

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM50052588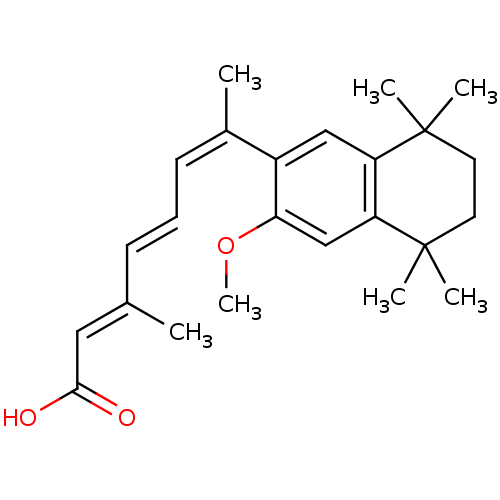 ((2E,4E,6Z)-7-(3-Methoxy-5,5,8,8-tetramethyl-5,6,7,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-Targretin binding to Retinoid X receptor RXR beta | J Med Chem 39: 3229-34 (1996) Article DOI: 10.1021/jm960311d BindingDB Entry DOI: 10.7270/Q2ZW1K14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50052588 ((2E,4E,6Z)-7-(3-Methoxy-5,5,8,8-tetramethyl-5,6,7,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-Targretin binding to Retinoid X receptor RXR alpha | J Med Chem 39: 3229-34 (1996) Article DOI: 10.1021/jm960311d BindingDB Entry DOI: 10.7270/Q2ZW1K14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50290192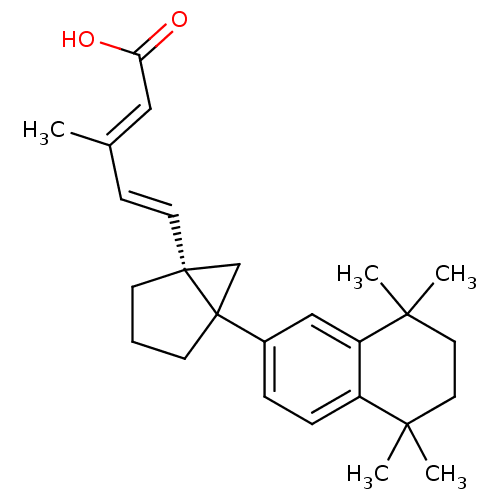 (3-Methyl-5-[5-(5,5,8,8-tetramethyl-5,6,7,8-tetrahy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-9-cis-RA binding to Retinoid X receptor RXR alpha | Bioorg Med Chem Lett 7: 2393-2398 (1997) Article DOI: 10.1016/S0960-894X(97)00437-X BindingDB Entry DOI: 10.7270/Q2FB52XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50290187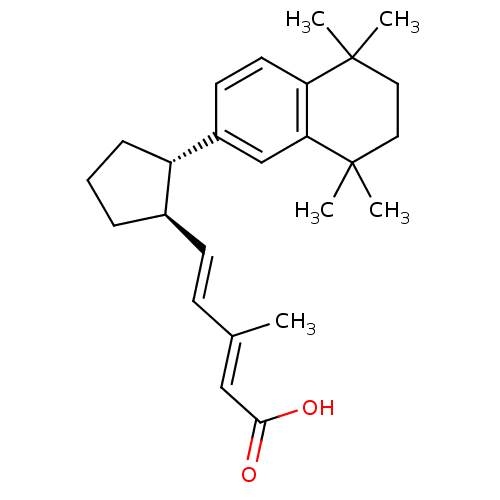 ((2E,4E)-3-Methyl-5-[(1R,2S)-2-(5,5,8,8-tetramethyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-9-cis-RA binding to Retinoid X receptor RXR alpha | Bioorg Med Chem Lett 7: 2393-2398 (1997) Article DOI: 10.1016/S0960-894X(97)00437-X BindingDB Entry DOI: 10.7270/Q2FB52XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50290188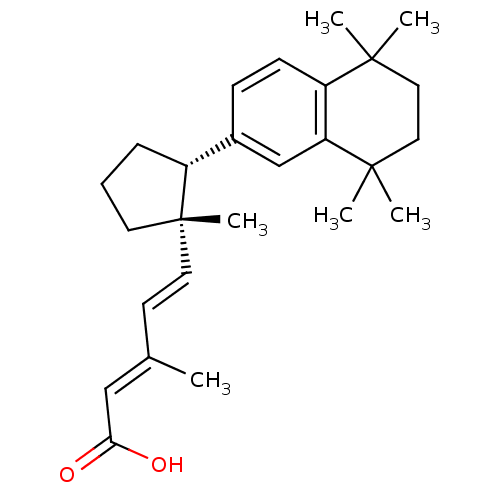 ((2E,4E)-3-Methyl-5-[(1S,2R)-1-methyl-2-(5,5,8,8-te...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-9-cis-RA binding to Retinoid X receptor RXR alpha | Bioorg Med Chem Lett 7: 2393-2398 (1997) Article DOI: 10.1016/S0960-894X(97)00437-X BindingDB Entry DOI: 10.7270/Q2FB52XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50052590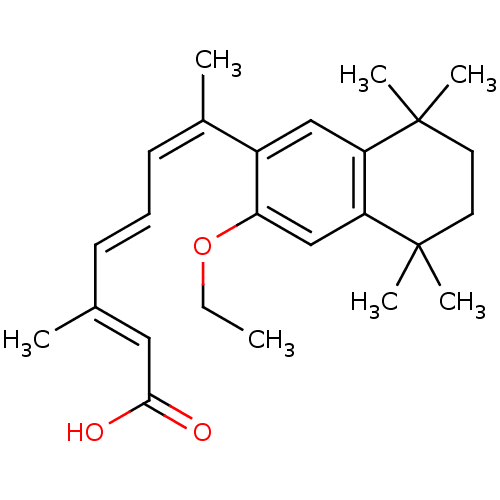 ((2E,4E,6Z)-7-(3-Ethoxy-5,5,8,8-tetramethyl-5,6,7,8...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-Targretin binding to Retinoid X receptor RXR alpha | J Med Chem 39: 3229-34 (1996) Article DOI: 10.1021/jm960311d BindingDB Entry DOI: 10.7270/Q2ZW1K14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Homo sapiens (Human)) | BDBM50052588 ((2E,4E,6Z)-7-(3-Methoxy-5,5,8,8-tetramethyl-5,6,7,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-Targretin binding to Retinoid X receptor RXR gamma | J Med Chem 39: 3229-34 (1996) Article DOI: 10.1021/jm960311d BindingDB Entry DOI: 10.7270/Q2ZW1K14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50290186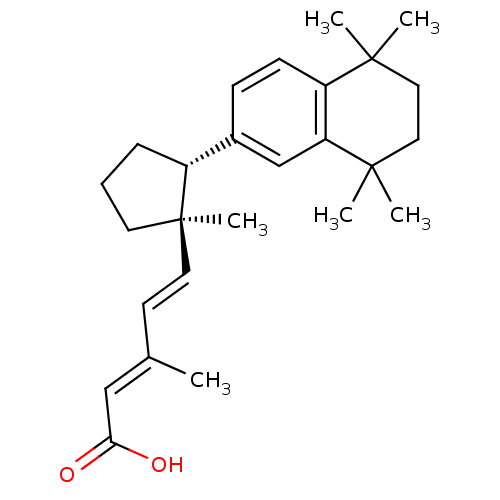 ((2E,4E)-3-Methyl-5-[(1R,2R)-1-methyl-2-(5,5,8,8-te...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-9-cis-RA binding to Retinoid X receptor RXR alpha | Bioorg Med Chem Lett 7: 2393-2398 (1997) Article DOI: 10.1016/S0960-894X(97)00437-X BindingDB Entry DOI: 10.7270/Q2FB52XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50290193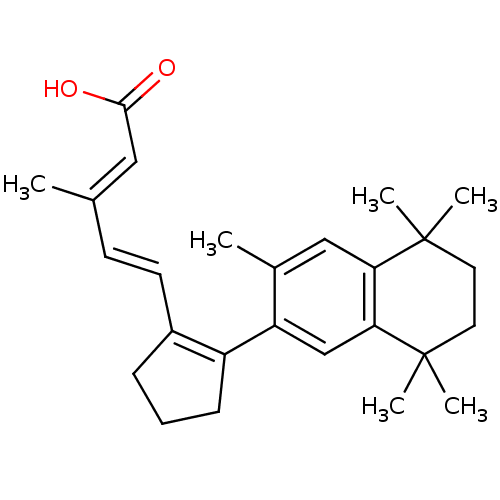 (3-Methyl-5-[2-(3,5,5,8,8-pentamethyl-5,6,7,8-tetra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-9-cis-RA binding to Retinoid X receptor RXR alpha | Bioorg Med Chem Lett 7: 2393-2398 (1997) Article DOI: 10.1016/S0960-894X(97)00437-X BindingDB Entry DOI: 10.7270/Q2FB52XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50290192 (3-Methyl-5-[5-(5,5,8,8-tetramethyl-5,6,7,8-tetrahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Effective potency in transcriptional activation assay in CV-1 cells expressing Retinoid X receptor RXR alpha | Bioorg Med Chem Lett 7: 2393-2398 (1997) Article DOI: 10.1016/S0960-894X(97)00437-X BindingDB Entry DOI: 10.7270/Q2FB52XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50290187 ((2E,4E)-3-Methyl-5-[(1R,2S)-2-(5,5,8,8-tetramethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Effective potency in transcriptional activation assay in CV-1 cells expressing retinoic acid receptor RAR gamma | Bioorg Med Chem Lett 7: 2393-2398 (1997) Article DOI: 10.1016/S0960-894X(97)00437-X BindingDB Entry DOI: 10.7270/Q2FB52XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Homo sapiens (Human)) | BDBM50074300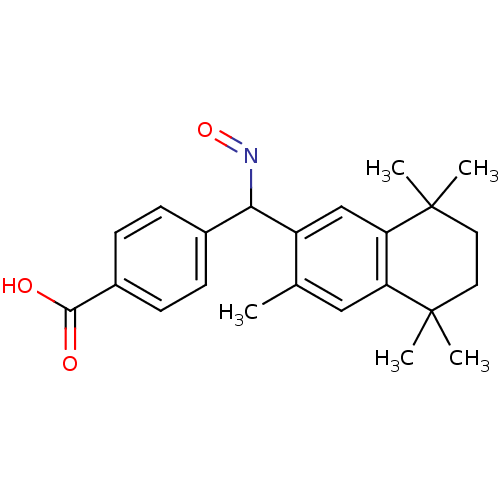 (4-[[(E)-Hydroxyimino]-(3,5,5,8,8-pentamethyl-5,6,7...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Binding affinity towards recombinantly expressed Retinoic acid receptor RXR-gamma in baculoviral sysytem, by using 5 nM [3H]-targretin in a competiti... | J Med Chem 42: 742-50 (1999) Article DOI: 10.1021/jm980621r BindingDB Entry DOI: 10.7270/Q2K936Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM27685 (2-{4-[(dimethylamino)methyl]phenyl}-3,10-diazatric...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity towards human poly(ADP-ribose) polymerase 1 (PARP-1). | J Med Chem 45: 4961-74 (2002) BindingDB Entry DOI: 10.7270/Q2T152ZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50120702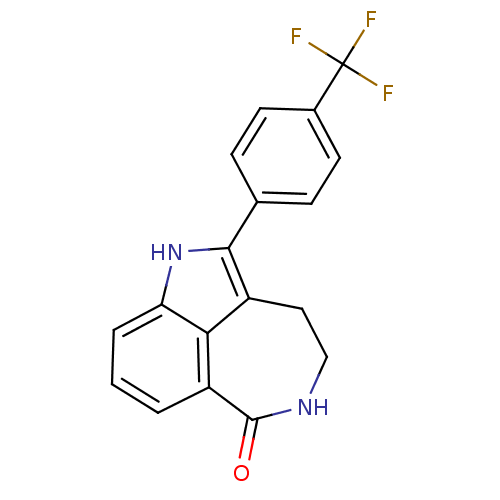 (2-(4-Trifluoromethyl-phenyl)-1,3,4,5-tetrahydro-az...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity towards human poly(ADP-ribose) polymerase 1 (PARP-1). | J Med Chem 45: 4961-74 (2002) BindingDB Entry DOI: 10.7270/Q2T152ZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50120704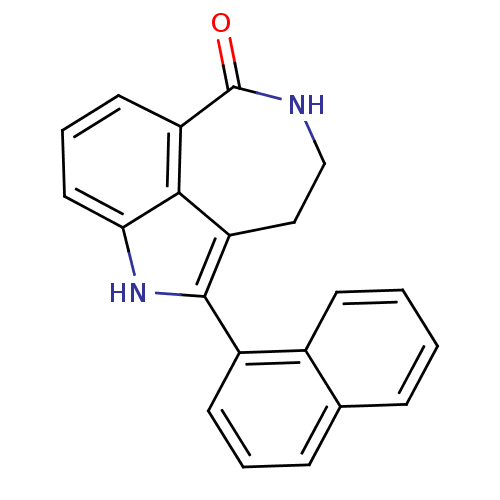 (2-Naphthalen-1-yl-1,3,4,5-tetrahydro-azepino[5,4,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity towards human poly(ADP-ribose) polymerase 1 (PARP-1). | J Med Chem 45: 4961-74 (2002) BindingDB Entry DOI: 10.7270/Q2T152ZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50120707 (2-Thiophen-2-yl-1,3,4,5-tetrahydro-azepino[5,4,3-c...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity towards human poly(ADP-ribose) polymerase 1 (PARP-1). | J Med Chem 45: 4961-74 (2002) BindingDB Entry DOI: 10.7270/Q2T152ZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM50074300 (4-[[(E)-Hydroxyimino]-(3,5,5,8,8-pentamethyl-5,6,7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Binding affinity towards recombinantly expressed Retinoic acid receptor RXR-beta in baculoviral sysytem, by using 5 nM [3H]-targretin in a competitiv... | J Med Chem 42: 742-50 (1999) Article DOI: 10.1021/jm980621r BindingDB Entry DOI: 10.7270/Q2K936Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50120724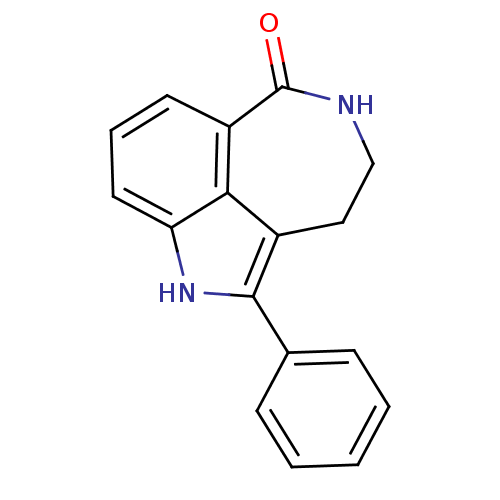 (2-Phenyl-1,3,4,5-tetrahydro-azepino[5,4,3-cd]indol...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity towards human poly(ADP-ribose) polymerase 1 (PARP-1). | J Med Chem 45: 4961-74 (2002) BindingDB Entry DOI: 10.7270/Q2T152ZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50120728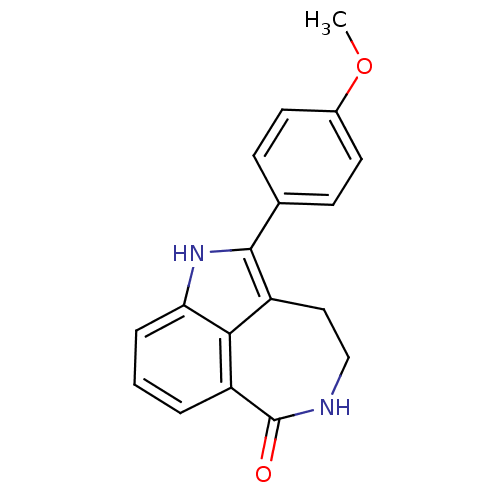 (2-(4-Methoxy-phenyl)-1,3,4,5-tetrahydro-azepino[5,...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity towards human poly(ADP-ribose) polymerase 1 (PARP-1). | J Med Chem 45: 4961-74 (2002) BindingDB Entry DOI: 10.7270/Q2T152ZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Mus musculus) | BDBM50290187 ((2E,4E)-3-Methyl-5-[(1R,2S)-2-(5,5,8,8-tetramethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-9-cis-RA binding to Retinoid X receptor RXR beta | Bioorg Med Chem Lett 7: 2393-2398 (1997) Article DOI: 10.1016/S0960-894X(97)00437-X BindingDB Entry DOI: 10.7270/Q2FB52XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50120726 (2-(3-Trifluoromethyl-phenyl)-1,3,4,5-tetrahydro-az...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity towards human poly(ADP-ribose) polymerase 1 (PARP-1). | J Med Chem 45: 4961-74 (2002) BindingDB Entry DOI: 10.7270/Q2T152ZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50074300 (4-[[(E)-Hydroxyimino]-(3,5,5,8,8-pentamethyl-5,6,7...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Binding affinity towards recombinantly expressed Retinoic acid receptor RXR-alpha in baculoviral sysytem, by using 5 nM [3H]-targretin in a competiti... | J Med Chem 42: 742-50 (1999) Article DOI: 10.1021/jm980621r BindingDB Entry DOI: 10.7270/Q2K936Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50074307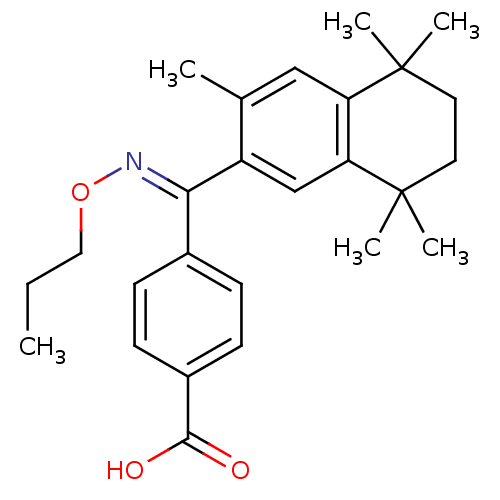 (4-{(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro-napht...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Binding affinity towards recombinantly expressed Retinoic acid receptor RXR-alpha in baculoviral sysytem, by using 5 nM [3H]-targretin in a competiti... | J Med Chem 42: 742-50 (1999) Article DOI: 10.1021/jm980621r BindingDB Entry DOI: 10.7270/Q2K936Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50120706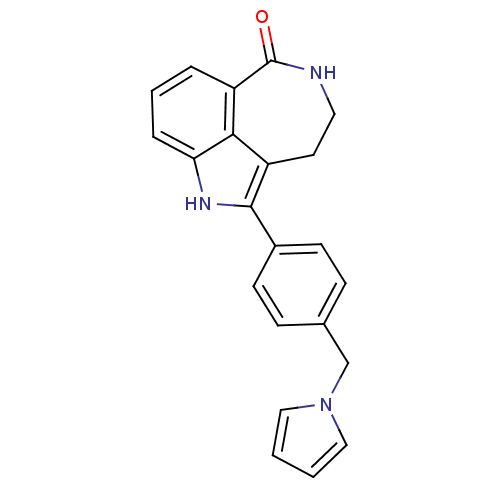 (2-(4-Pyrrol-1-ylmethyl-phenyl)-1,3,4,5-tetrahydro-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound towards human Poly (ADP-ribose) polymerase 1 (PARP-1) | J Med Chem 45: 4961-74 (2002) BindingDB Entry DOI: 10.7270/Q2T152ZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM50074304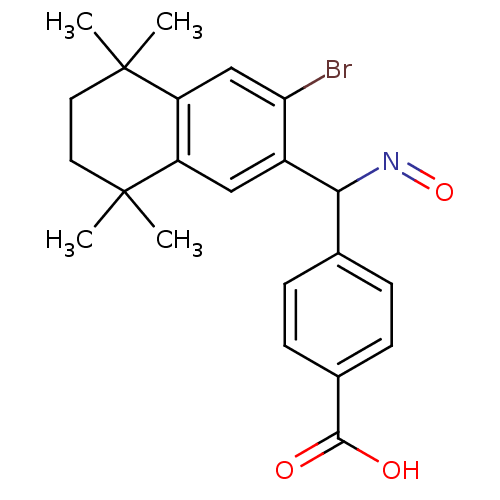 (4-{(3-Bromo-5,5,8,8-tetramethyl-5,6,7,8-tetrahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Binding affinity towards recombinantly expressed Retinoic acid receptor RXR-beta in baculoviral sysytem, by using 5 nM [3H]-targretin in a competitiv... | J Med Chem 42: 742-50 (1999) Article DOI: 10.1021/jm980621r BindingDB Entry DOI: 10.7270/Q2K936Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50290188 ((2E,4E)-3-Methyl-5-[(1S,2R)-1-methyl-2-(5,5,8,8-te...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-9-cis-RA binding to Retinoid X receptor RXR gamma | Bioorg Med Chem Lett 7: 2393-2398 (1997) Article DOI: 10.1016/S0960-894X(97)00437-X BindingDB Entry DOI: 10.7270/Q2FB52XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50290186 ((2E,4E)-3-Methyl-5-[(1R,2R)-1-methyl-2-(5,5,8,8-te...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Effective potency in transcriptional activation assay in CV-1 cells expressing Retinoid X receptor RXR alpha | Bioorg Med Chem Lett 7: 2393-2398 (1997) Article DOI: 10.1016/S0960-894X(97)00437-X BindingDB Entry DOI: 10.7270/Q2FB52XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50120721 (1-Methyl-2-phenyl-1,3,4,5-tetrahydro-azepino[5,4,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity towards human poly(ADP-ribose) polymerase 1 (PARP-1). | J Med Chem 45: 4961-74 (2002) BindingDB Entry DOI: 10.7270/Q2T152ZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50120710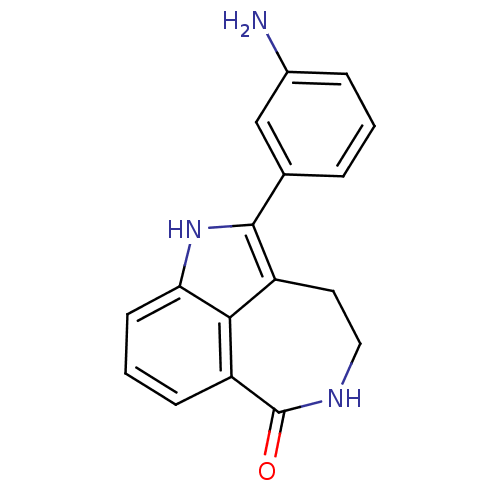 (2-(3-Amino-phenyl)-1,3,4,5-tetrahydro-azepino[5,4,...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity towards human poly(ADP-ribose) polymerase 1 (PARP-1). | J Med Chem 45: 4961-74 (2002) BindingDB Entry DOI: 10.7270/Q2T152ZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50120718 (2-Pyridin-3-yl-1,3,4,5-tetrahydro-azepino[5,4,3-cd...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity towards human poly(ADP-ribose) polymerase 1 (PARP-1). | J Med Chem 45: 4961-74 (2002) BindingDB Entry DOI: 10.7270/Q2T152ZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50074306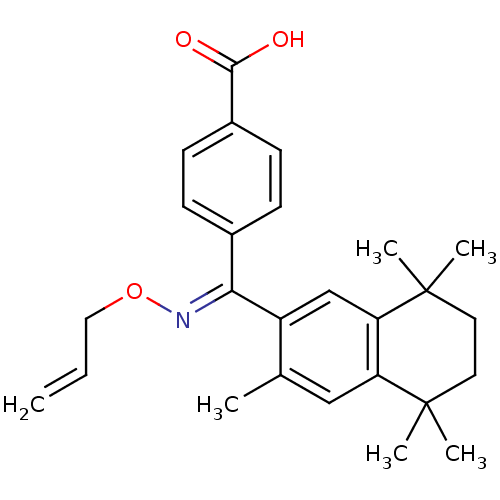 (4-[[(E)-Allyloxyimino]-(3,5,5,8,8-pentamethyl-5,6,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Binding affinity towards recombinantly expressed Retinoic acid receptor RXR-alpha in baculoviral sysytem, by using 5 nM [3H]-targretin in a competiti... | J Med Chem 42: 742-50 (1999) Article DOI: 10.1021/jm980621r BindingDB Entry DOI: 10.7270/Q2K936Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50120723 (2-(3-Dimethylaminomethyl-phenyl)-1,3,4,5-tetrahydr...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity towards human poly(ADP-ribose) polymerase 1 (PARP-1). | J Med Chem 45: 4961-74 (2002) BindingDB Entry DOI: 10.7270/Q2T152ZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50120731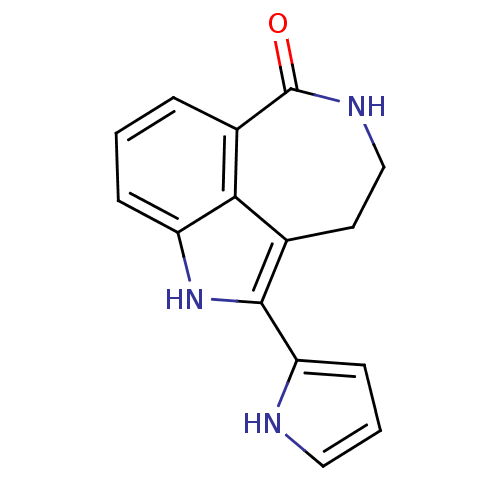 (2-(1H-Pyrrol-2-yl)-1,3,4,5-tetrahydro-azepino[5,4,...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity towards human poly(ADP-ribose) polymerase 1 (PARP-1). | J Med Chem 45: 4961-74 (2002) BindingDB Entry DOI: 10.7270/Q2T152ZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50179116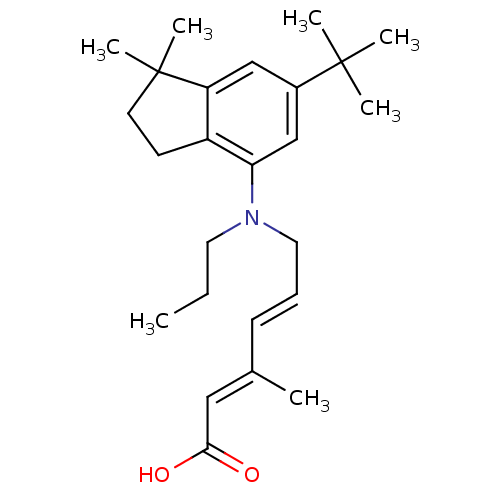 ((2E,4E)-6-((6-tert-butyl-1,1-dimethyl-2,3-dihydro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding affinity to RXRalpha | Bioorg Med Chem Lett 16: 2352-6 (2006) Article DOI: 10.1016/j.bmcl.2005.12.003 BindingDB Entry DOI: 10.7270/Q2S75FW3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50120720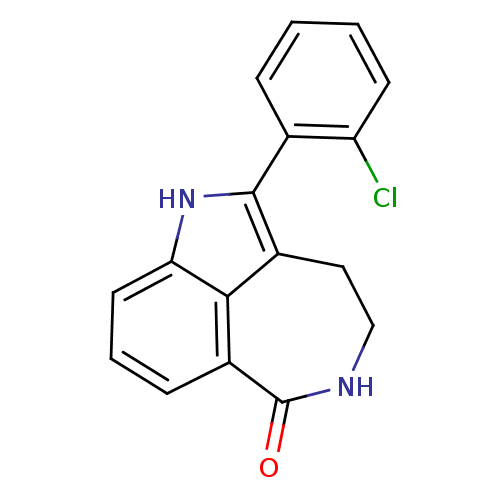 (2-(2-Chloro-phenyl)-1,3,4,5-tetrahydro-azepino[5,4...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity towards human poly(ADP-ribose) polymerase 1 (PARP-1). | J Med Chem 45: 4961-74 (2002) BindingDB Entry DOI: 10.7270/Q2T152ZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM31892 (9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-Targretin binding to Retinoid X receptor RXR alpha | J Med Chem 39: 3229-34 (1996) Article DOI: 10.1021/jm960311d BindingDB Entry DOI: 10.7270/Q2ZW1K14 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM50074295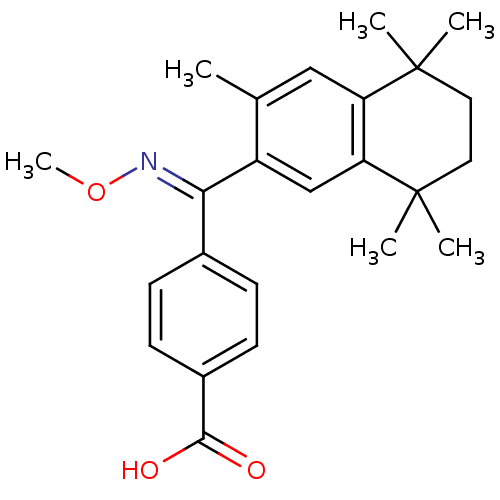 (4-[[(E)-Methoxyimino]-(3,5,5,8,8-pentamethyl-5,6,7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Binding affinity towards recombinantly expressed Retinoic acid receptor RXR-beta in baculoviral sysytem, by using 5 nM [3H]-targretin in a competitiv... | J Med Chem 42: 742-50 (1999) Article DOI: 10.1021/jm980621r BindingDB Entry DOI: 10.7270/Q2K936Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Homo sapiens (Human)) | BDBM50074295 (4-[[(E)-Methoxyimino]-(3,5,5,8,8-pentamethyl-5,6,7...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Binding affinity towards recombinantly expressed Retinoic acid receptor RXR-gamma in baculoviral sysytem, by using 5 nM [3H]-targretin in a competiti... | J Med Chem 42: 742-50 (1999) Article DOI: 10.1021/jm980621r BindingDB Entry DOI: 10.7270/Q2K936Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50052589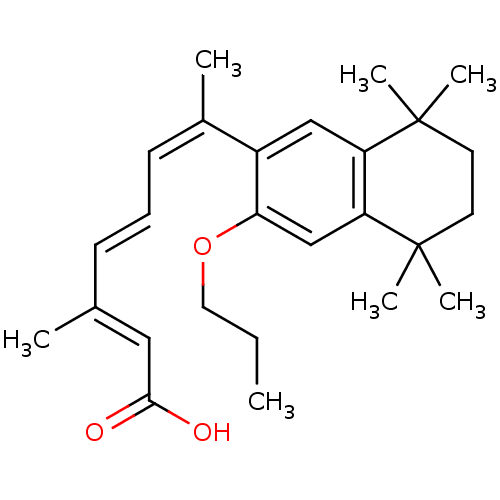 ((2E,4E,6Z)-3-Methyl-7-(5,5,8,8-tetramethyl-3-propo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-Targretin binding to Retinoid X receptor RXR alpha | J Med Chem 39: 3229-34 (1996) Article DOI: 10.1021/jm960311d BindingDB Entry DOI: 10.7270/Q2ZW1K14 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50120722 (2-(1H-Indol-5-yl)-1,3,4,5-tetrahydro-azepino[5,4,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity towards human poly(ADP-ribose) polymerase 1 (PARP-1). | J Med Chem 45: 4961-74 (2002) BindingDB Entry DOI: 10.7270/Q2T152ZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50074295 (4-[[(E)-Methoxyimino]-(3,5,5,8,8-pentamethyl-5,6,7...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Binding affinity towards recombinantly expressed Retinoic acid receptor RXR-alpha in baculoviral sysytem, by using 5 nM [3H]-targretin in a competiti... | J Med Chem 42: 742-50 (1999) Article DOI: 10.1021/jm980621r BindingDB Entry DOI: 10.7270/Q2K936Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM31892 (9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-Targretin binding to Retinoid X receptor RXR beta | J Med Chem 39: 3229-34 (1996) Article DOI: 10.1021/jm960311d BindingDB Entry DOI: 10.7270/Q2ZW1K14 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM50052589 ((2E,4E,6Z)-3-Methyl-7-(5,5,8,8-tetramethyl-3-propo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-Targretin binding to Retinoid X receptor RXR beta | J Med Chem 39: 3229-34 (1996) Article DOI: 10.1021/jm960311d BindingDB Entry DOI: 10.7270/Q2ZW1K14 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Retinoic acid receptor RXR-gamma (Homo sapiens (Human)) | BDBM50179116 ((2E,4E)-6-((6-tert-butyl-1,1-dimethyl-2,3-dihydro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding affinity to RXRgamma | Bioorg Med Chem Lett 16: 2352-6 (2006) Article DOI: 10.1016/j.bmcl.2005.12.003 BindingDB Entry DOI: 10.7270/Q2S75FW3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Mus musculus) | BDBM50290188 ((2E,4E)-3-Methyl-5-[(1S,2R)-1-methyl-2-(5,5,8,8-te...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Effective potency in transcriptional activation assay in CV-1 cells expressing retinoic acid receptor RAR beta | Bioorg Med Chem Lett 7: 2393-2398 (1997) Article DOI: 10.1016/S0960-894X(97)00437-X BindingDB Entry DOI: 10.7270/Q2FB52XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50120712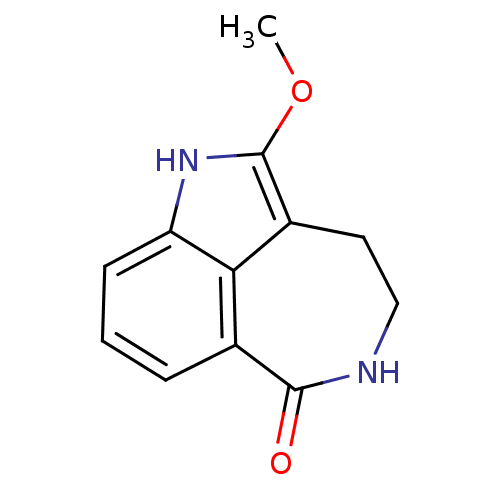 (2-Methoxy-1,3,4,5-tetrahydro-azepino[5,4,3-cd]indo...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity towards human poly(ADP-ribose) polymerase 1 (PARP-1). | J Med Chem 45: 4961-74 (2002) BindingDB Entry DOI: 10.7270/Q2T152ZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Mus musculus) | BDBM50290186 ((2E,4E)-3-Methyl-5-[(1R,2R)-1-methyl-2-(5,5,8,8-te...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Effective potency in transcriptional activation assay in CV-1 cells expressing Retinoid X receptor RXR beta | Bioorg Med Chem Lett 7: 2393-2398 (1997) Article DOI: 10.1016/S0960-894X(97)00437-X BindingDB Entry DOI: 10.7270/Q2FB52XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50120700 (6-Oxo-3,4,5,6-tetrahydro-1H-azepino[5,4,3-cd]indol...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity towards human poly(ADP-ribose) polymerase 1 (PARP-1). | J Med Chem 45: 4961-74 (2002) BindingDB Entry DOI: 10.7270/Q2T152ZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Mus musculus) | BDBM50290192 (3-Methyl-5-[5-(5,5,8,8-tetramethyl-5,6,7,8-tetrahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Effective potency in transcriptional activation assay in CV-1 cells expressing Retinoid X receptor RXR alpha | Bioorg Med Chem Lett 7: 2393-2398 (1997) Article DOI: 10.1016/S0960-894X(97)00437-X BindingDB Entry DOI: 10.7270/Q2FB52XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50120730 (6-Oxo-3,4,5,6-tetrahydro-1H-azepino[5,4,3-cd]indol...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity towards human poly(ADP-ribose) polymerase 1 (PARP-1). | J Med Chem 45: 4961-74 (2002) BindingDB Entry DOI: 10.7270/Q2T152ZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 407 total ) | Next | Last >> |