

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190273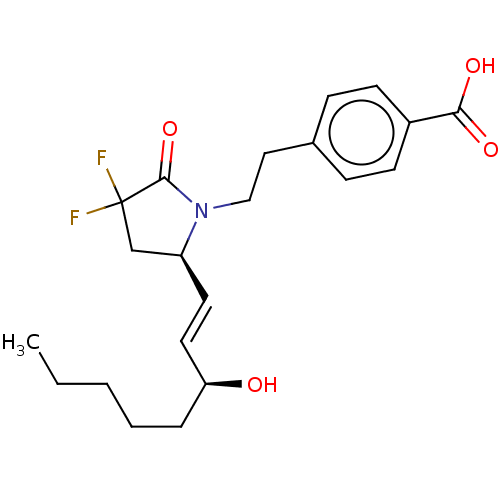 (US9180116, 21C) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0820 | n/a | 0.220 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190282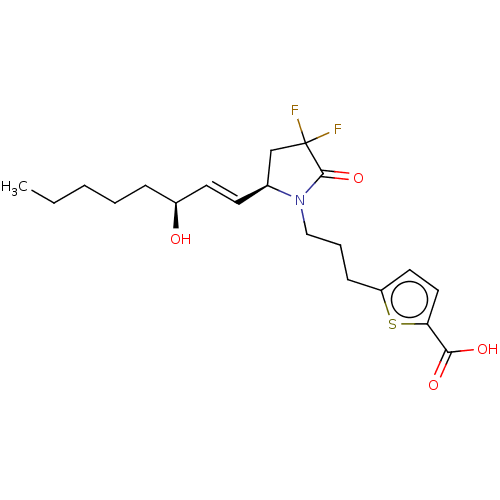 (US9180116, 33C) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | n/a | 0.280 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM35847 ((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human recombinant EP4 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... | J Med Chem 62: 4731-4741 (2019) Article DOI: 10.1021/acs.jmedchem.9b00336 BindingDB Entry DOI: 10.7270/Q22R3W32 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190272 (US9180116, 12D) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.120 | n/a | 0.320 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50101822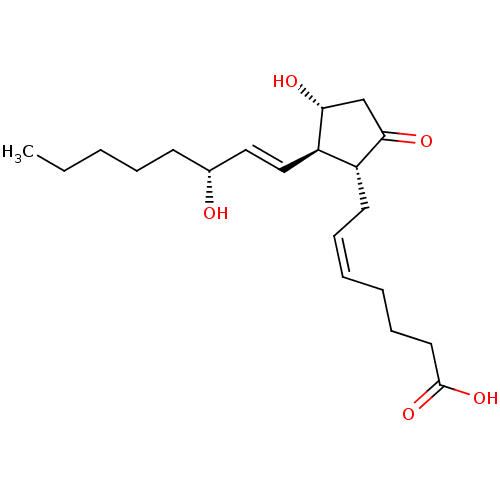 ((Z)-7-[(1R,2R,3R)-3-Hydroxy-2-((E)-(R)-3-hydroxy-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | 0.140 | n/a | 0.380 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190270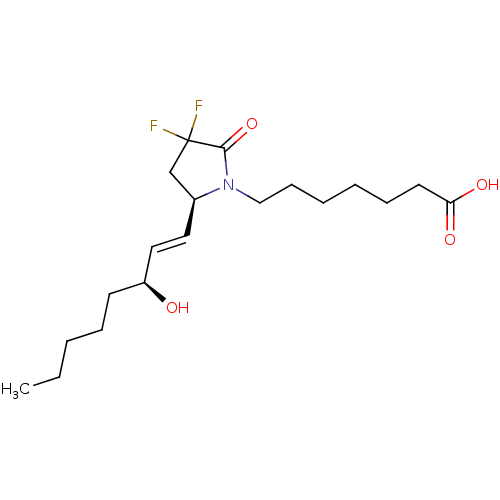 (US9180116, 9C) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human recombinant EP4 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... | J Med Chem 62: 4731-4741 (2019) Article DOI: 10.1021/acs.jmedchem.9b00336 BindingDB Entry DOI: 10.7270/Q22R3W32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190270 (US9180116, 9C) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.210 | n/a | 0.570 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190275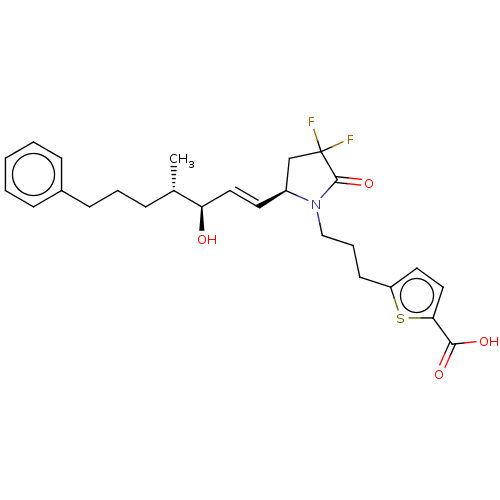 (US9180116, 28C | US9180116, 28H) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.280 | n/a | 0.740 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190268 (US9180116, 2C) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.378 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human recombinant EP4 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... | J Med Chem 62: 4731-4741 (2019) Article DOI: 10.1021/acs.jmedchem.9b00336 BindingDB Entry DOI: 10.7270/Q22R3W32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50101858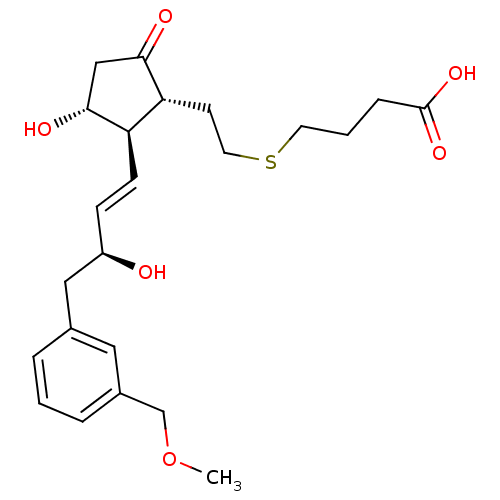 (4-(2-((1R,2R,3R)-3-hydroxy-2-((S,E)-3-hydroxy-4-(3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.396 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human recombinant EP4 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... | J Med Chem 62: 4731-4741 (2019) Article DOI: 10.1021/acs.jmedchem.9b00336 BindingDB Entry DOI: 10.7270/Q22R3W32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190267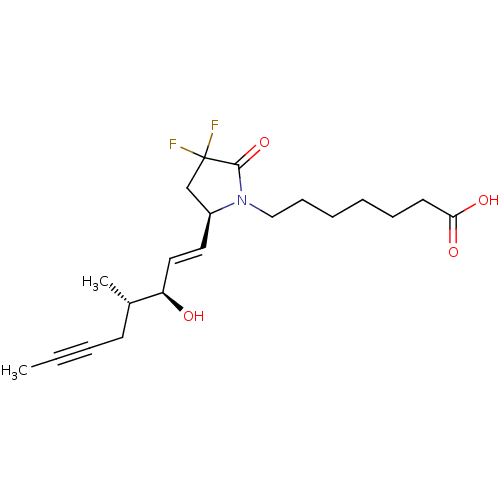 (US9180116, 1F) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.440 | n/a | 1.20 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190268 (US9180116, 2C) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.490 | n/a | 1.30 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190269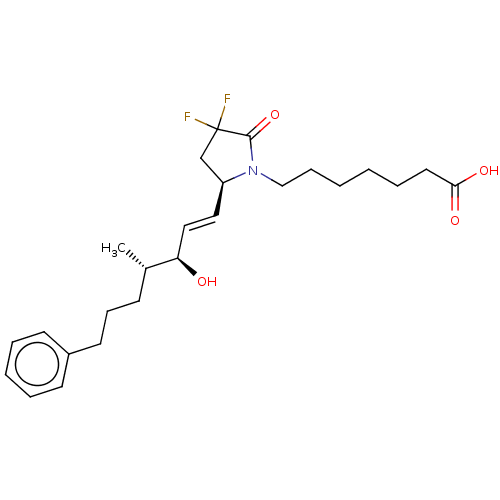 (US9180116, 6D) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.890 | n/a | 2.40 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM569294 (US11427563, Compound 36) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description All compounds were initially prepared as 10 mM stocks in anhydrous dimethylsulfoxide (DMSO). A 20 μl aliquot of the 10 mM solutions was transfer... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DJ5JVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM35847 ((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human recombinant EP2 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... | J Med Chem 62: 4731-4741 (2019) Article DOI: 10.1021/acs.jmedchem.9b00336 BindingDB Entry DOI: 10.7270/Q22R3W32 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190274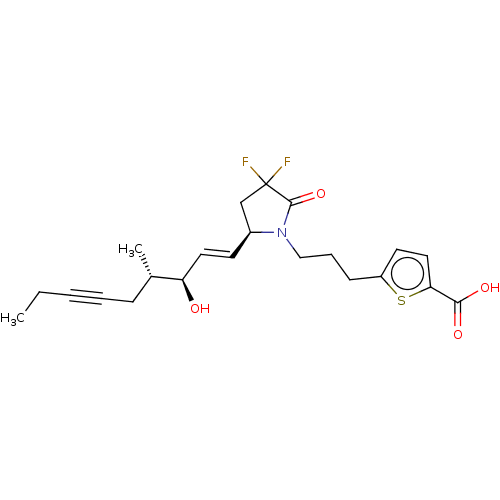 (US9180116, 24D) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.20 | n/a | 3.30 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50142481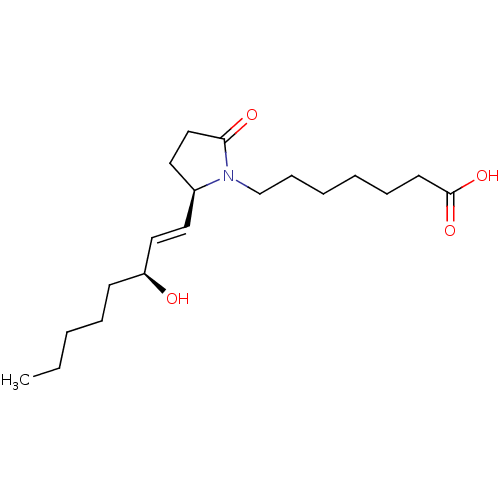 (7-[(R)-2-((E)-(S)-3-Hydroxy-oct-1-enyl)-5-oxo-pyrr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human recombinant EP4 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... | J Med Chem 62: 4731-4741 (2019) Article DOI: 10.1021/acs.jmedchem.9b00336 BindingDB Entry DOI: 10.7270/Q22R3W32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190271 (US9180116, 10C) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.80 | n/a | 4.90 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM179834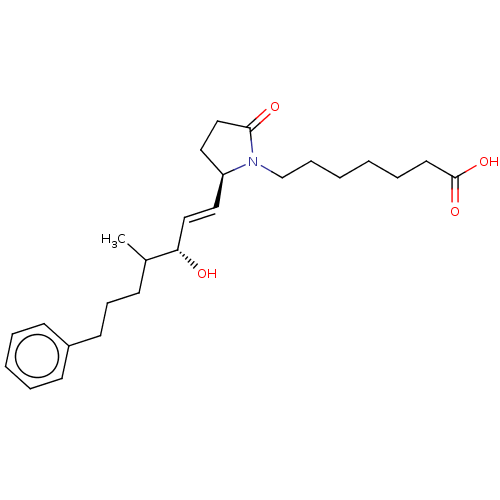 (US9676712, 2C) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.10 | n/a | 5.70 | n/a | n/a | n/a | n/a | 6.0 | n/a |
CAYMAN CHEMICAL COMPANY, INC. US Patent | Assay Description 200 μl in 96-well plate. Cell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3H]PGE2 in the absence ... | US Patent US9676712 (2017) BindingDB Entry DOI: 10.7270/Q23X84T9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM35847 ((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human recombinant EP3 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... | J Med Chem 62: 4731-4741 (2019) Article DOI: 10.1021/acs.jmedchem.9b00336 BindingDB Entry DOI: 10.7270/Q22R3W32 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50521600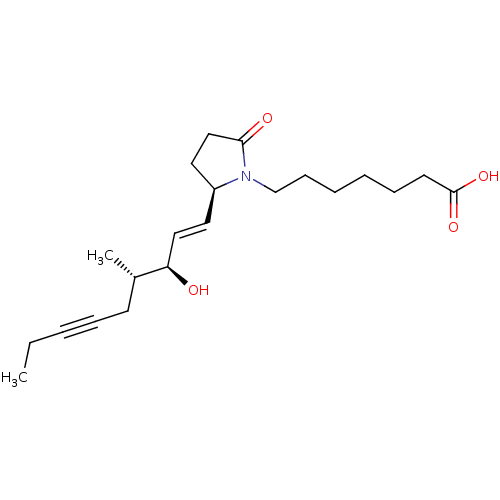 (CHEMBL4558749) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human recombinant EP4 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... | J Med Chem 62: 4731-4741 (2019) Article DOI: 10.1021/acs.jmedchem.9b00336 BindingDB Entry DOI: 10.7270/Q22R3W32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM179827 (US9676712, 1C) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.80 | n/a | 10 | n/a | n/a | n/a | n/a | 6.0 | n/a |
CAYMAN CHEMICAL COMPANY, INC. US Patent | Assay Description 200 μl in 96-well plate. Cell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3H]PGE2 in the absence ... | US Patent US9676712 (2017) BindingDB Entry DOI: 10.7270/Q23X84T9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM569294 (US11427563, Compound 36) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were prepared in the exact same manner as described in the ROCK Kinase Assay with the exception to the substrate and enzyme. The JAK 2× sub... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DJ5JVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM569294 (US11427563, Compound 36) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 211 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were prepared in the exact same manner as described in the ROCK Kinase Assay with the exception to the substrate and enzyme. The JAK 2× sub... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DJ5JVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM190268 (US9180116, 2C) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 258 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human recombinant EP3 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... | J Med Chem 62: 4731-4741 (2019) Article DOI: 10.1021/acs.jmedchem.9b00336 BindingDB Entry DOI: 10.7270/Q22R3W32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM190270 (US9180116, 9C) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 329 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human recombinant EP2 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... | J Med Chem 62: 4731-4741 (2019) Article DOI: 10.1021/acs.jmedchem.9b00336 BindingDB Entry DOI: 10.7270/Q22R3W32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM190270 (US9180116, 9C) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human recombinant EP3 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... | J Med Chem 62: 4731-4741 (2019) Article DOI: 10.1021/acs.jmedchem.9b00336 BindingDB Entry DOI: 10.7270/Q22R3W32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50521600 (CHEMBL4558749) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human recombinant EP3 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... | J Med Chem 62: 4731-4741 (2019) Article DOI: 10.1021/acs.jmedchem.9b00336 BindingDB Entry DOI: 10.7270/Q22R3W32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50142481 (7-[(R)-2-((E)-(S)-3-Hydroxy-oct-1-enyl)-5-oxo-pyrr...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human recombinant EP3 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... | J Med Chem 62: 4731-4741 (2019) Article DOI: 10.1021/acs.jmedchem.9b00336 BindingDB Entry DOI: 10.7270/Q22R3W32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM569294 (US11427563, Compound 36) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description All compounds were initially prepared as 10 mM stocks in anhydrous dimethylsulfoxide (DMSO). A 20 μl aliquot of the 10 mM solutions was transfer... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DJ5JVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50142481 (7-[(R)-2-((E)-(S)-3-Hydroxy-oct-1-enyl)-5-oxo-pyrr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human recombinant EP2 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... | J Med Chem 62: 4731-4741 (2019) Article DOI: 10.1021/acs.jmedchem.9b00336 BindingDB Entry DOI: 10.7270/Q22R3W32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM190268 (US9180116, 2C) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human recombinant EP2 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... | J Med Chem 62: 4731-4741 (2019) Article DOI: 10.1021/acs.jmedchem.9b00336 BindingDB Entry DOI: 10.7270/Q22R3W32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50521600 (CHEMBL4558749) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human recombinant EP2 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... | J Med Chem 62: 4731-4741 (2019) Article DOI: 10.1021/acs.jmedchem.9b00336 BindingDB Entry DOI: 10.7270/Q22R3W32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM569235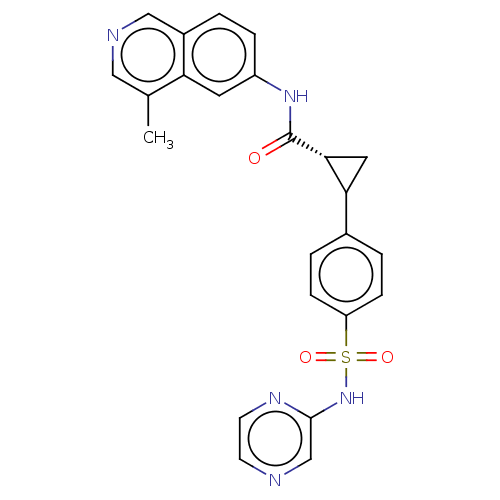 (US11427563, Compound 10 | US11427563, Compound 102...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were prepared in the exact same manner as described in the ROCK Kinase Assay with the exception to the substrate and enzyme. The JAK 2× sub... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DJ5JVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM569296 (US11427563, Compound 51) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were prepared in the exact same manner as described in the ROCK Kinase Assay with the exception to the substrate and enzyme. The JAK 2× sub... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DJ5JVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM569325 (US11427563, Compound 121 | US11427563, Compound 81) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description All compounds were initially prepared as 10 mM stocks in anhydrous dimethylsulfoxide (DMSO). A 20 μl aliquot of the 10 mM solutions was transfer... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DJ5JVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM569281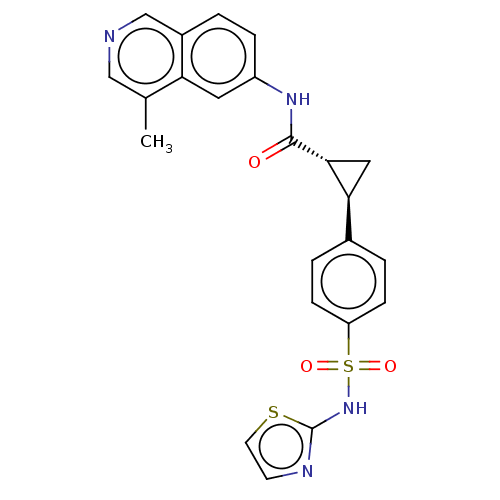 (US11427563, Compound 170 | US11427563, Compound 32...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were prepared in the exact same manner as described in the ROCK Kinase Assay with the exception to the substrate and enzyme. The JAK 2× sub... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DJ5JVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM569235 (US11427563, Compound 10 | US11427563, Compound 102...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were prepared in the exact same manner as described in the ROCK Kinase Assay with the exception to the substrate and enzyme. The JAK 2× sub... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DJ5JVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM569243 (US11427563, Compound 110 | US11427563, Compound 52) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description All compounds were initially prepared as 10 mM stocks in anhydrous dimethylsulfoxide (DMSO). A 20 μl aliquot of the 10 mM solutions was transfer... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DJ5JVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM569281 (US11427563, Compound 170 | US11427563, Compound 32...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were prepared in the exact same manner as described in the ROCK Kinase Assay with the exception to the substrate and enzyme. The JAK 2× sub... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DJ5JVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM569236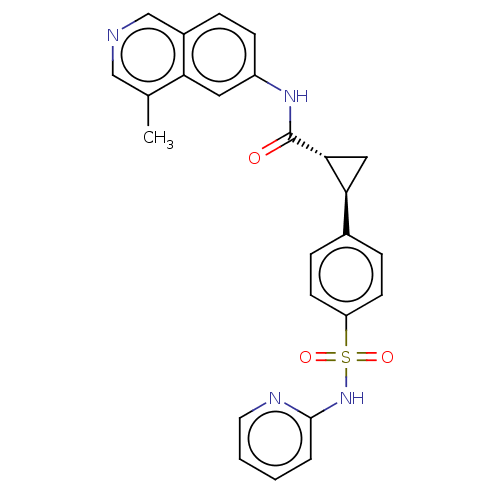 (US11427563, Compound 103) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description All compounds were initially prepared as 10 mM stocks in anhydrous dimethylsulfoxide (DMSO). A 20 μl aliquot of the 10 mM solutions was transfer... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DJ5JVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM569239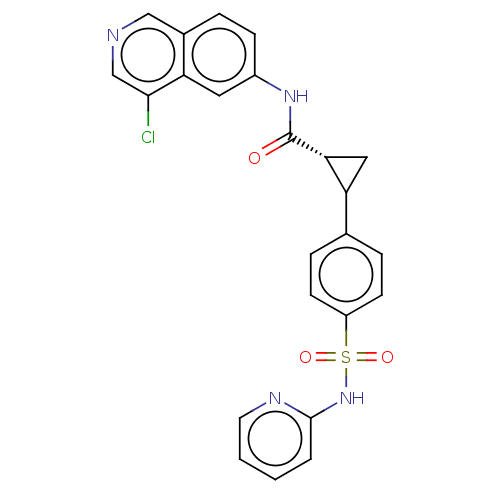 (US11427563, Compound 106 | US11427563, Compound 11...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description All compounds were initially prepared as 10 mM stocks in anhydrous dimethylsulfoxide (DMSO). A 20 μl aliquot of the 10 mM solutions was transfer... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DJ5JVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM569288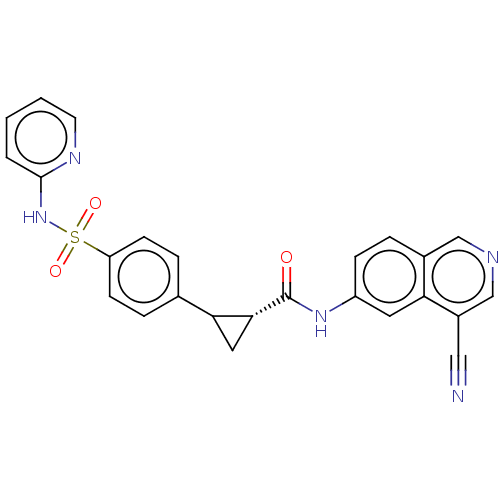 (US11427563, Compound 20 | US11427563, Compound 93) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description All compounds were initially prepared as 10 mM stocks in anhydrous dimethylsulfoxide (DMSO). A 20 μl aliquot of the 10 mM solutions was transfer... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DJ5JVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM569236 (US11427563, Compound 103) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were prepared in the exact same manner as described in the ROCK Kinase Assay with the exception to the substrate and enzyme. The JAK 2× sub... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DJ5JVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM569312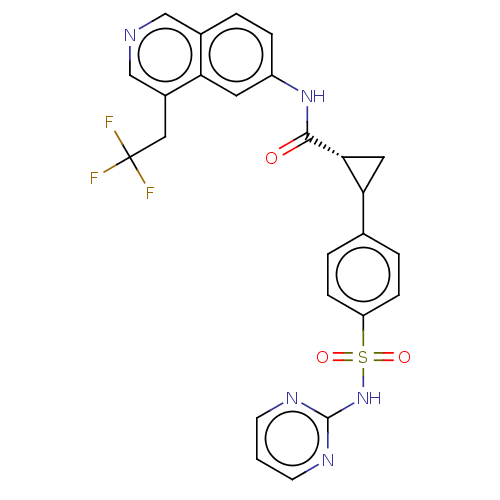 (US11427563, Compound 68) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were prepared in the exact same manner as described in the ROCK Kinase Assay with the exception to the substrate and enzyme. The JAK 2× sub... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DJ5JVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM569313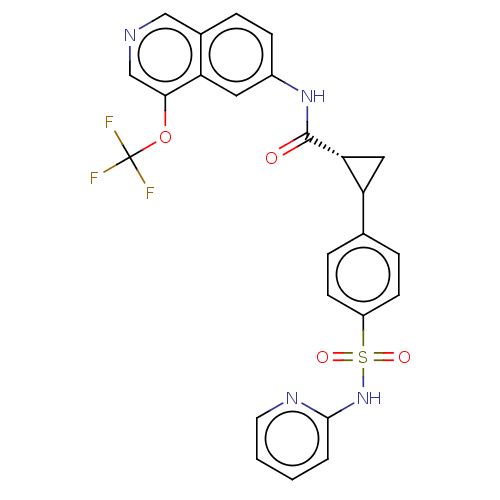 (US11427563, Compound 69) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were prepared in the exact same manner as described in the ROCK Kinase Assay with the exception to the substrate and enzyme. The JAK 2× sub... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DJ5JVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM569288 (US11427563, Compound 20 | US11427563, Compound 93) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were prepared in the exact same manner as described in the ROCK Kinase Assay with the exception to the substrate and enzyme. The JAK 2× sub... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DJ5JVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM569315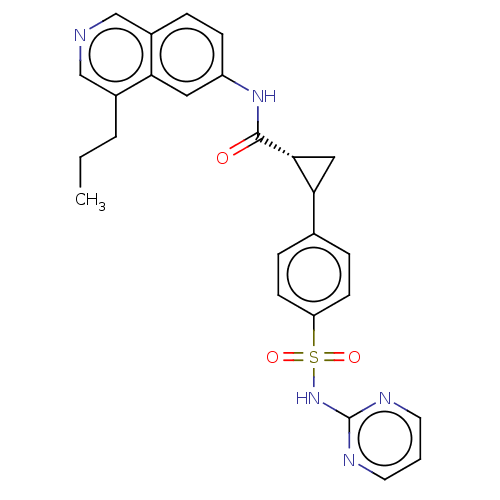 (US11427563, Compound 70) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description All compounds were initially prepared as 10 mM stocks in anhydrous dimethylsulfoxide (DMSO). A 20 μl aliquot of the 10 mM solutions was transfer... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DJ5JVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM569295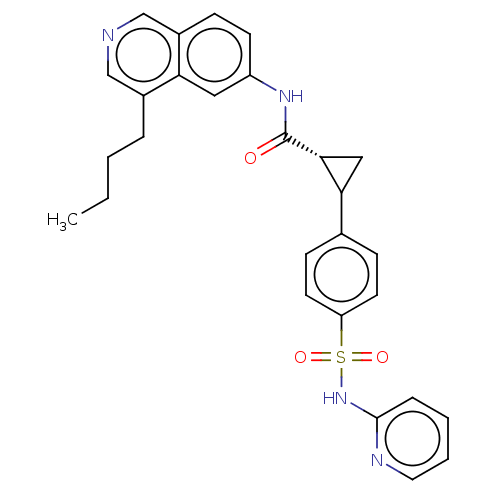 (US11427563, Compound 49) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description All compounds were initially prepared as 10 mM stocks in anhydrous dimethylsulfoxide (DMSO). A 20 μl aliquot of the 10 mM solutions was transfer... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DJ5JVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM569298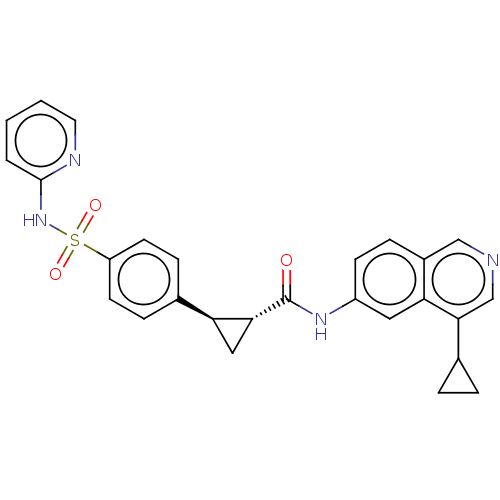 (US11427563, Compound 53 | US11427563, Compound 62) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description All compounds were initially prepared as 10 mM stocks in anhydrous dimethylsulfoxide (DMSO). A 20 μl aliquot of the 10 mM solutions was transfer... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DJ5JVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 886 total ) | Next | Last >> |