 Found 30 hits with Last Name = 'kohlbacher' and Initial = 'o'
Found 30 hits with Last Name = 'kohlbacher' and Initial = 'o' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM47167

(CHEMBL201511 | US8957103, Z1 | US9364459, A)Show SMILES COc1cc(cc(OC)c1OC)C1=C(C(=O)NC1=O)c1c[nH]c2ccccc12 |t:13| Show InChI InChI=1S/C21H18N2O5/c1-26-15-8-11(9-16(27-2)19(15)28-3)17-18(21(25)23-20(17)24)13-10-22-14-7-5-4-6-12(13)14/h4-10,22H,1-3H3,(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University
Curated by ChEMBL
| Assay Description
Inhibition of human VEGFR2 |
J Med Chem 49: 7549-53 (2006)
Article DOI: 10.1021/jm0609871
BindingDB Entry DOI: 10.7270/Q2NZ8794 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 3
(Homo sapiens (Human)) | BDBM47167

(CHEMBL201511 | US8957103, Z1 | US9364459, A)Show SMILES COc1cc(cc(OC)c1OC)C1=C(C(=O)NC1=O)c1c[nH]c2ccccc12 |t:13| Show InChI InChI=1S/C21H18N2O5/c1-26-15-8-11(9-16(27-2)19(15)28-3)17-18(21(25)23-20(17)24)13-10-22-14-7-5-4-6-12(13)14/h4-10,22H,1-3H3,(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University
Curated by ChEMBL
| Assay Description
Inhibition of human VEGFR3 |
J Med Chem 49: 7549-53 (2006)
Article DOI: 10.1021/jm0609871
BindingDB Entry DOI: 10.7270/Q2NZ8794 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50206421
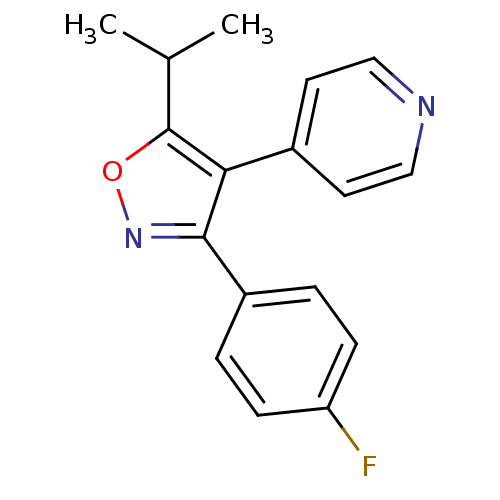
(3-(4-fluorophenyl)-5-isopropyl-4-(pyridin-4-yl)iso...)Show InChI InChI=1S/C17H15FN2O/c1-11(2)17-15(12-7-9-19-10-8-12)16(20-21-17)13-3-5-14(18)6-4-13/h3-11H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem Lett 18: 1431-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.073
BindingDB Entry DOI: 10.7270/Q208652M |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50206421

(3-(4-fluorophenyl)-5-isopropyl-4-(pyridin-4-yl)iso...)Show InChI InChI=1S/C17H15FN2O/c1-11(2)17-15(12-7-9-19-10-8-12)16(20-21-17)13-3-5-14(18)6-4-13/h3-11H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University
Curated by ChEMBL
| Assay Description
Inhibition of JNK3 |
Bioorg Med Chem Lett 18: 1431-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.073
BindingDB Entry DOI: 10.7270/Q208652M |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM47167

(CHEMBL201511 | US8957103, Z1 | US9364459, A)Show SMILES COc1cc(cc(OC)c1OC)C1=C(C(=O)NC1=O)c1c[nH]c2ccccc12 |t:13| Show InChI InChI=1S/C21H18N2O5/c1-26-15-8-11(9-16(27-2)19(15)28-3)17-18(21(25)23-20(17)24)13-10-22-14-7-5-4-6-12(13)14/h4-10,22H,1-3H3,(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University
Curated by ChEMBL
| Assay Description
Inhibition of human PDGFR beta |
J Med Chem 49: 7549-53 (2006)
Article DOI: 10.1021/jm0609871
BindingDB Entry DOI: 10.7270/Q2NZ8794 |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM47167

(CHEMBL201511 | US8957103, Z1 | US9364459, A)Show SMILES COc1cc(cc(OC)c1OC)C1=C(C(=O)NC1=O)c1c[nH]c2ccccc12 |t:13| Show InChI InChI=1S/C21H18N2O5/c1-26-15-8-11(9-16(27-2)19(15)28-3)17-18(21(25)23-20(17)24)13-10-22-14-7-5-4-6-12(13)14/h4-10,22H,1-3H3,(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University
Curated by ChEMBL
| Assay Description
Inhibition of human FAK |
J Med Chem 49: 7549-53 (2006)
Article DOI: 10.1021/jm0609871
BindingDB Entry DOI: 10.7270/Q2NZ8794 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM47167

(CHEMBL201511 | US8957103, Z1 | US9364459, A)Show SMILES COc1cc(cc(OC)c1OC)C1=C(C(=O)NC1=O)c1c[nH]c2ccccc12 |t:13| Show InChI InChI=1S/C21H18N2O5/c1-26-15-8-11(9-16(27-2)19(15)28-3)17-18(21(25)23-20(17)24)13-10-22-14-7-5-4-6-12(13)14/h4-10,22H,1-3H3,(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3 beta |
J Med Chem 49: 7549-53 (2006)
Article DOI: 10.1021/jm0609871
BindingDB Entry DOI: 10.7270/Q2NZ8794 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50206421

(3-(4-fluorophenyl)-5-isopropyl-4-(pyridin-4-yl)iso...)Show InChI InChI=1S/C17H15FN2O/c1-11(2)17-15(12-7-9-19-10-8-12)16(20-21-17)13-3-5-14(18)6-4-13/h3-11H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University
Curated by ChEMBL
| Assay Description
Inhibition of JNK2 |
Bioorg Med Chem Lett 18: 1431-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.073
BindingDB Entry DOI: 10.7270/Q208652M |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50206422
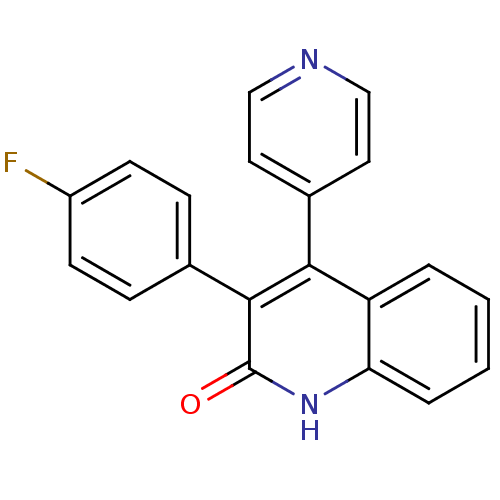
(3-(4-fluorophenyl)-4-(pyridin-4-yl)quinolin-2(1H)-...)Show InChI InChI=1S/C20H13FN2O/c21-15-7-5-13(6-8-15)19-18(14-9-11-22-12-10-14)16-3-1-2-4-17(16)23-20(19)24/h1-12H,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem Lett 18: 1431-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.073
BindingDB Entry DOI: 10.7270/Q208652M |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50232725

(1-ethyl-3-(4-fluorophenyl)-4-(pyridin-4-yl)quinoli...)Show SMILES CCn1c2ccccc2c(-c2ccncc2)c(-c2ccc(F)cc2)c1=O Show InChI InChI=1S/C22H17FN2O/c1-2-25-19-6-4-3-5-18(19)20(16-11-13-24-14-12-16)21(22(25)26)15-7-9-17(23)10-8-15/h3-14H,2H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem Lett 18: 1431-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.073
BindingDB Entry DOI: 10.7270/Q208652M |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50206423
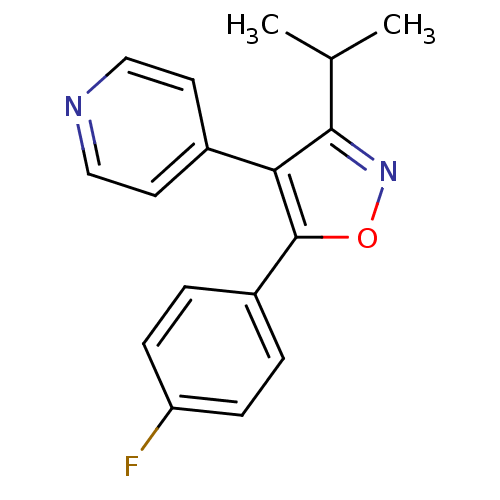
(4-(5-(4-fluorophenyl)-3-isopropylisoxazol-4-yl)pyr...)Show InChI InChI=1S/C17H15FN2O/c1-11(2)16-15(12-7-9-19-10-8-12)17(21-20-16)13-3-5-14(18)6-4-13/h3-11H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem Lett 18: 1431-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.073
BindingDB Entry DOI: 10.7270/Q208652M |
More data for this
Ligand-Target Pair | |
Angiopoietin-1 receptor
(Homo sapiens (Human)) | BDBM47167

(CHEMBL201511 | US8957103, Z1 | US9364459, A)Show SMILES COc1cc(cc(OC)c1OC)C1=C(C(=O)NC1=O)c1c[nH]c2ccccc12 |t:13| Show InChI InChI=1S/C21H18N2O5/c1-26-15-8-11(9-16(27-2)19(15)28-3)17-18(21(25)23-20(17)24)13-10-22-14-7-5-4-6-12(13)14/h4-10,22H,1-3H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University
Curated by ChEMBL
| Assay Description
Inhibition of human TIE2 |
J Med Chem 49: 7549-53 (2006)
Article DOI: 10.1021/jm0609871
BindingDB Entry DOI: 10.7270/Q2NZ8794 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50232724
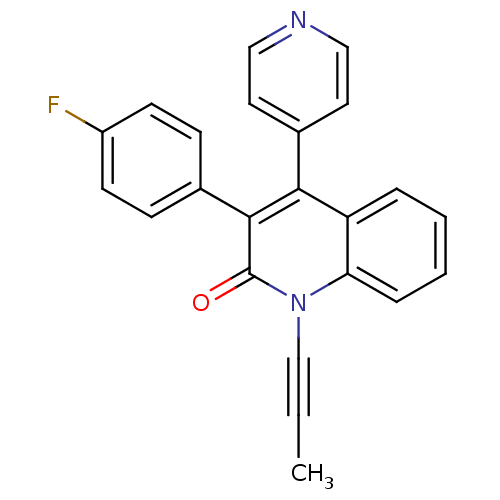
(3-(4-fluorophenyl)-1-(prop-1-ynyl)-4-(pyridin-4-yl...)Show SMILES CC#Cn1c2ccccc2c(-c2ccncc2)c(-c2ccc(F)cc2)c1=O Show InChI InChI=1S/C23H15FN2O/c1-2-15-26-20-6-4-3-5-19(20)21(17-11-13-25-14-12-17)22(23(26)27)16-7-9-18(24)10-8-16/h3-14H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem Lett 18: 1431-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.073
BindingDB Entry DOI: 10.7270/Q208652M |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50206423

(4-(5-(4-fluorophenyl)-3-isopropylisoxazol-4-yl)pyr...)Show InChI InChI=1S/C17H15FN2O/c1-11(2)16-15(12-7-9-19-10-8-12)17(21-20-16)13-3-5-14(18)6-4-13/h3-11H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University
Curated by ChEMBL
| Assay Description
Inhibition of JNK3 |
Bioorg Med Chem Lett 18: 1431-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.073
BindingDB Entry DOI: 10.7270/Q208652M |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50232726

(3-(4-fluorophenyl)-1-methyl-4-(pyridin-4-yl)quinol...)Show InChI InChI=1S/C21H15FN2O/c1-24-18-5-3-2-4-17(18)19(15-10-12-23-13-11-15)20(21(24)25)14-6-8-16(22)9-7-14/h2-13H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem Lett 18: 1431-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.073
BindingDB Entry DOI: 10.7270/Q208652M |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM47167

(CHEMBL201511 | US8957103, Z1 | US9364459, A)Show SMILES COc1cc(cc(OC)c1OC)C1=C(C(=O)NC1=O)c1c[nH]c2ccccc12 |t:13| Show InChI InChI=1S/C21H18N2O5/c1-26-15-8-11(9-16(27-2)19(15)28-3)17-18(21(25)23-20(17)24)13-10-22-14-7-5-4-6-12(13)14/h4-10,22H,1-3H3,(H,23,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University
Curated by ChEMBL
| Assay Description
Inhibition of human CDK2 (with cyclin A) |
J Med Chem 49: 7549-53 (2006)
Article DOI: 10.1021/jm0609871
BindingDB Entry DOI: 10.7270/Q2NZ8794 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50232723
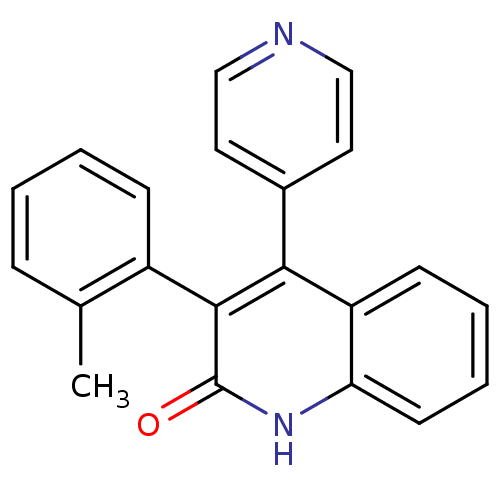
(4-(pyridin-4-yl)-3-o-tolylquinolin-2(1H)-one | CHE...)Show SMILES Cc1ccccc1-c1c(-c2ccncc2)c2ccccc2[nH]c1=O |(1.24,-15.15,;-.1,-14.38,;-.1,-12.84,;-1.44,-12.08,;-2.78,-12.87,;-2.76,-14.4,;-1.43,-15.16,;-1.42,-16.7,;-2.74,-17.47,;-4.07,-16.7,;-5.41,-17.47,;-6.75,-16.7,;-6.74,-15.16,;-5.41,-14.38,;-4.08,-15.15,;-2.74,-19.01,;-4.06,-19.78,;-4.06,-21.31,;-2.72,-22.08,;-1.4,-21.31,;-1.41,-19.78,;-.07,-19.01,;-.08,-17.46,;1.25,-16.68,)| Show InChI InChI=1S/C21H16N2O/c1-14-6-2-3-7-16(14)20-19(15-10-12-22-13-11-15)17-8-4-5-9-18(17)23-21(20)24/h2-13H,1H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem Lett 18: 1431-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.073
BindingDB Entry DOI: 10.7270/Q208652M |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM47167

(CHEMBL201511 | US8957103, Z1 | US9364459, A)Show SMILES COc1cc(cc(OC)c1OC)C1=C(C(=O)NC1=O)c1c[nH]c2ccccc12 |t:13| Show InChI InChI=1S/C21H18N2O5/c1-26-15-8-11(9-16(27-2)19(15)28-3)17-18(21(25)23-20(17)24)13-10-22-14-7-5-4-6-12(13)14/h4-10,22H,1-3H3,(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University
Curated by ChEMBL
| Assay Description
Inhibition of human CDK4 (with cyclin D1) |
J Med Chem 49: 7549-53 (2006)
Article DOI: 10.1021/jm0609871
BindingDB Entry DOI: 10.7270/Q2NZ8794 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM47167

(CHEMBL201511 | US8957103, Z1 | US9364459, A)Show SMILES COc1cc(cc(OC)c1OC)C1=C(C(=O)NC1=O)c1c[nH]c2ccccc12 |t:13| Show InChI InChI=1S/C21H18N2O5/c1-26-15-8-11(9-16(27-2)19(15)28-3)17-18(21(25)23-20(17)24)13-10-22-14-7-5-4-6-12(13)14/h4-10,22H,1-3H3,(H,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University
Curated by ChEMBL
| Assay Description
Inhibition of human IGF1-R |
J Med Chem 49: 7549-53 (2006)
Article DOI: 10.1021/jm0609871
BindingDB Entry DOI: 10.7270/Q2NZ8794 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM47167

(CHEMBL201511 | US8957103, Z1 | US9364459, A)Show SMILES COc1cc(cc(OC)c1OC)C1=C(C(=O)NC1=O)c1c[nH]c2ccccc12 |t:13| Show InChI InChI=1S/C21H18N2O5/c1-26-15-8-11(9-16(27-2)19(15)28-3)17-18(21(25)23-20(17)24)13-10-22-14-7-5-4-6-12(13)14/h4-10,22H,1-3H3,(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora-A |
J Med Chem 49: 7549-53 (2006)
Article DOI: 10.1021/jm0609871
BindingDB Entry DOI: 10.7270/Q2NZ8794 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM47167

(CHEMBL201511 | US8957103, Z1 | US9364459, A)Show SMILES COc1cc(cc(OC)c1OC)C1=C(C(=O)NC1=O)c1c[nH]c2ccccc12 |t:13| Show InChI InChI=1S/C21H18N2O5/c1-26-15-8-11(9-16(27-2)19(15)28-3)17-18(21(25)23-20(17)24)13-10-22-14-7-5-4-6-12(13)14/h4-10,22H,1-3H3,(H,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
J Med Chem 49: 7549-53 (2006)
Article DOI: 10.1021/jm0609871
BindingDB Entry DOI: 10.7270/Q2NZ8794 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM47167

(CHEMBL201511 | US8957103, Z1 | US9364459, A)Show SMILES COc1cc(cc(OC)c1OC)C1=C(C(=O)NC1=O)c1c[nH]c2ccccc12 |t:13| Show InChI InChI=1S/C21H18N2O5/c1-26-15-8-11(9-16(27-2)19(15)28-3)17-18(21(25)23-20(17)24)13-10-22-14-7-5-4-6-12(13)14/h4-10,22H,1-3H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University
Curated by ChEMBL
| Assay Description
Inhibition of human SRC |
J Med Chem 49: 7549-53 (2006)
Article DOI: 10.1021/jm0609871
BindingDB Entry DOI: 10.7270/Q2NZ8794 |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM47167

(CHEMBL201511 | US8957103, Z1 | US9364459, A)Show SMILES COc1cc(cc(OC)c1OC)C1=C(C(=O)NC1=O)c1c[nH]c2ccccc12 |t:13| Show InChI InChI=1S/C21H18N2O5/c1-26-15-8-11(9-16(27-2)19(15)28-3)17-18(21(25)23-20(17)24)13-10-22-14-7-5-4-6-12(13)14/h4-10,22H,1-3H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University
Curated by ChEMBL
| Assay Description
Inhibition of human Ins-R |
J Med Chem 49: 7549-53 (2006)
Article DOI: 10.1021/jm0609871
BindingDB Entry DOI: 10.7270/Q2NZ8794 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM47167

(CHEMBL201511 | US8957103, Z1 | US9364459, A)Show SMILES COc1cc(cc(OC)c1OC)C1=C(C(=O)NC1=O)c1c[nH]c2ccccc12 |t:13| Show InChI InChI=1S/C21H18N2O5/c1-26-15-8-11(9-16(27-2)19(15)28-3)17-18(21(25)23-20(17)24)13-10-22-14-7-5-4-6-12(13)14/h4-10,22H,1-3H3,(H,23,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora-B |
J Med Chem 49: 7549-53 (2006)
Article DOI: 10.1021/jm0609871
BindingDB Entry DOI: 10.7270/Q2NZ8794 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM47167

(CHEMBL201511 | US8957103, Z1 | US9364459, A)Show SMILES COc1cc(cc(OC)c1OC)C1=C(C(=O)NC1=O)c1c[nH]c2ccccc12 |t:13| Show InChI InChI=1S/C21H18N2O5/c1-26-15-8-11(9-16(27-2)19(15)28-3)17-18(21(25)23-20(17)24)13-10-22-14-7-5-4-6-12(13)14/h4-10,22H,1-3H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University
Curated by ChEMBL
| Assay Description
Inhibition of human EGFR |
J Med Chem 49: 7549-53 (2006)
Article DOI: 10.1021/jm0609871
BindingDB Entry DOI: 10.7270/Q2NZ8794 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM47167

(CHEMBL201511 | US8957103, Z1 | US9364459, A)Show SMILES COc1cc(cc(OC)c1OC)C1=C(C(=O)NC1=O)c1c[nH]c2ccccc12 |t:13| Show InChI InChI=1S/C21H18N2O5/c1-26-15-8-11(9-16(27-2)19(15)28-3)17-18(21(25)23-20(17)24)13-10-22-14-7-5-4-6-12(13)14/h4-10,22H,1-3H3,(H,23,24,25) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University
Curated by ChEMBL
| Assay Description
Inhibition of human ERbB2 |
J Med Chem 49: 7549-53 (2006)
Article DOI: 10.1021/jm0609871
BindingDB Entry DOI: 10.7270/Q2NZ8794 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM47167

(CHEMBL201511 | US8957103, Z1 | US9364459, A)Show SMILES COc1cc(cc(OC)c1OC)C1=C(C(=O)NC1=O)c1c[nH]c2ccccc12 |t:13| Show InChI InChI=1S/C21H18N2O5/c1-26-15-8-11(9-16(27-2)19(15)28-3)17-18(21(25)23-20(17)24)13-10-22-14-7-5-4-6-12(13)14/h4-10,22H,1-3H3,(H,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University
Curated by ChEMBL
| Assay Description
Inhibition of human ABL1 |
J Med Chem 49: 7549-53 (2006)
Article DOI: 10.1021/jm0609871
BindingDB Entry DOI: 10.7270/Q2NZ8794 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Nek2
(Homo sapiens (Human)) | BDBM47167

(CHEMBL201511 | US8957103, Z1 | US9364459, A)Show SMILES COc1cc(cc(OC)c1OC)C1=C(C(=O)NC1=O)c1c[nH]c2ccccc12 |t:13| Show InChI InChI=1S/C21H18N2O5/c1-26-15-8-11(9-16(27-2)19(15)28-3)17-18(21(25)23-20(17)24)13-10-22-14-7-5-4-6-12(13)14/h4-10,22H,1-3H3,(H,23,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University
Curated by ChEMBL
| Assay Description
Inhibition of human NEK2 |
J Med Chem 49: 7549-53 (2006)
Article DOI: 10.1021/jm0609871
BindingDB Entry DOI: 10.7270/Q2NZ8794 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM47167

(CHEMBL201511 | US8957103, Z1 | US9364459, A)Show SMILES COc1cc(cc(OC)c1OC)C1=C(C(=O)NC1=O)c1c[nH]c2ccccc12 |t:13| Show InChI InChI=1S/C21H18N2O5/c1-26-15-8-11(9-16(27-2)19(15)28-3)17-18(21(25)23-20(17)24)13-10-22-14-7-5-4-6-12(13)14/h4-10,22H,1-3H3,(H,23,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University
Curated by ChEMBL
| Assay Description
Inhibition of human AKT1 |
J Med Chem 49: 7549-53 (2006)
Article DOI: 10.1021/jm0609871
BindingDB Entry DOI: 10.7270/Q2NZ8794 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM47167

(CHEMBL201511 | US8957103, Z1 | US9364459, A)Show SMILES COc1cc(cc(OC)c1OC)C1=C(C(=O)NC1=O)c1c[nH]c2ccccc12 |t:13| Show InChI InChI=1S/C21H18N2O5/c1-26-15-8-11(9-16(27-2)19(15)28-3)17-18(21(25)23-20(17)24)13-10-22-14-7-5-4-6-12(13)14/h4-10,22H,1-3H3,(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University
Curated by ChEMBL
| Assay Description
Inhibition of human PLK1 |
J Med Chem 49: 7549-53 (2006)
Article DOI: 10.1021/jm0609871
BindingDB Entry DOI: 10.7270/Q2NZ8794 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data