Q2Q23XJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM19008 (1-(4-methoxyphenyl)-6-(4-{2-[(methylamino)methyl]p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 50: 5339-56 (2007) Article DOI: 10.1021/jm070245n BindingDB Entry DOI: 10.7270/Q2Q23XJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12743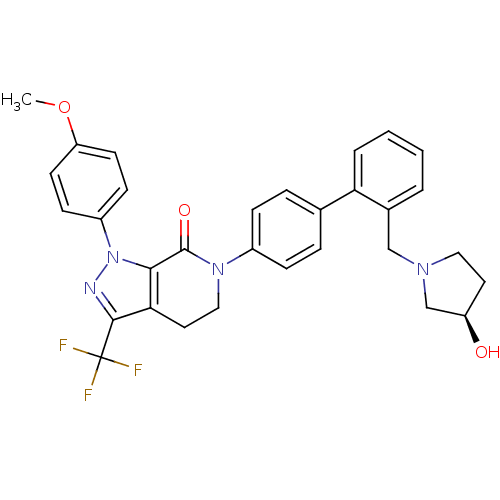 (6-[4-(2-{[(3R)-3-hydroxypyrrolidin-1-yl]methyl}phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | -55.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 50: 5339-56 (2007) Article DOI: 10.1021/jm070245n BindingDB Entry DOI: 10.7270/Q2Q23XJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM19020 (1-{4-[1-(4-methoxyphenyl)-7-oxo-3-(trifluoromethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 50: 5339-56 (2007) Article DOI: 10.1021/jm070245n BindingDB Entry DOI: 10.7270/Q2Q23XJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM19009 (6-(4-{2-[(dimethylamino)methyl]phenyl}phenyl)-1-(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 50: 5339-56 (2007) Article DOI: 10.1021/jm070245n BindingDB Entry DOI: 10.7270/Q2Q23XJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM18988 (6-[4-(2-{[(3R)-3-hydroxypyrrolidin-1-yl]methyl}phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.25 | -54.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 50: 5339-56 (2007) Article DOI: 10.1021/jm070245n BindingDB Entry DOI: 10.7270/Q2Q23XJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM19004 (6-[4-(2-{[(3R)-3-hydroxypyrrolidin-1-yl]methyl}phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.25 | -54.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 50: 5339-56 (2007) Article DOI: 10.1021/jm070245n BindingDB Entry DOI: 10.7270/Q2Q23XJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM19012 (6-(4-{2-[(4-hydroxypiperidin-1-yl)methyl]phenyl}ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 50: 5339-56 (2007) Article DOI: 10.1021/jm070245n BindingDB Entry DOI: 10.7270/Q2Q23XJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM19011 (1-(4-methoxyphenyl)-7-oxo-6-{4-[2-(pyrrolidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 50: 5339-56 (2007) Article DOI: 10.1021/jm070245n BindingDB Entry DOI: 10.7270/Q2Q23XJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM18999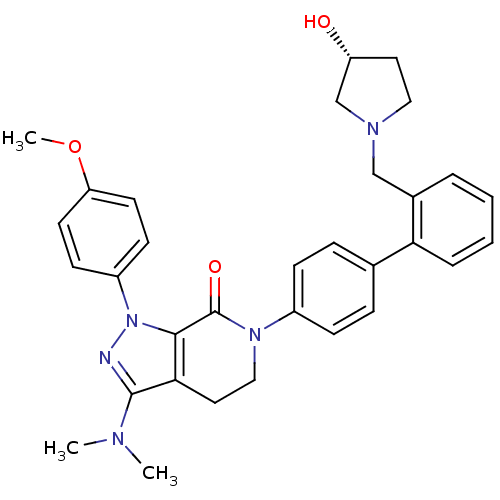 (3-(dimethylamino)-6-[4-(2-{[(3R)-3-hydroxypyrrolid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.310 | -53.7 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 50: 5339-56 (2007) Article DOI: 10.1021/jm070245n BindingDB Entry DOI: 10.7270/Q2Q23XJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM18994 (6-[4-(2-{[(3R)-3-hydroxypyrrolidin-1-yl]methyl}phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.330 | -53.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 50: 5339-56 (2007) Article DOI: 10.1021/jm070245n BindingDB Entry DOI: 10.7270/Q2Q23XJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM19013 (6-[4-(2-{[bis(2-hydroxyethyl)amino]methyl}phenyl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 50: 5339-56 (2007) Article DOI: 10.1021/jm070245n BindingDB Entry DOI: 10.7270/Q2Q23XJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM19021 (1-{4-[1-(4-methoxyphenyl)-7-oxo-3-(trifluoromethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 50: 5339-56 (2007) Article DOI: 10.1021/jm070245n BindingDB Entry DOI: 10.7270/Q2Q23XJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM19003 (6-[4-(2-{[(3R)-3-hydroxypyrrolidin-1-yl]methyl}phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.480 | -52.7 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 50: 5339-56 (2007) Article DOI: 10.1021/jm070245n BindingDB Entry DOI: 10.7270/Q2Q23XJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM19017 (N-{4-[1-(4-methoxyphenyl)-7-oxo-3-(trifluoromethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 50: 5339-56 (2007) Article DOI: 10.1021/jm070245n BindingDB Entry DOI: 10.7270/Q2Q23XJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM19022 (1-(4-methoxyphenyl)-6-[4-(N-methylacetamido)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 50: 5339-56 (2007) Article DOI: 10.1021/jm070245n BindingDB Entry DOI: 10.7270/Q2Q23XJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM19001 (6-[4-(2-{[(3R)-3-hydroxypyrrolidin-1-yl]methyl}phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.630 | -52.0 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 50: 5339-56 (2007) Article DOI: 10.1021/jm070245n BindingDB Entry DOI: 10.7270/Q2Q23XJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM19002 (6-[4-(2-{[(3R)-3-hydroxypyrrolidin-1-yl]methyl}phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.670 | -51.8 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 50: 5339-56 (2007) Article DOI: 10.1021/jm070245n BindingDB Entry DOI: 10.7270/Q2Q23XJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50136680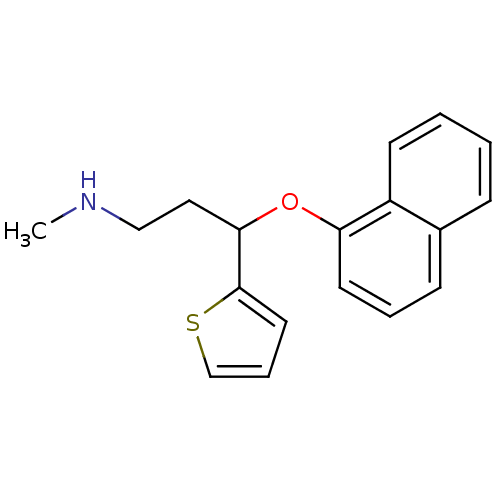 (CHEMBL424660 | N-methyl-3-(1-naphthyloxy)-3-(2-thi...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by PDSP Ki Database | Neuropharmacology 45: 935-44 (2003) Article DOI: 10.1016/s0028-3908(03)00268-5 BindingDB Entry DOI: 10.7270/Q2VQ318R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM19005 (6-[4-(2-{[(3R)-3-hydroxypyrrolidin-1-yl]methyl}phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.850 | -51.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 50: 5339-56 (2007) Article DOI: 10.1021/jm070245n BindingDB Entry DOI: 10.7270/Q2Q23XJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM86421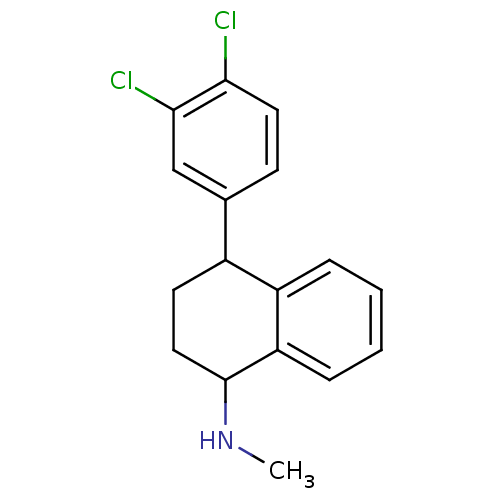 (CAS_79617-96-2 | NSC_68617 | SERTRALINE) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by PDSP Ki Database | Neuropharmacology 45: 935-44 (2003) Article DOI: 10.1016/s0028-3908(03)00268-5 BindingDB Entry DOI: 10.7270/Q2VQ318R | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM19007 (6-{4-[2-(aminomethyl)phenyl]phenyl}-1-(4-methoxyph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 50: 5339-56 (2007) Article DOI: 10.1021/jm070245n BindingDB Entry DOI: 10.7270/Q2Q23XJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM19006 (6-[4-(2-{[(3R)-3-hydroxypyrrolidin-1-yl]methyl}phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.10 | -50.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 50: 5339-56 (2007) Article DOI: 10.1021/jm070245n BindingDB Entry DOI: 10.7270/Q2Q23XJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM18993 (6-[4-(2-{[(3R)-3-hydroxypyrrolidin-1-yl]methyl}phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.70 | -49.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 50: 5339-56 (2007) Article DOI: 10.1021/jm070245n BindingDB Entry DOI: 10.7270/Q2Q23XJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM18998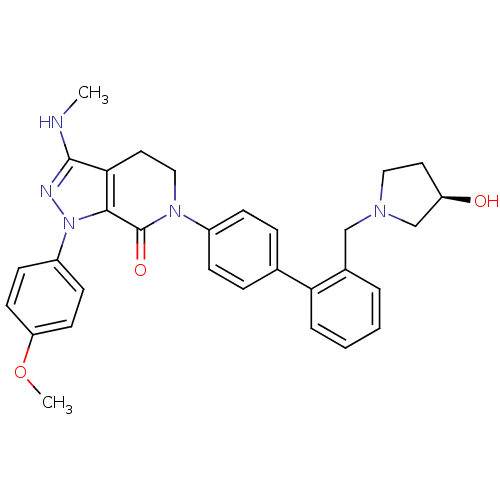 (6-[4-(2-{[(3R)-3-hydroxypyrrolidin-1-yl]methyl}phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.70 | -49.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 50: 5339-56 (2007) Article DOI: 10.1021/jm070245n BindingDB Entry DOI: 10.7270/Q2Q23XJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM18997 (Pyrazolopyridinone analogue, 23 | tert-butyl N-{6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | -49.2 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 50: 5339-56 (2007) Article DOI: 10.1021/jm070245n BindingDB Entry DOI: 10.7270/Q2Q23XJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50052588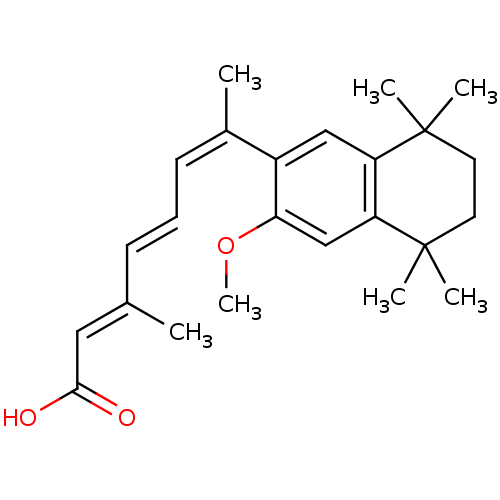 ((2E,4E,6Z)-7-(3-Methoxy-5,5,8,8-tetramethyl-5,6,7,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-Targretin binding to Retinoid X receptor RXR alpha | J Med Chem 39: 3229-34 (1996) Article DOI: 10.1021/jm960311d BindingDB Entry DOI: 10.7270/Q2ZW1K14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM50052588 ((2E,4E,6Z)-7-(3-Methoxy-5,5,8,8-tetramethyl-5,6,7,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-Targretin binding to Retinoid X receptor RXR beta | J Med Chem 39: 3229-34 (1996) Article DOI: 10.1021/jm960311d BindingDB Entry DOI: 10.7270/Q2ZW1K14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM19019 (1-(4-methoxyphenyl)-6-[4-(piperidin-1-yl)phenyl]-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 50: 5339-56 (2007) Article DOI: 10.1021/jm070245n BindingDB Entry DOI: 10.7270/Q2Q23XJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM19000 (6-[4-(2-{[(3R)-3-hydroxypyrrolidin-1-yl]methyl}phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.30 | -48.8 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 50: 5339-56 (2007) Article DOI: 10.1021/jm070245n BindingDB Entry DOI: 10.7270/Q2Q23XJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50426352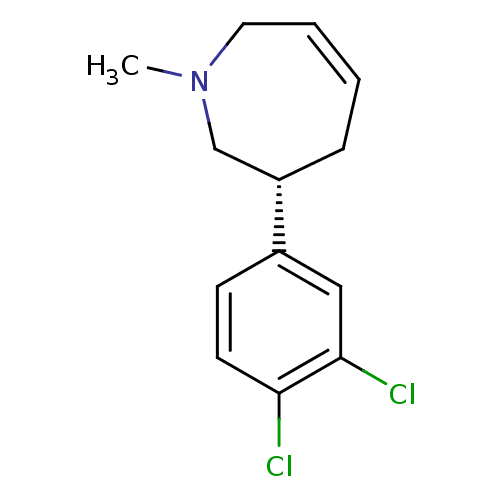 (CHEMBL2321996) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant DAT expressed in HEK cells | ACS Med Chem Lett 4: 46-51 (2013) Article DOI: 10.1021/ml300262e BindingDB Entry DOI: 10.7270/Q2FQ9XXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50290187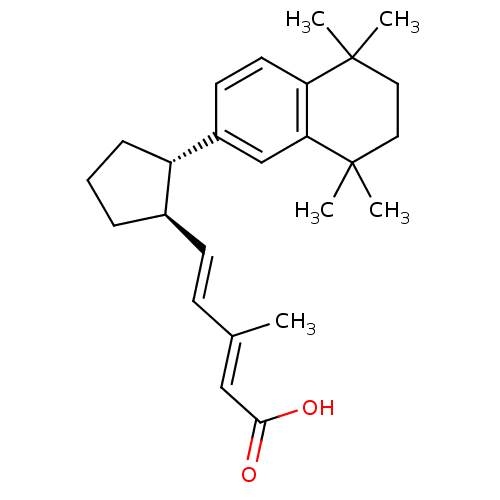 ((2E,4E)-3-Methyl-5-[(1R,2S)-2-(5,5,8,8-tetramethyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-9-cis-RA binding to Retinoid X receptor RXR alpha | Bioorg Med Chem Lett 7: 2393-2398 (1997) Article DOI: 10.1016/S0960-894X(97)00437-X BindingDB Entry DOI: 10.7270/Q2FB52XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50290192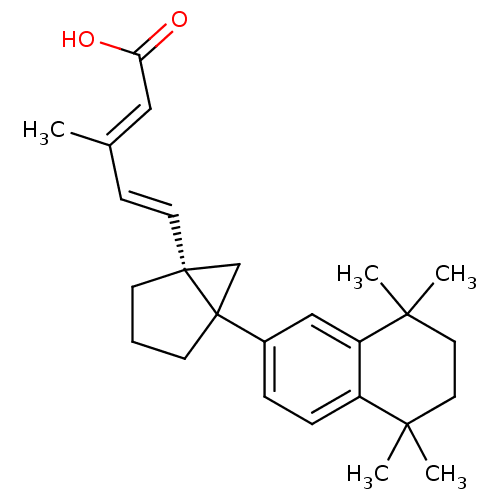 (3-Methyl-5-[5-(5,5,8,8-tetramethyl-5,6,7,8-tetrahy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-9-cis-RA binding to Retinoid X receptor RXR alpha | Bioorg Med Chem Lett 7: 2393-2398 (1997) Article DOI: 10.1016/S0960-894X(97)00437-X BindingDB Entry DOI: 10.7270/Q2FB52XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50426355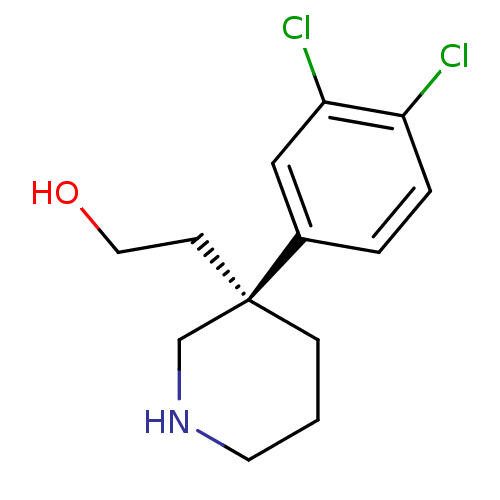 (CHEMBL2321991) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant NET expressed in HEK cells | ACS Med Chem Lett 4: 46-51 (2013) Article DOI: 10.1021/ml300262e BindingDB Entry DOI: 10.7270/Q2FQ9XXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM35229 (3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by PDSP Ki Database | Neuropharmacology 45: 935-44 (2003) Article DOI: 10.1016/s0028-3908(03)00268-5 BindingDB Entry DOI: 10.7270/Q2VQ318R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM18989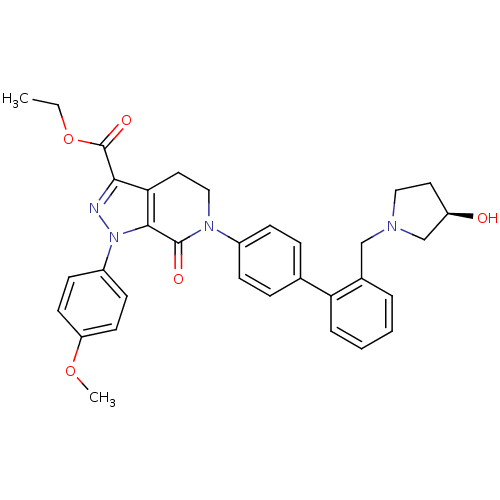 (Pyrazolopyridinone analogue, 11b | ethyl 6-[4-(2-{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.90 | -47.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 50: 5339-56 (2007) Article DOI: 10.1021/jm070245n BindingDB Entry DOI: 10.7270/Q2Q23XJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50021218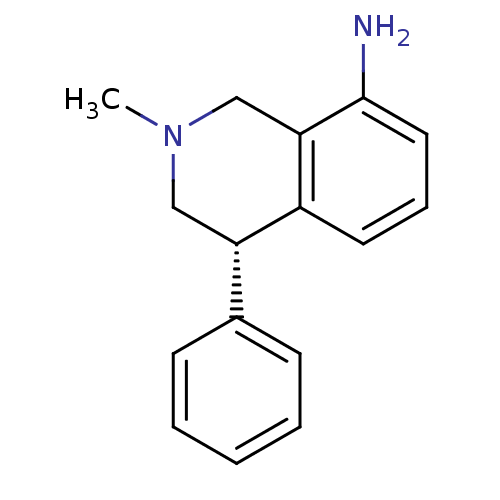 ((S)-2-methyl-4-phenyl-1,2,3,4-tetrahydroisoquinoli...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant NET expressed in HEK cells | ACS Med Chem Lett 4: 46-51 (2013) Article DOI: 10.1021/ml300262e BindingDB Entry DOI: 10.7270/Q2FQ9XXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transporter (Rattus norvegicus (rat)) | BDBM50005548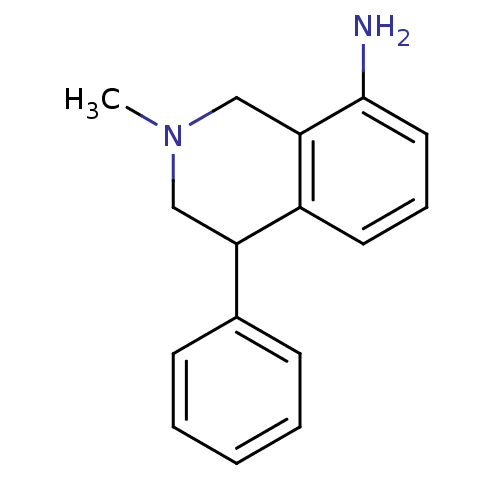 ((+)-2-Methyl-4-phenyl-1,2,3,4-tetrahydro-isoquinol...) | Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of NET in rat synaptosomal membrane | ACS Med Chem Lett 4: 46-51 (2013) Article DOI: 10.1021/ml300262e BindingDB Entry DOI: 10.7270/Q2FQ9XXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50426353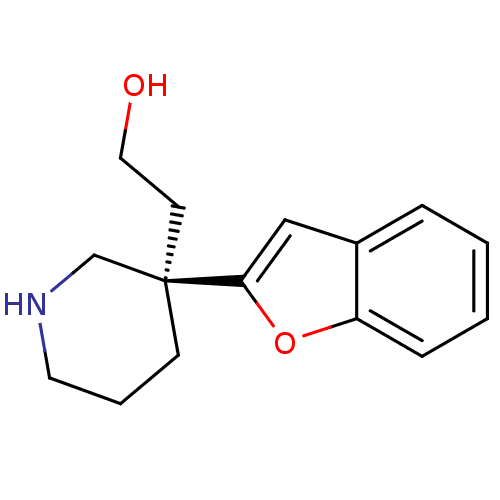 (CHEMBL2321993) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant NET expressed in HEK cells | ACS Med Chem Lett 4: 46-51 (2013) Article DOI: 10.1021/ml300262e BindingDB Entry DOI: 10.7270/Q2FQ9XXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50052590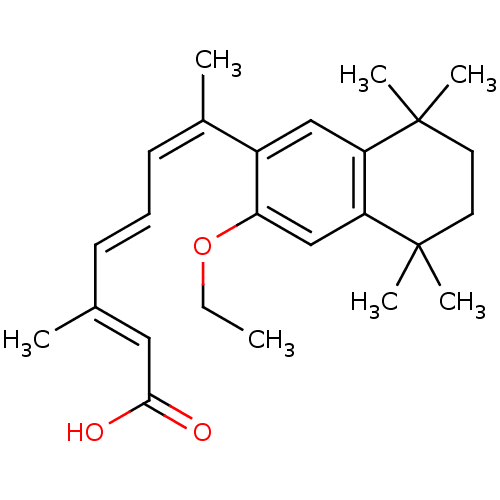 ((2E,4E,6Z)-7-(3-Ethoxy-5,5,8,8-tetramethyl-5,6,7,8...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-Targretin binding to Retinoid X receptor RXR alpha | J Med Chem 39: 3229-34 (1996) Article DOI: 10.1021/jm960311d BindingDB Entry DOI: 10.7270/Q2ZW1K14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50290188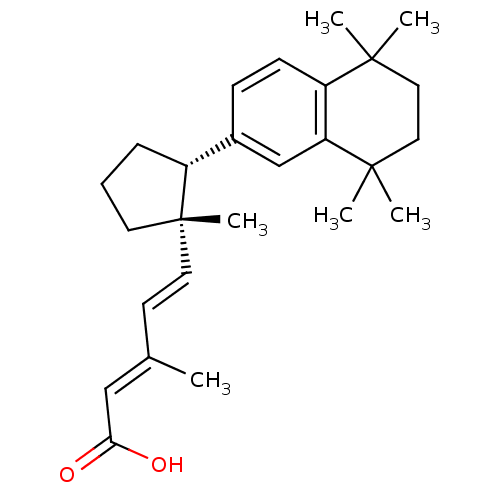 ((2E,4E)-3-Methyl-5-[(1S,2R)-1-methyl-2-(5,5,8,8-te...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-9-cis-RA binding to Retinoid X receptor RXR alpha | Bioorg Med Chem Lett 7: 2393-2398 (1997) Article DOI: 10.1016/S0960-894X(97)00437-X BindingDB Entry DOI: 10.7270/Q2FB52XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50290186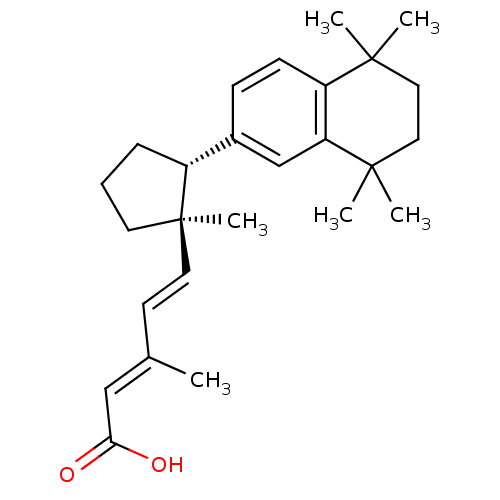 ((2E,4E)-3-Methyl-5-[(1R,2R)-1-methyl-2-(5,5,8,8-te...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-9-cis-RA binding to Retinoid X receptor RXR alpha | Bioorg Med Chem Lett 7: 2393-2398 (1997) Article DOI: 10.1016/S0960-894X(97)00437-X BindingDB Entry DOI: 10.7270/Q2FB52XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Homo sapiens (Human)) | BDBM50052588 ((2E,4E,6Z)-7-(3-Methoxy-5,5,8,8-tetramethyl-5,6,7,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-Targretin binding to Retinoid X receptor RXR gamma | J Med Chem 39: 3229-34 (1996) Article DOI: 10.1021/jm960311d BindingDB Entry DOI: 10.7270/Q2ZW1K14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50426357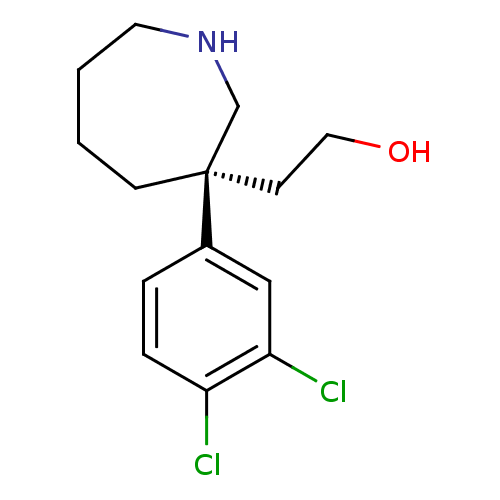 (CHEMBL2326695) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant DAT expressed in HEK cells | ACS Med Chem Lett 4: 46-51 (2013) Article DOI: 10.1021/ml300262e BindingDB Entry DOI: 10.7270/Q2FQ9XXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM18992 (6-[4-(2-{[(3R)-3-hydroxypyrrolidin-1-yl]methyl}phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.80 | -47.0 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 50: 5339-56 (2007) Article DOI: 10.1021/jm070245n BindingDB Entry DOI: 10.7270/Q2Q23XJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50005548 ((+)-2-Methyl-4-phenyl-1,2,3,4-tetrahydro-isoquinol...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant NET expressed in HEK cells | ACS Med Chem Lett 4: 46-51 (2013) Article DOI: 10.1021/ml300262e BindingDB Entry DOI: 10.7270/Q2FQ9XXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50426352 (CHEMBL2321996) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant DAT expressed in HEK cells | ACS Med Chem Lett 4: 46-51 (2013) Article DOI: 10.1021/ml300262e BindingDB Entry DOI: 10.7270/Q2FQ9XXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50120704 (2-Naphthalen-1-yl-1,3,4,5-tetrahydro-azepino[5,4,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity towards human poly(ADP-ribose) polymerase 1 (PARP-1). | J Med Chem 45: 4961-74 (2002) BindingDB Entry DOI: 10.7270/Q2T152ZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 772 total ) | Next | Last >> |