

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M-phase inducer phosphatase 2 (Mus musculus) | BDBM50104684 ((4-Hexadecanoyl-3-hydroxy-5-oxo-2,5-dihydro-furan-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University Curated by ChEMBL | Assay Description Inhibitory activity against cell division cycle 25B | J Med Chem 44: 3216-22 (2001) BindingDB Entry DOI: 10.7270/Q2WD3ZVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Mus musculus) | BDBM50104691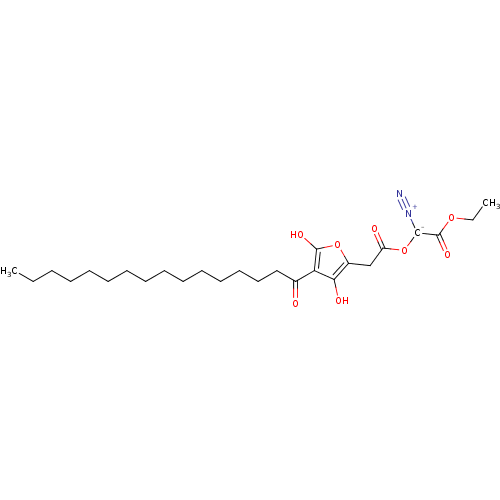 (CHEMBL423508 | Diazo-[2-(4-hexadecanoyl-3-hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University Curated by ChEMBL | Assay Description Inhibitory activity against cell division cycle 25B | J Med Chem 44: 3216-22 (2001) BindingDB Entry DOI: 10.7270/Q2WD3ZVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Mus musculus) | BDBM50104690 ((4-Hexadecanoyl-3-hydroxy-5-oxo-2,5-dihydro-furan-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University Curated by ChEMBL | Assay Description Inhibitory activity against cell division cycle 25B | J Med Chem 44: 3216-22 (2001) BindingDB Entry DOI: 10.7270/Q2WD3ZVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM125988 (US8772297, Y244) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University US Patent | Assay Description The biological activity of a compound was determined by measuring the activation of Smad3/Smad4 complex which is a transcription factor showing activ... | US Patent US8772297 (2014) BindingDB Entry DOI: 10.7270/Q2XS5T2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Mus musculus) | BDBM50104674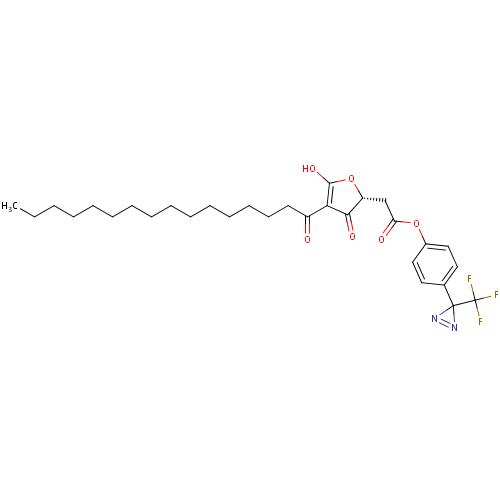 ((4-Hexadecanoyl-3-hydroxy-5-oxo-2,5-dihydro-furan-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University Curated by ChEMBL | Assay Description Inhibitory activity against cell division cycle 25B | J Med Chem 44: 3216-22 (2001) BindingDB Entry DOI: 10.7270/Q2WD3ZVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM125985 (US8772297, Y241) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University US Patent | Assay Description The biological activity of a compound was determined by measuring the activation of Smad3/Smad4 complex which is a transcription factor showing activ... | US Patent US8772297 (2014) BindingDB Entry DOI: 10.7270/Q2XS5T2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Mus musculus) | BDBM50104693 (4-Hydroxy-5-hydroxymethyl-3-octadec-9-enoyl-5H-fur...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University Curated by ChEMBL | Assay Description Inhibitory activity against cell division cycle 25B | J Med Chem 44: 3216-22 (2001) BindingDB Entry DOI: 10.7270/Q2WD3ZVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50324539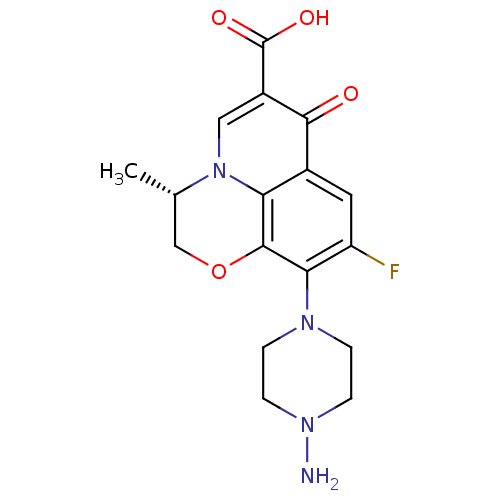 ((S)-(-)-9-(4-aminopiperazin-1-yl)-8-fluoro-3-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | MMDB Article PubMed | n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Piemonte Orientale Amedeo Avogadro Curated by ChEMBL | Assay Description Inhibition of human recombinant MBP-KAT2 expressed in HEK293 cells assessed as conversion of L-kynurenine to kynurenic acid after 1 hr | J Med Chem 53: 5684-9 (2010) Article DOI: 10.1021/jm100464k BindingDB Entry DOI: 10.7270/Q2HM58N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Rattus norvegicus) | BDBM50324539 ((S)-(-)-9-(4-aminopiperazin-1-yl)-8-fluoro-3-methy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Piemonte Orientale Amedeo Avogadro Curated by ChEMBL | Assay Description Inhibition of rat brain KAT2 | J Med Chem 53: 5684-9 (2010) Article DOI: 10.1021/jm100464k BindingDB Entry DOI: 10.7270/Q2HM58N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50324539 ((S)-(-)-9-(4-aminopiperazin-1-yl)-8-fluoro-3-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | MMDB Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Piemonte Orientale Amedeo Avogadro Curated by ChEMBL | Assay Description Inhibition of KAT2 in human prefrontal cortex homogenates | J Med Chem 53: 5684-9 (2010) Article DOI: 10.1021/jm100464k BindingDB Entry DOI: 10.7270/Q2HM58N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Mus musculus) | BDBM50104677 (3-Hexadecanoyl-4-hydroxy-5-methylene-5H-furan-2-on...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University Curated by ChEMBL | Assay Description Inhibitory activity against cell division cycle 25B | J Med Chem 44: 3216-22 (2001) BindingDB Entry DOI: 10.7270/Q2WD3ZVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50567332 (CHEMBL4853586) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hypoxia-induced HIF1 alpha transcriptional activity in human HeLa cells transfected with luciferase reporter containing of HRE assessed... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116375 BindingDB Entry DOI: 10.7270/Q2542S9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50567336 (CHEMBL4876055) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hypoxia-induced HIF1 alpha transcriptional activity in human HeLa cells transfected with luciferase reporter containing of HRE assessed... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116375 BindingDB Entry DOI: 10.7270/Q2542S9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Mus musculus) | BDBM50104676 ((4-Hexadecanoyl-3-hydroxy-5-oxo-2,5-dihydro-furan-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University Curated by ChEMBL | Assay Description Inhibitory activity against cell division cycle 25B | J Med Chem 44: 3216-22 (2001) BindingDB Entry DOI: 10.7270/Q2WD3ZVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Mus musculus) | BDBM50104681 (CHEMBL109357 | Methanesulfonic acid 4-hexadecanoyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University Curated by ChEMBL | Assay Description Inhibitory activity against cell division cycle 25B | J Med Chem 44: 3216-22 (2001) BindingDB Entry DOI: 10.7270/Q2WD3ZVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50567334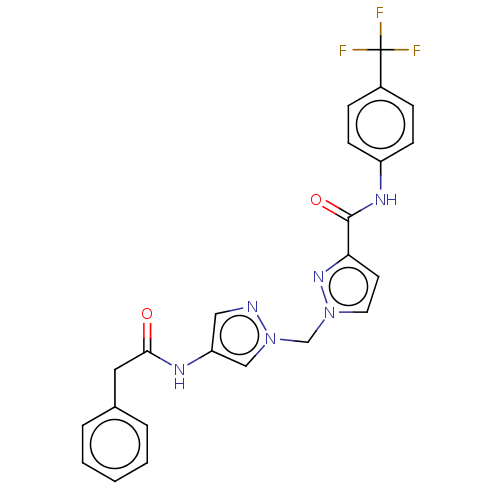 (CHEMBL4869045) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hypoxia-induced HIF1 alpha transcriptional activity in human HeLa cells transfected with luciferase reporter containing of HRE assessed... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116375 BindingDB Entry DOI: 10.7270/Q2542S9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM125986 (US8772297, Y260) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University US Patent | Assay Description The biological activity of a compound was determined by measuring the activation of Smad3/Smad4 complex which is a transcription factor showing activ... | US Patent US8772297 (2014) BindingDB Entry DOI: 10.7270/Q2XS5T2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50416560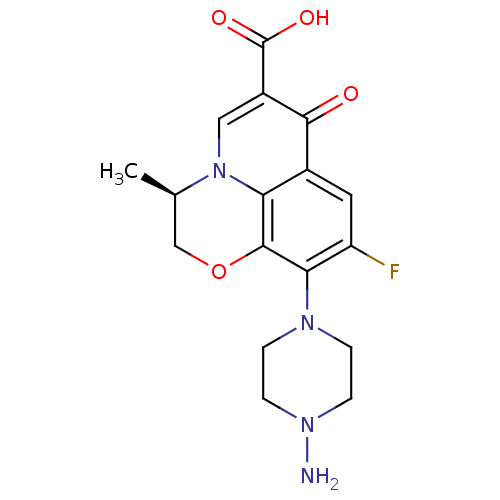 (CHEMBL1215660) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Piemonte Orientale Amedeo Avogadro Curated by ChEMBL | Assay Description Inhibition of human recombinant MBP-KAT2 expressed in HEK293 cells assessed as conversion of L-kynurenine to kynurenic acid after 1 hr | J Med Chem 53: 5684-9 (2010) Article DOI: 10.1021/jm100464k BindingDB Entry DOI: 10.7270/Q2HM58N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50012280 (CHEMBL3260030) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Science and Technology Agency Curated by ChEMBL | Assay Description Inhibition of Vaccinia H1-related phosphatase (unknown origin) by fluorescence emission assay | Bioorg Med Chem 22: 2771-82 (2014) Article DOI: 10.1016/j.bmc.2014.03.012 BindingDB Entry DOI: 10.7270/Q24T6KXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Mus musculus) | BDBM50104696 (4-Hydroxy-5-hydroxymethyl-2-oxo-2,5-dihydro-furan-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University Curated by ChEMBL | Assay Description Inhibitory activity against cell division cycle 25B | J Med Chem 44: 3216-22 (2001) BindingDB Entry DOI: 10.7270/Q2WD3ZVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50567333 (CHEMBL4865589) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hypoxia-induced HIF1 alpha transcriptional activity in human HeLa cells transfected with luciferase reporter containing of HRE assessed... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116375 BindingDB Entry DOI: 10.7270/Q2542S9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Mus musculus) | BDBM50104688 (4-Hydroxy-5-hydroxymethyl-2-oxo-2,5-dihydro-furan-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University Curated by ChEMBL | Assay Description Inhibitory activity against cell division cycle 25B | J Med Chem 44: 3216-22 (2001) BindingDB Entry DOI: 10.7270/Q2WD3ZVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM125979 (US8772297, Y177) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University US Patent | Assay Description The biological activity of a compound was determined by measuring the activation of Smad3/Smad4 complex which is a transcription factor showing activ... | US Patent US8772297 (2014) BindingDB Entry DOI: 10.7270/Q2XS5T2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Mus musculus) | BDBM50104694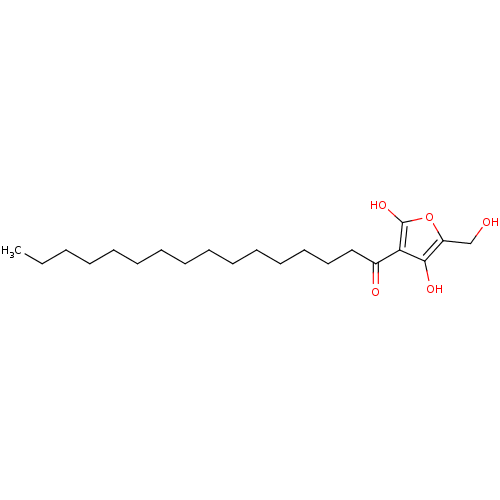 ((R)-4-hydroxy-5-(hydroxymethyl)-3-palmitoylfuran-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University Curated by ChEMBL | Assay Description Inhibitory activity against cell division cycle 25B | J Med Chem 44: 3216-22 (2001) BindingDB Entry DOI: 10.7270/Q2WD3ZVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Mus musculus) | BDBM50104697 ((4-Hexadecanoyl-3-hydroxy-5-oxo-2,5-dihydro-furan-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University Curated by ChEMBL | Assay Description Inhibitory activity against cell division cycle 25B | J Med Chem 44: 3216-22 (2001) BindingDB Entry DOI: 10.7270/Q2WD3ZVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM125980 (US8772297, Y224) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University US Patent | Assay Description The biological activity of a compound was determined by measuring the activation of Smad3/Smad4 complex which is a transcription factor showing activ... | US Patent US8772297 (2014) BindingDB Entry DOI: 10.7270/Q2XS5T2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM125989 (US8772297, Y250) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University US Patent | Assay Description The biological activity of a compound was determined by measuring the activation of Smad3/Smad4 complex which is a transcription factor showing activ... | US Patent US8772297 (2014) BindingDB Entry DOI: 10.7270/Q2XS5T2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Mus musculus) | BDBM50104692 (4-Hydroxy-5-nonyloxycarbonylmethyl-2-oxo-2,5-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University Curated by ChEMBL | Assay Description Inhibitory activity against cell division cycle 25B | J Med Chem 44: 3216-22 (2001) BindingDB Entry DOI: 10.7270/Q2WD3ZVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Mus musculus) | BDBM50104700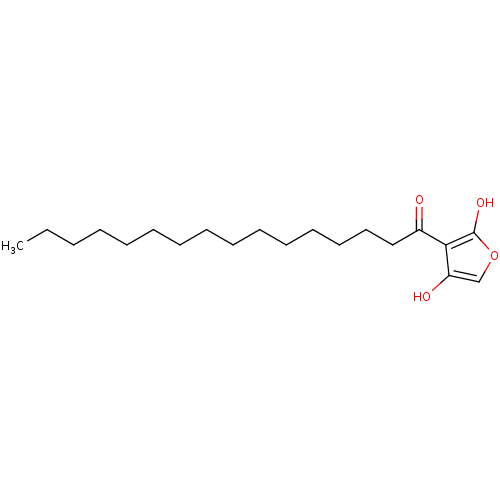 (3-Hexadecanoyl-4-hydroxy-5H-furan-2-one | CHEMBL32...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University Curated by ChEMBL | Assay Description Inhibitory activity against cell division cycle 25B | J Med Chem 44: 3216-22 (2001) BindingDB Entry DOI: 10.7270/Q2WD3ZVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Mus musculus) | BDBM50104703 (4-Hydroxy-5-hydroxymethyl-2-oxo-2,5-dihydro-furan-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University Curated by ChEMBL | Assay Description Inhibitory activity against cell division cycle 25B | J Med Chem 44: 3216-22 (2001) BindingDB Entry DOI: 10.7270/Q2WD3ZVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Mus musculus) | BDBM50104673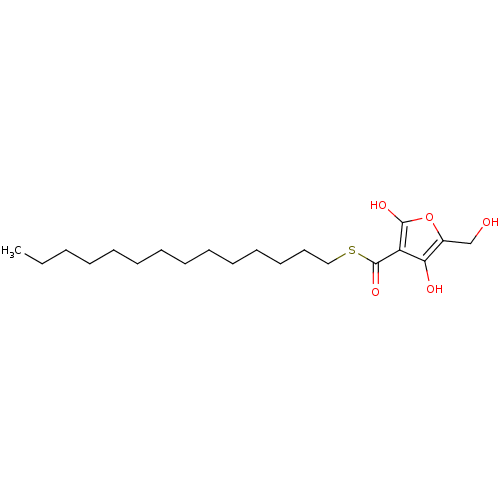 (4-Hydroxy-5-hydroxymethyl-2-oxo-2,5-dihydro-furan-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University Curated by ChEMBL | Assay Description Inhibitory activity against cell division cycle 25B | J Med Chem 44: 3216-22 (2001) BindingDB Entry DOI: 10.7270/Q2WD3ZVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM125981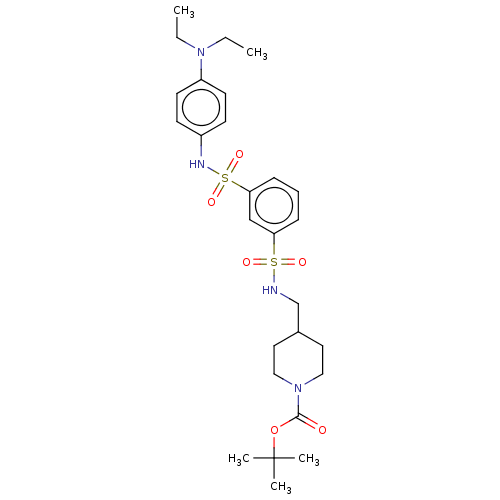 (US8772297, Y186) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University US Patent | Assay Description The biological activity of a compound was determined by measuring the activation of Smad3/Smad4 complex which is a transcription factor showing activ... | US Patent US8772297 (2014) BindingDB Entry DOI: 10.7270/Q2XS5T2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Mus musculus) | BDBM50104683 (3-Hexadecanoyl-4-hydroxy-5-methyl-5H-furan-2-one |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University Curated by ChEMBL | Assay Description Inhibitory activity against cell division cycle 25B | J Med Chem 44: 3216-22 (2001) BindingDB Entry DOI: 10.7270/Q2WD3ZVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50104684 ((4-Hexadecanoyl-3-hydroxy-5-oxo-2,5-dihydro-furan-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University Curated by ChEMBL | Assay Description Inhibitory activity against vaccinia VH1-related phosphatase(VHR) | J Med Chem 44: 3216-22 (2001) BindingDB Entry DOI: 10.7270/Q2WD3ZVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50104690 ((4-Hexadecanoyl-3-hydroxy-5-oxo-2,5-dihydro-furan-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University Curated by ChEMBL | Assay Description Inhibitory activity against vaccinia VH1-related phosphatase(VHR) | J Med Chem 44: 3216-22 (2001) BindingDB Entry DOI: 10.7270/Q2WD3ZVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50012323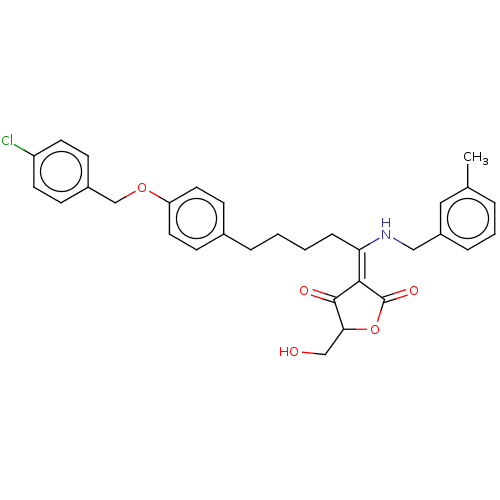 (CHEMBL3260378) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Science and Technology Agency Curated by ChEMBL | Assay Description Inhibition of Vaccinia H1-related phosphatase (unknown origin) by fluorescence emission assay in presence of 0.001% NP-40 | Bioorg Med Chem 22: 2771-82 (2014) Article DOI: 10.1016/j.bmc.2014.03.012 BindingDB Entry DOI: 10.7270/Q24T6KXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM125991 (US8772297, Y296) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University US Patent | Assay Description The biological activity of a compound was determined by measuring the activation of Smad3/Smad4 complex which is a transcription factor showing activ... | US Patent US8772297 (2014) BindingDB Entry DOI: 10.7270/Q2XS5T2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50104674 ((4-Hexadecanoyl-3-hydroxy-5-oxo-2,5-dihydro-furan-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University Curated by ChEMBL | Assay Description Inhibitory activity against vaccinia VH1-related phosphatase(VHR) | J Med Chem 44: 3216-22 (2001) BindingDB Entry DOI: 10.7270/Q2WD3ZVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Mus musculus) | BDBM50104694 ((R)-4-hydroxy-5-(hydroxymethyl)-3-palmitoylfuran-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University Curated by ChEMBL | Assay Description Inhibitory activity against cell division cycle 25B | J Med Chem 44: 3216-22 (2001) BindingDB Entry DOI: 10.7270/Q2WD3ZVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50567331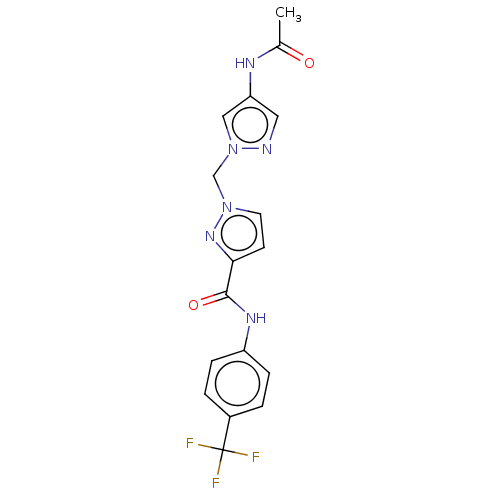 (CHEMBL4850885) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hypoxia-induced HIF1 alpha transcriptional activity in human HeLa cells transfected with luciferase reporter containing of HRE assessed... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116375 BindingDB Entry DOI: 10.7270/Q2542S9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Mus musculus) | BDBM50104699 (4-Hydroxy-5-methoxycarbonylmethyl-2-oxo-2,5-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University Curated by ChEMBL | Assay Description Inhibitory activity against cell division cycle 25B | J Med Chem 44: 3216-22 (2001) BindingDB Entry DOI: 10.7270/Q2WD3ZVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM125990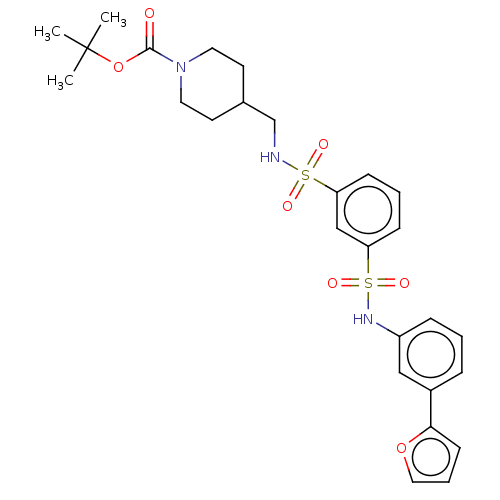 (US8772297, Y284) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University US Patent | Assay Description The biological activity of a compound was determined by measuring the activation of Smad3/Smad4 complex which is a transcription factor showing activ... | US Patent US8772297 (2014) BindingDB Entry DOI: 10.7270/Q2XS5T2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50567335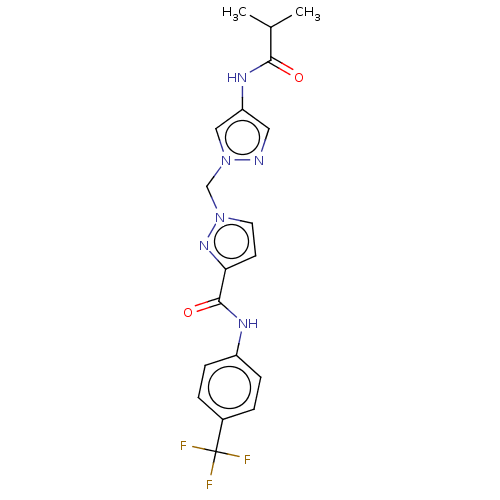 (CHEMBL4854814) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hypoxia-induced HIF1 alpha transcriptional activity in human HeLa cells transfected with luciferase reporter containing of HRE assessed... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116375 BindingDB Entry DOI: 10.7270/Q2542S9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM125987 (US8772297, Y366) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University US Patent | Assay Description The biological activity of a compound was determined by measuring the activation of Smad3/Smad4 complex which is a transcription factor showing activ... | US Patent US8772297 (2014) BindingDB Entry DOI: 10.7270/Q2XS5T2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50012321 (CHEMBL3260377) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Science and Technology Agency Curated by ChEMBL | Assay Description Inhibition of Vaccinia H1-related phosphatase (unknown origin) by fluorescence emission assay in presence of 0.001% NP-40 | Bioorg Med Chem 22: 2771-82 (2014) Article DOI: 10.1016/j.bmc.2014.03.012 BindingDB Entry DOI: 10.7270/Q24T6KXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Mus musculus) | BDBM50104686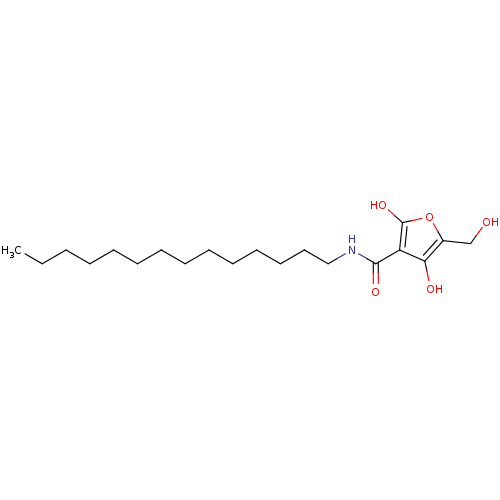 (4-Hydroxy-5-hydroxymethyl-2-oxo-2,5-dihydro-furan-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University Curated by ChEMBL | Assay Description Inhibitory activity against cell division cycle 25B | J Med Chem 44: 3216-22 (2001) BindingDB Entry DOI: 10.7270/Q2WD3ZVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM125976 (US8772297, Y145) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University US Patent | Assay Description The biological activity of a compound was determined by measuring the activation of Smad3/Smad4 complex which is a transcription factor showing activ... | US Patent US8772297 (2014) BindingDB Entry DOI: 10.7270/Q2XS5T2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50012320 (CHEMBL3260376) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Science and Technology Agency Curated by ChEMBL | Assay Description Inhibition of Vaccinia H1-related phosphatase (unknown origin) by fluorescence emission assay in presence of 0.001% NP-40 | Bioorg Med Chem 22: 2771-82 (2014) Article DOI: 10.1016/j.bmc.2014.03.012 BindingDB Entry DOI: 10.7270/Q24T6KXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50104681 (CHEMBL109357 | Methanesulfonic acid 4-hexadecanoyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University Curated by ChEMBL | Assay Description Inhibitory activity against vaccinia VH1-related phosphatase(VHR) | J Med Chem 44: 3216-22 (2001) BindingDB Entry DOI: 10.7270/Q2WD3ZVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50104677 (3-Hexadecanoyl-4-hydroxy-5-methylene-5H-furan-2-on...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University Curated by ChEMBL | Assay Description Inhibitory activity against vaccinia VH1-related phosphatase(VHR) | J Med Chem 44: 3216-22 (2001) BindingDB Entry DOI: 10.7270/Q2WD3ZVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 130 total ) | Next | Last >> |