 Found 686 hits with Last Name = 'kou' and Initial = 'k'
Found 686 hits with Last Name = 'kou' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50053929
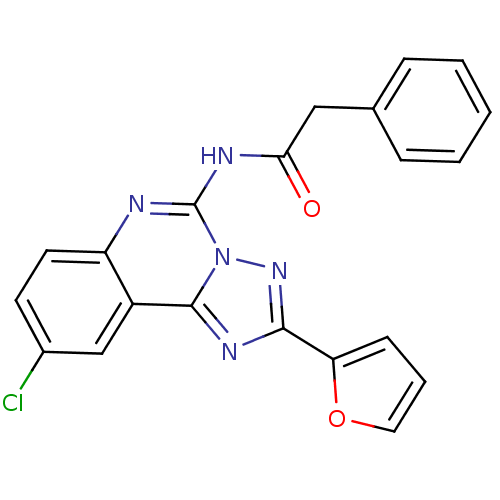
(CHEMBL88147 | N-(9-Chloro-2-furan-2-yl-[1,2,4]tria...)Show SMILES Clc1ccc2nc(NC(=O)Cc3ccccc3)n3nc(nc3c2c1)-c1ccco1 Show InChI InChI=1S/C21H14ClN5O2/c22-14-8-9-16-15(12-14)20-25-19(17-7-4-10-29-17)26-27(20)21(23-16)24-18(28)11-13-5-2-1-3-6-13/h1-10,12H,11H2,(H,23,24,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.115 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00052
BindingDB Entry DOI: 10.7270/Q20G3Q6Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM21173

(1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...)Show InChI InChI=1S/C16H24N4O2/c1-3-9-19-14-12(15(21)20(10-4-2)16(19)22)17-13(18-14)11-7-5-6-8-11/h11H,3-10H2,1-2H3,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.589 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00052
BindingDB Entry DOI: 10.7270/Q20G3Q6Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50597191
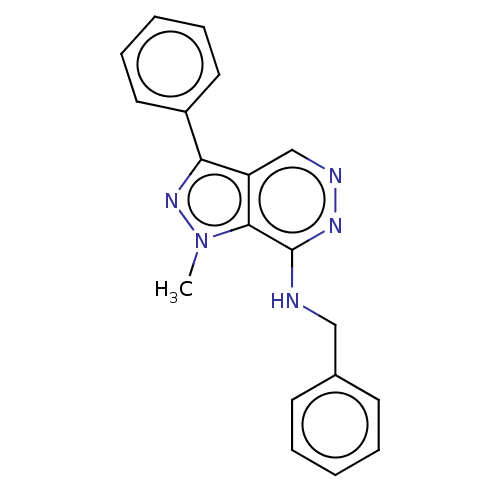
(CHEMBL5195631) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00052
BindingDB Entry DOI: 10.7270/Q20G3Q6Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50597191

(CHEMBL5195631) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00052
BindingDB Entry DOI: 10.7270/Q20G3Q6Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50597191

(CHEMBL5195631) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00052
BindingDB Entry DOI: 10.7270/Q20G3Q6Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50597191

(CHEMBL5195631) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00052
BindingDB Entry DOI: 10.7270/Q20G3Q6Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50597191

(CHEMBL5195631) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00052
BindingDB Entry DOI: 10.7270/Q20G3Q6Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50597191

(CHEMBL5195631) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00052
BindingDB Entry DOI: 10.7270/Q20G3Q6Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50053929

(CHEMBL88147 | N-(9-Chloro-2-furan-2-yl-[1,2,4]tria...)Show SMILES Clc1ccc2nc(NC(=O)Cc3ccccc3)n3nc(nc3c2c1)-c1ccco1 Show InChI InChI=1S/C21H14ClN5O2/c22-14-8-9-16-15(12-14)20-25-19(17-7-4-10-29-17)26-27(20)21(23-16)24-18(28)11-13-5-2-1-3-6-13/h1-10,12H,11H2,(H,23,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00052
BindingDB Entry DOI: 10.7270/Q20G3Q6Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM21220

((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C12H16N6O4/c1-2-14-11(21)8-6(19)7(20)12(22-8)18-4-17-5-9(13)15-3-16-10(5)18/h3-4,6-8,12,19-20H,2H2,1H3,(H,14,21)(H2,13,15,16)/t6-,7+,8-,12+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00052
BindingDB Entry DOI: 10.7270/Q20G3Q6Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM21220

((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C12H16N6O4/c1-2-14-11(21)8-6(19)7(20)12(22-8)18-4-17-5-9(13)15-3-16-10(5)18/h3-4,6-8,12,19-20H,2H2,1H3,(H,14,21)(H2,13,15,16)/t6-,7+,8-,12+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 204 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00052
BindingDB Entry DOI: 10.7270/Q20G3Q6Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50597195
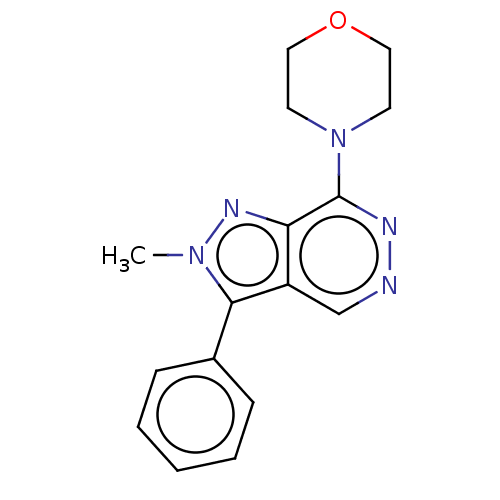
(CHEMBL5183118) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 363 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00052
BindingDB Entry DOI: 10.7270/Q20G3Q6Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50597190

(CHEMBL5185411) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00052
BindingDB Entry DOI: 10.7270/Q20G3Q6Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50597193
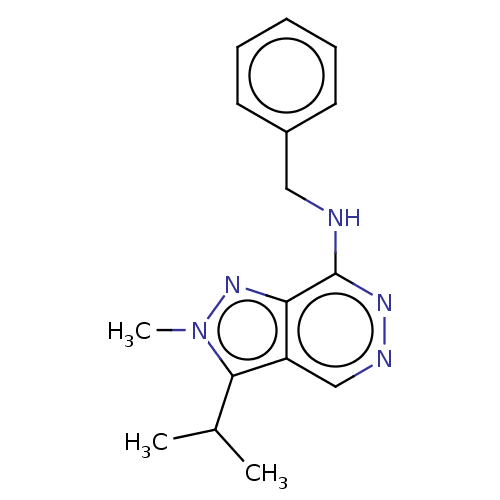
(CHEMBL5203865) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00052
BindingDB Entry DOI: 10.7270/Q20G3Q6Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50597190

(CHEMBL5185411) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00052
BindingDB Entry DOI: 10.7270/Q20G3Q6Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50597194
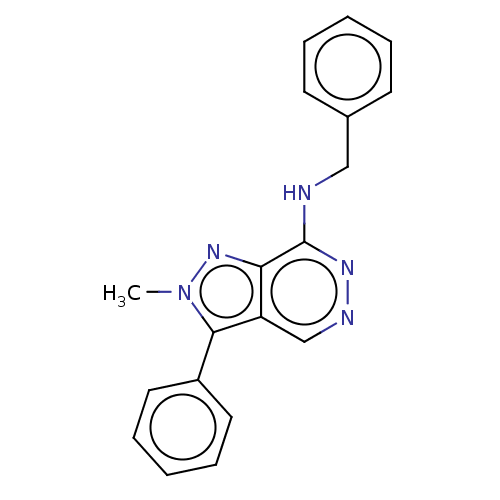
(CHEMBL5197567) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00052
BindingDB Entry DOI: 10.7270/Q20G3Q6Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50597195

(CHEMBL5183118) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00052
BindingDB Entry DOI: 10.7270/Q20G3Q6Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50597194

(CHEMBL5197567) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00052
BindingDB Entry DOI: 10.7270/Q20G3Q6Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50597193

(CHEMBL5203865) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00052
BindingDB Entry DOI: 10.7270/Q20G3Q6Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50597192

(CHEMBL5175347) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00052
BindingDB Entry DOI: 10.7270/Q20G3Q6Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50597192

(CHEMBL5175347) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00052
BindingDB Entry DOI: 10.7270/Q20G3Q6Z |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Mycobacterium smegmatis) | BDBM50010376
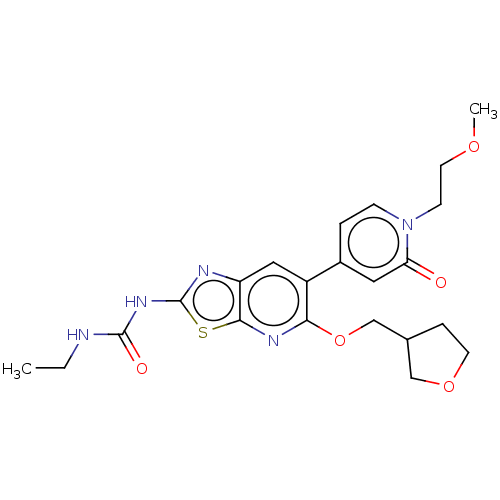
(CHEMBL3263618)Show SMILES CCNC(=O)Nc1nc2cc(-c3ccn(CCOC)c(=O)c3)c(OCC3CCOC3)nc2s1 Show InChI InChI=1S/C22H27N5O5S/c1-3-23-21(29)26-22-24-17-11-16(15-4-6-27(7-9-30-2)18(28)10-15)19(25-20(17)33-22)32-13-14-5-8-31-12-14/h4,6,10-11,14H,3,5,7-9,12-13H2,1-2H3,(H2,23,24,26,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca India Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium smegmatis DNA gyrase B ATPase activity assessed as inorganic phosphate release using ATP as substrate by colorimetric ana... |
Cell Chem Biol 56: 8834-48 (2013)
Article DOI: 10.1021/jm401268f
BindingDB Entry DOI: 10.7270/Q2RV0Q7W |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Mycobacterium smegmatis) | BDBM50010373
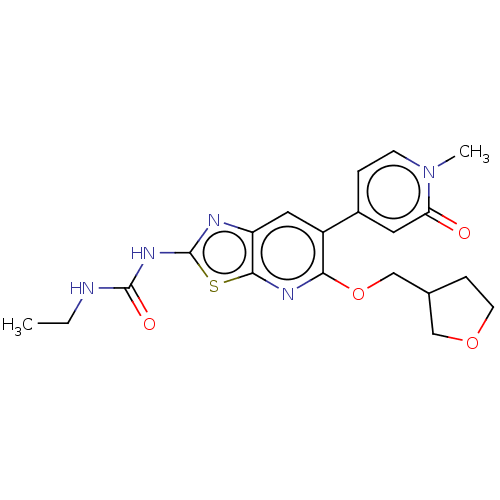
(CHEMBL3263615)Show SMILES CCNC(=O)Nc1nc2cc(-c3ccn(C)c(=O)c3)c(OCC3CCOC3)nc2s1 Show InChI InChI=1S/C20H23N5O4S/c1-3-21-19(27)24-20-22-15-9-14(13-4-6-25(2)16(26)8-13)17(23-18(15)30-20)29-11-12-5-7-28-10-12/h4,6,8-9,12H,3,5,7,10-11H2,1-2H3,(H2,21,22,24,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca India Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium smegmatis DNA gyrase B ATPase activity assessed as inorganic phosphate release using ATP as substrate by colorimetric ana... |
Cell Chem Biol 56: 8834-48 (2013)
Article DOI: 10.1021/jm401268f
BindingDB Entry DOI: 10.7270/Q2RV0Q7W |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Mycobacterium smegmatis) | BDBM50010340
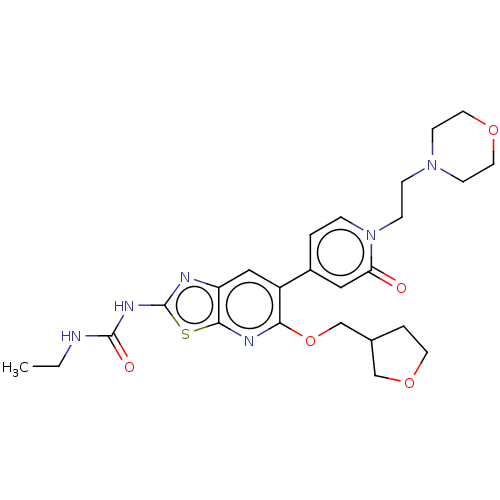
(CHEMBL3263621)Show SMILES CCNC(=O)Nc1nc2cc(c(OCC3CCOC3)nc2s1)-c1ccn(CCN2CCOCC2)c(=O)c1 Show InChI InChI=1S/C25H32N6O5S/c1-2-26-24(33)29-25-27-20-14-19(22(28-23(20)37-25)36-16-17-4-10-35-15-17)18-3-5-31(21(32)13-18)7-6-30-8-11-34-12-9-30/h3,5,13-14,17H,2,4,6-12,15-16H2,1H3,(H2,26,27,29,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca India Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium smegmatis DNA gyrase B ATPase activity assessed as inorganic phosphate release using ATP as substrate by colorimetric ana... |
Cell Chem Biol 56: 8834-48 (2013)
Article DOI: 10.1021/jm401268f
BindingDB Entry DOI: 10.7270/Q2RV0Q7W |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Mycobacterium smegmatis) | BDBM50447299
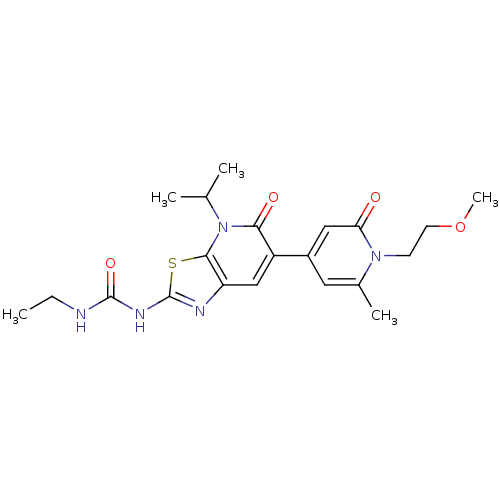
(CHEMBL3114208)Show SMILES CCNC(=O)Nc1nc2cc(-c3cc(C)n(CCOC)c(=O)c3)c(=O)n(C(C)C)c2s1 Show InChI InChI=1S/C21H27N5O4S/c1-6-22-20(29)24-21-23-16-11-15(18(28)26(12(2)3)19(16)31-21)14-9-13(4)25(7-8-30-5)17(27)10-14/h9-12H,6-8H2,1-5H3,(H2,22,23,24,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium smegmatis DNA gyrase B ATPase activity after 100 mins |
Bioorg Med Chem Lett 24: 870-9 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.080
BindingDB Entry DOI: 10.7270/Q2K9390V |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Mycobacterium smegmatis) | BDBM50010377
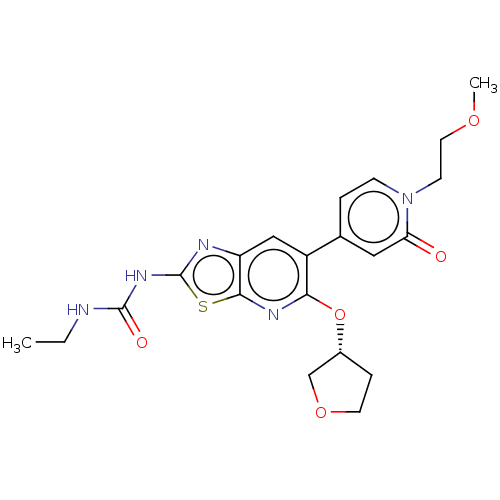
(CHEMBL3263619)Show SMILES CCNC(=O)Nc1nc2cc(-c3ccn(CCOC)c(=O)c3)c(O[C@@H]3CCOC3)nc2s1 |r| Show InChI InChI=1S/C21H25N5O5S/c1-3-22-20(28)25-21-23-16-11-15(13-4-6-26(7-9-29-2)17(27)10-13)18(24-19(16)32-21)31-14-5-8-30-12-14/h4,6,10-11,14H,3,5,7-9,12H2,1-2H3,(H2,22,23,25,28)/t14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca India Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium smegmatis DNA gyrase B ATPase activity assessed as inorganic phosphate release using ATP as substrate by colorimetric ana... |
Cell Chem Biol 56: 8834-48 (2013)
Article DOI: 10.1021/jm401268f
BindingDB Entry DOI: 10.7270/Q2RV0Q7W |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Mycobacterium smegmatis) | BDBM50010339

(CHEMBL3263620)Show SMILES CCNC(=O)Nc1nc2cc(-c3ccn(CCN(C)C)c(=O)c3)c(OCC3CCOC3)nc2s1 Show InChI InChI=1S/C23H30N6O4S/c1-4-24-22(31)27-23-25-18-12-17(16-5-7-29(19(30)11-16)9-8-28(2)3)20(26-21(18)34-23)33-14-15-6-10-32-13-15/h5,7,11-12,15H,4,6,8-10,13-14H2,1-3H3,(H2,24,25,27,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca India Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium smegmatis DNA gyrase B ATPase activity assessed as inorganic phosphate release using ATP as substrate by colorimetric ana... |
Cell Chem Biol 56: 8834-48 (2013)
Article DOI: 10.1021/jm401268f
BindingDB Entry DOI: 10.7270/Q2RV0Q7W |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Mycobacterium smegmatis) | BDBM50010338
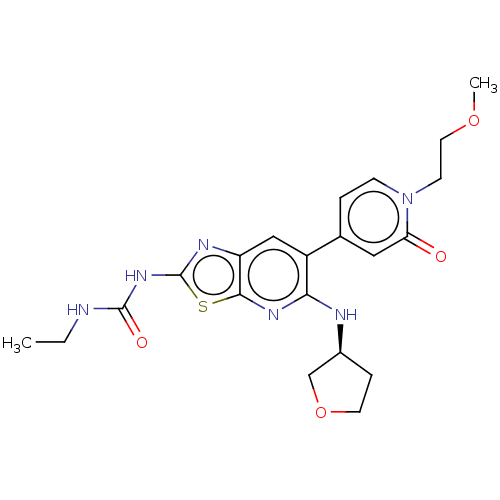
(CHEMBL3259858)Show SMILES CCNC(=O)Nc1nc2cc(c(N[C@H]3CCOC3)nc2s1)-c1ccn(CCOC)c(=O)c1 |r| Show InChI InChI=1S/C21H26N6O4S/c1-3-22-20(29)26-21-24-16-11-15(13-4-6-27(7-9-30-2)17(28)10-13)18(25-19(16)32-21)23-14-5-8-31-12-14/h4,6,10-11,14H,3,5,7-9,12H2,1-2H3,(H,23,25)(H2,22,24,26,29)/t14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca India Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium smegmatis DNA gyrase B ATPase activity assessed as inorganic phosphate release using ATP as substrate by colorimetric ana... |
Cell Chem Biol 56: 8834-48 (2013)
Article DOI: 10.1021/jm401268f
BindingDB Entry DOI: 10.7270/Q2RV0Q7W |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Mycobacterium smegmatis) | BDBM50010371
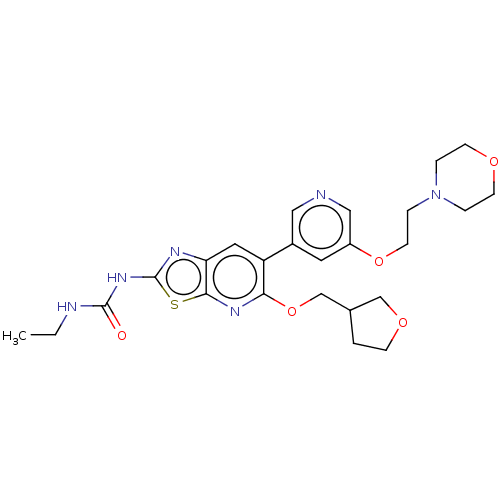
(CHEMBL3263613)Show SMILES CCNC(=O)Nc1nc2cc(c(OCC3CCOC3)nc2s1)-c1cncc(OCCN2CCOCC2)c1 Show InChI InChI=1S/C25H32N6O5S/c1-2-27-24(32)30-25-28-21-12-20(22(29-23(21)37-25)36-16-17-3-7-34-15-17)18-11-19(14-26-13-18)35-10-6-31-4-8-33-9-5-31/h11-14,17H,2-10,15-16H2,1H3,(H2,27,28,30,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca India Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium smegmatis DNA gyrase B ATPase activity assessed as inorganic phosphate release using ATP as substrate by colorimetric ana... |
Cell Chem Biol 56: 8834-48 (2013)
Article DOI: 10.1021/jm401268f
BindingDB Entry DOI: 10.7270/Q2RV0Q7W |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Mycobacterium smegmatis) | BDBM50447303

(CHEMBL3114202)Show SMILES CCNC(=O)Nc1nc2cc(-c3cncc(c3)C#N)c(=O)n(C(C)C)c2s1 Show InChI InChI=1S/C18H18N6O2S/c1-4-21-17(26)23-18-22-14-6-13(12-5-11(7-19)8-20-9-12)15(25)24(10(2)3)16(14)27-18/h5-6,8-10H,4H2,1-3H3,(H2,21,22,23,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium smegmatis DNA gyrase B ATPase activity after 100 mins |
Bioorg Med Chem Lett 24: 870-9 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.080
BindingDB Entry DOI: 10.7270/Q2K9390V |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Mycobacterium smegmatis) | BDBM50010374
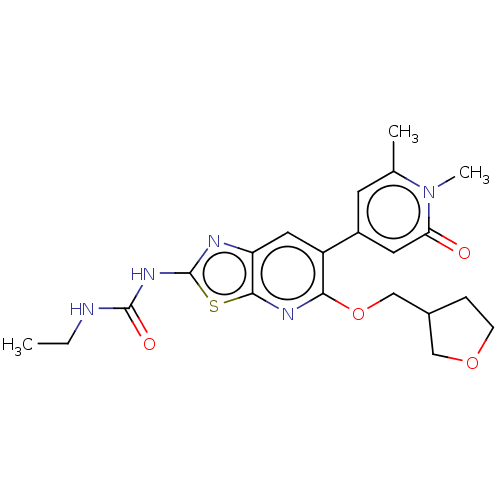
(CHEMBL3263616)Show SMILES CCNC(=O)Nc1nc2cc(-c3cc(C)n(C)c(=O)c3)c(OCC3CCOC3)nc2s1 Show InChI InChI=1S/C21H25N5O4S/c1-4-22-20(28)25-21-23-16-9-15(14-7-12(2)26(3)17(27)8-14)18(24-19(16)31-21)30-11-13-5-6-29-10-13/h7-9,13H,4-6,10-11H2,1-3H3,(H2,22,23,25,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca India Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium smegmatis DNA gyrase B ATPase activity assessed as inorganic phosphate release using ATP as substrate by colorimetric ana... |
Cell Chem Biol 56: 8834-48 (2013)
Article DOI: 10.1021/jm401268f
BindingDB Entry DOI: 10.7270/Q2RV0Q7W |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Mycobacterium smegmatis) | BDBM50447300
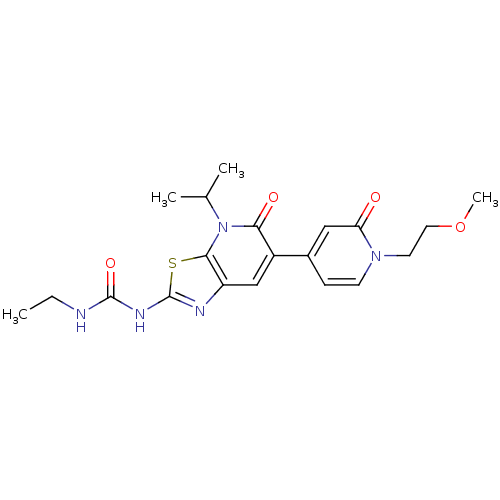
(CHEMBL3114207)Show SMILES CCNC(=O)Nc1nc2cc(-c3ccn(CCOC)c(=O)c3)c(=O)n(C(C)C)c2s1 Show InChI InChI=1S/C20H25N5O4S/c1-5-21-19(28)23-20-22-15-11-14(17(27)25(12(2)3)18(15)30-20)13-6-7-24(8-9-29-4)16(26)10-13/h6-7,10-12H,5,8-9H2,1-4H3,(H2,21,22,23,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium smegmatis DNA gyrase B ATPase activity after 100 mins |
Bioorg Med Chem Lett 24: 870-9 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.080
BindingDB Entry DOI: 10.7270/Q2K9390V |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Mycobacterium smegmatis) | BDBM50447301

(CHEMBL3114206)Show SMILES CCNC(=O)Nc1nc2cc(-c3cc(C)n(CC)c(=O)c3)c(=O)n(C(C)C)c2s1 Show InChI InChI=1S/C20H25N5O3S/c1-6-21-19(28)23-20-22-15-10-14(17(27)25(11(3)4)18(15)29-20)13-8-12(5)24(7-2)16(26)9-13/h8-11H,6-7H2,1-5H3,(H2,21,22,23,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium smegmatis DNA gyrase B ATPase activity after 100 mins |
Bioorg Med Chem Lett 24: 870-9 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.080
BindingDB Entry DOI: 10.7270/Q2K9390V |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Mycobacterium smegmatis) | BDBM50010347
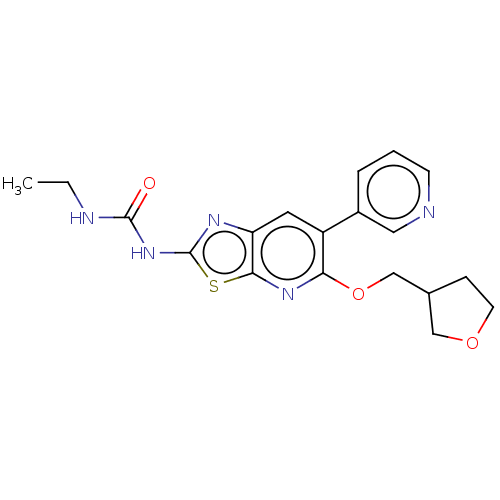
(CHEMBL3263591)Show SMILES CCNC(=O)Nc1nc2cc(-c3cccnc3)c(OCC3CCOC3)nc2s1 Show InChI InChI=1S/C19H21N5O3S/c1-2-21-18(25)24-19-22-15-8-14(13-4-3-6-20-9-13)16(23-17(15)28-19)27-11-12-5-7-26-10-12/h3-4,6,8-9,12H,2,5,7,10-11H2,1H3,(H2,21,22,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca India Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium smegmatis DNA gyrase B ATPase activity assessed as inorganic phosphate release using ATP as substrate by colorimetric ana... |
Cell Chem Biol 56: 8834-48 (2013)
Article DOI: 10.1021/jm401268f
BindingDB Entry DOI: 10.7270/Q2RV0Q7W |
More data for this
Ligand-Target Pair | |
Beta-1,4-mannosyl-glycoprotein 4-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM50117663

(CHEMBL3613707)Show SMILES COc1cccc(CC(=O)N2Cc3ccc(cc3C2)S(=O)(=O)Nc2cnn(n2)-c2ccc(F)cc2)c1 Show InChI InChI=1S/C25H22FN5O4S/c1-35-22-4-2-3-17(11-22)12-25(32)30-15-18-5-10-23(13-19(18)16-30)36(33,34)29-24-14-27-31(28-24)21-8-6-20(26)7-9-21/h2-11,13-14H,12,15-16H2,1H3,(H,28,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MGAT3 (unknown origin) assessed effect on incorporation of [1-14C]decanoyl moiety into triacylglycerol using [1-14C]decanoyl-CoA and 1,... |
J Med Chem 58: 7164-72 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01008
BindingDB Entry DOI: 10.7270/Q2F191HJ |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Mycobacterium smegmatis) | BDBM50447305
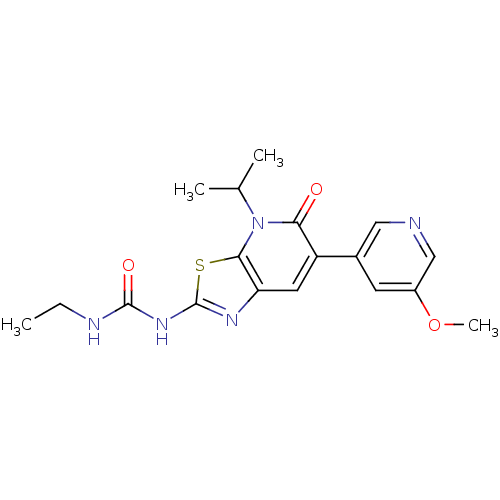
(CHEMBL3114200)Show SMILES CCNC(=O)Nc1nc2cc(-c3cncc(OC)c3)c(=O)n(C(C)C)c2s1 Show InChI InChI=1S/C18H21N5O3S/c1-5-20-17(25)22-18-21-14-7-13(11-6-12(26-4)9-19-8-11)15(24)23(10(2)3)16(14)27-18/h6-10H,5H2,1-4H3,(H2,20,21,22,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium smegmatis DNA gyrase B ATPase activity after 100 mins |
Bioorg Med Chem Lett 24: 870-9 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.080
BindingDB Entry DOI: 10.7270/Q2K9390V |
More data for this
Ligand-Target Pair | |
Beta-1,4-mannosyl-glycoprotein 4-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM50117662
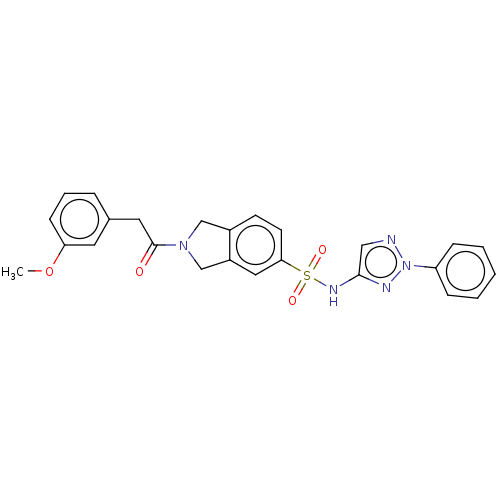
(CHEMBL3613706)Show SMILES COc1cccc(CC(=O)N2Cc3ccc(cc3C2)S(=O)(=O)Nc2cnn(n2)-c2ccccc2)c1 Show InChI InChI=1S/C25H23N5O4S/c1-34-22-9-5-6-18(12-22)13-25(31)29-16-19-10-11-23(14-20(19)17-29)35(32,33)28-24-15-26-30(27-24)21-7-3-2-4-8-21/h2-12,14-15H,13,16-17H2,1H3,(H,27,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MGAT3 (unknown origin) assessed effect on incorporation of [1-14C]decanoyl moiety into triacylglycerol using [1-14C]decanoyl-CoA and 1,... |
J Med Chem 58: 7164-72 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01008
BindingDB Entry DOI: 10.7270/Q2F191HJ |
More data for this
Ligand-Target Pair | |
Beta-1,4-mannosyl-glycoprotein 4-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM50117663

(CHEMBL3613707)Show SMILES COc1cccc(CC(=O)N2Cc3ccc(cc3C2)S(=O)(=O)Nc2cnn(n2)-c2ccc(F)cc2)c1 Show InChI InChI=1S/C25H22FN5O4S/c1-35-22-4-2-3-17(11-22)12-25(32)30-15-18-5-10-23(13-19(18)16-30)36(33,34)29-24-14-27-31(28-24)21-8-6-20(26)7-9-21/h2-11,13-14H,12,15-16H2,1H3,(H,28,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MGAT3 (unknown origin) assessed effect on incorporation of [1-14C]decanoyl moiety into triacylglycerol using [1-14C]decanoyl-CoA and 1,... |
J Med Chem 58: 7164-72 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01008
BindingDB Entry DOI: 10.7270/Q2F191HJ |
More data for this
Ligand-Target Pair | |
Beta-1,4-mannosyl-glycoprotein 4-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM50117662

(CHEMBL3613706)Show SMILES COc1cccc(CC(=O)N2Cc3ccc(cc3C2)S(=O)(=O)Nc2cnn(n2)-c2ccccc2)c1 Show InChI InChI=1S/C25H23N5O4S/c1-34-22-9-5-6-18(12-22)13-25(31)29-16-19-10-11-23(14-20(19)17-29)35(32,33)28-24-15-26-30(27-24)21-7-3-2-4-8-21/h2-12,14-15H,13,16-17H2,1H3,(H,27,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MGAT3 (unknown origin) assessed effect on incorporation of [1-14C]decanoyl moiety into triacylglycerol using [1-14C]decanoyl-CoA and 1,... |
J Med Chem 58: 7164-72 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01008
BindingDB Entry DOI: 10.7270/Q2F191HJ |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM276748
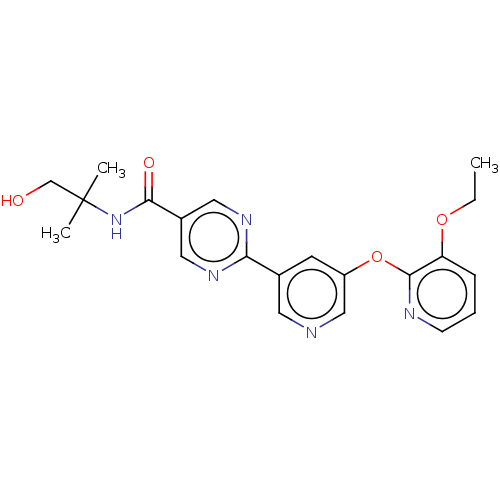
(US10071992, Example 3.5 | US10071992, Example 5 | ...)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)NC(C)(C)CO Show InChI InChI=1S/C21H23N5O4/c1-4-29-17-6-5-7-23-20(17)30-16-8-14(9-22-12-16)18-24-10-15(11-25-18)19(28)26-21(2,3)13-27/h5-12,27H,4,13H2,1-3H3,(H,26,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Mycobacterium smegmatis) | BDBM50010362
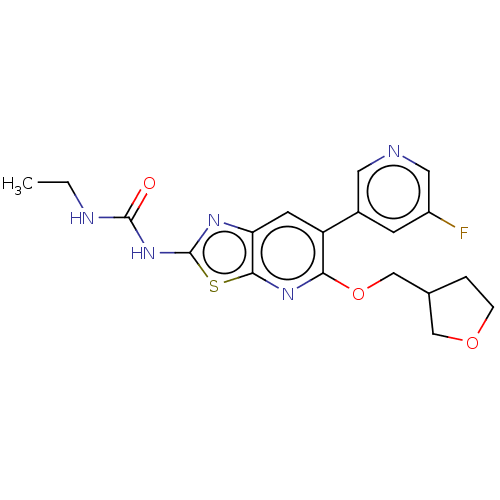
(CHEMBL3263604)Show SMILES CCNC(=O)Nc1nc2cc(-c3cncc(F)c3)c(OCC3CCOC3)nc2s1 Show InChI InChI=1S/C19H20FN5O3S/c1-2-22-18(26)25-19-23-15-6-14(12-5-13(20)8-21-7-12)16(24-17(15)29-19)28-10-11-3-4-27-9-11/h5-8,11H,2-4,9-10H2,1H3,(H2,22,23,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca India Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium smegmatis DNA gyrase B ATPase activity assessed as inorganic phosphate release using ATP as substrate by colorimetric ana... |
Cell Chem Biol 56: 8834-48 (2013)
Article DOI: 10.1021/jm401268f
BindingDB Entry DOI: 10.7270/Q2RV0Q7W |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Mycobacterium smegmatis) | BDBM50010365
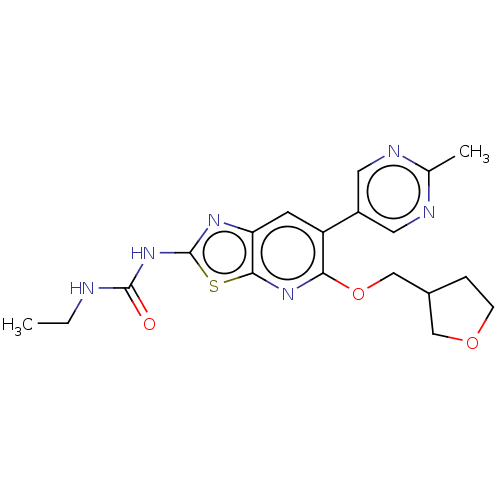
(CHEMBL3263607)Show SMILES CCNC(=O)Nc1nc2cc(-c3cnc(C)nc3)c(OCC3CCOC3)nc2s1 Show InChI InChI=1S/C19H22N6O3S/c1-3-20-18(26)25-19-23-15-6-14(13-7-21-11(2)22-8-13)16(24-17(15)29-19)28-10-12-4-5-27-9-12/h6-8,12H,3-5,9-10H2,1-2H3,(H2,20,23,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca India Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium smegmatis DNA gyrase B ATPase activity assessed as inorganic phosphate release using ATP as substrate by colorimetric ana... |
Cell Chem Biol 56: 8834-48 (2013)
Article DOI: 10.1021/jm401268f
BindingDB Entry DOI: 10.7270/Q2RV0Q7W |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM276749
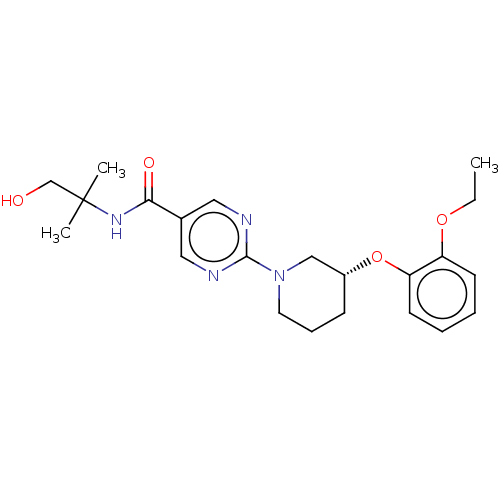
(US10188653, Example 19.21 | US11034678, WO20151406...)Show SMILES CCOc1ccccc1O[C@@H]1CCCN(C1)c1ncc(cn1)C(=O)NC(C)(C)CO |r| Show InChI InChI=1S/C22H30N4O4/c1-4-29-18-9-5-6-10-19(18)30-17-8-7-11-26(14-17)21-23-12-16(13-24-21)20(28)25-22(2,3)15-27/h5-6,9-10,12-13,17,27H,4,7-8,11,14-15H2,1-3H3,(H,25,28)/t17-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Mycobacterium smegmatis) | BDBM50010364
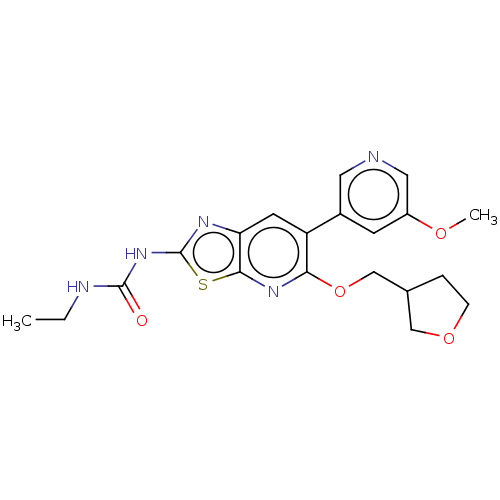
(CHEMBL3263606)Show SMILES CCNC(=O)Nc1nc2cc(-c3cncc(OC)c3)c(OCC3CCOC3)nc2s1 Show InChI InChI=1S/C20H23N5O4S/c1-3-22-19(26)25-20-23-16-7-15(13-6-14(27-2)9-21-8-13)17(24-18(16)30-20)29-11-12-4-5-28-10-12/h6-9,12H,3-5,10-11H2,1-2H3,(H2,22,23,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca India Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium smegmatis DNA gyrase B ATPase activity assessed as inorganic phosphate release using ATP as substrate by colorimetric ana... |
Cell Chem Biol 56: 8834-48 (2013)
Article DOI: 10.1021/jm401268f
BindingDB Entry DOI: 10.7270/Q2RV0Q7W |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Mycobacterium smegmatis) | BDBM50447297
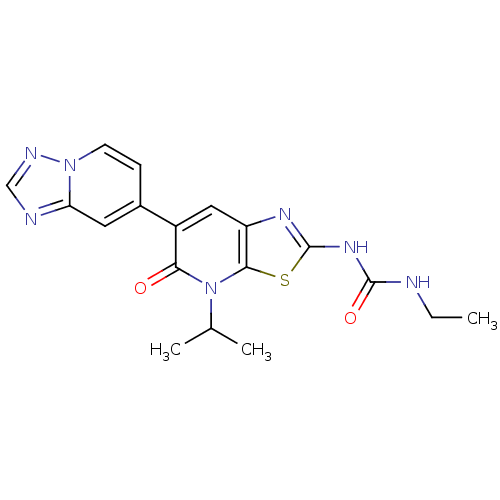
(CHEMBL3114210)Show SMILES CCNC(=O)Nc1nc2cc(-c3ccn4ncnc4c3)c(=O)n(C(C)C)c2s1 Show InChI InChI=1S/C18H19N7O2S/c1-4-19-17(27)23-18-22-13-8-12(15(26)25(10(2)3)16(13)28-18)11-5-6-24-14(7-11)20-9-21-24/h5-10H,4H2,1-3H3,(H2,19,22,23,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium smegmatis DNA gyrase B ATPase activity after 100 mins |
Bioorg Med Chem Lett 24: 870-9 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.080
BindingDB Entry DOI: 10.7270/Q2K9390V |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Mycobacterium smegmatis) | BDBM50010375
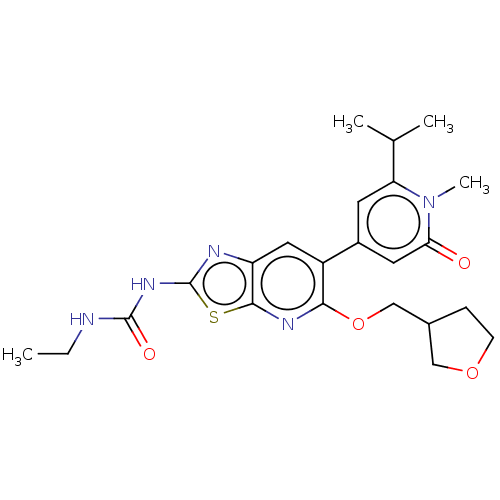
(CHEMBL3263617)Show SMILES CCNC(=O)Nc1nc2cc(-c3cc(C(C)C)n(C)c(=O)c3)c(OCC3CCOC3)nc2s1 Show InChI InChI=1S/C23H29N5O4S/c1-5-24-22(30)27-23-25-17-10-16(15-8-18(13(2)3)28(4)19(29)9-15)20(26-21(17)33-23)32-12-14-6-7-31-11-14/h8-10,13-14H,5-7,11-12H2,1-4H3,(H2,24,25,27,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca India Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium smegmatis DNA gyrase B ATPase activity assessed as inorganic phosphate release using ATP as substrate by colorimetric ana... |
Cell Chem Biol 56: 8834-48 (2013)
Article DOI: 10.1021/jm401268f
BindingDB Entry DOI: 10.7270/Q2RV0Q7W |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Mycobacterium smegmatis) | BDBM50010348

(CHEMBL3263592)Show SMILES CCNC(=O)Nc1nc2cc(-c3cccnc3)c(OC3CCOCC3)nc2s1 Show InChI InChI=1S/C19H21N5O3S/c1-2-21-18(25)24-19-22-15-10-14(12-4-3-7-20-11-12)16(23-17(15)28-19)27-13-5-8-26-9-6-13/h3-4,7,10-11,13H,2,5-6,8-9H2,1H3,(H2,21,22,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca India Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium smegmatis DNA gyrase B ATPase activity assessed as inorganic phosphate release using ATP as substrate by colorimetric ana... |
Cell Chem Biol 56: 8834-48 (2013)
Article DOI: 10.1021/jm401268f
BindingDB Entry DOI: 10.7270/Q2RV0Q7W |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Mycobacterium smegmatis) | BDBM50010360
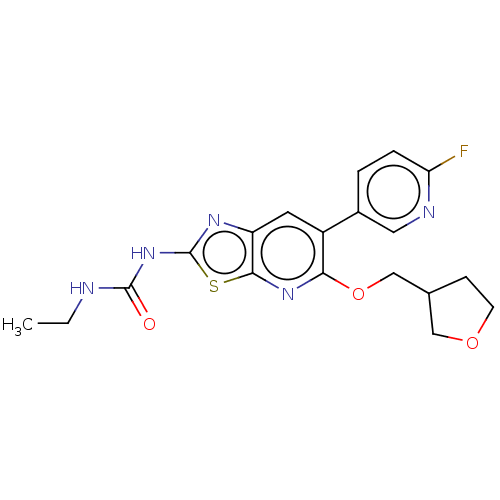
(CHEMBL3263603)Show SMILES CCNC(=O)Nc1nc2cc(-c3ccc(F)nc3)c(OCC3CCOC3)nc2s1 Show InChI InChI=1S/C19H20FN5O3S/c1-2-21-18(26)25-19-23-14-7-13(12-3-4-15(20)22-8-12)16(24-17(14)29-19)28-10-11-5-6-27-9-11/h3-4,7-8,11H,2,5-6,9-10H2,1H3,(H2,21,23,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca India Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium smegmatis DNA gyrase B ATPase activity assessed as inorganic phosphate release using ATP as substrate by colorimetric ana... |
Cell Chem Biol 56: 8834-48 (2013)
Article DOI: 10.1021/jm401268f
BindingDB Entry DOI: 10.7270/Q2RV0Q7W |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM160788
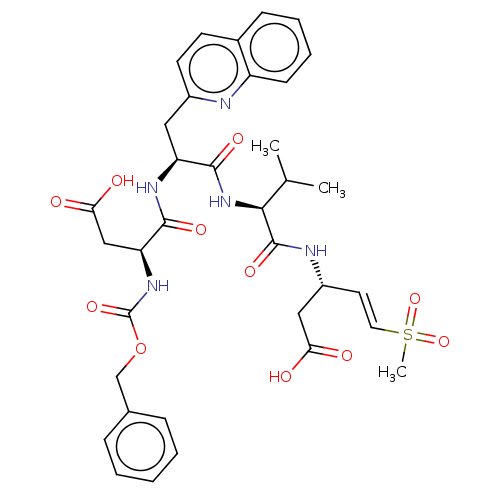
(US10167313, Compound 55 | US9045524, 55)Show SMILES CC(C)[C@H](NC(=O)[C@H](Cc1ccc2ccccc2n1)NC(=O)[C@H](CC(O)=O)NC(=O)OCc1ccccc1)C(=O)N[C@@H](CC(O)=O)\C=C\S(C)(=O)=O |r| Show InChI InChI=1S/C35H41N5O11S/c1-21(2)31(34(47)37-25(18-29(41)42)15-16-52(3,49)50)40-33(46)27(17-24-14-13-23-11-7-8-12-26(23)36-24)38-32(45)28(19-30(43)44)39-35(48)51-20-22-9-5-4-6-10-22/h4-16,21,25,27-28,31H,17-20H2,1-3H3,(H,37,47)(H,38,45)(H,39,48)(H,40,46)(H,41,42)(H,43,44)/b16-15+/t25-,27+,28+,31+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVAGENESIS FOUNDATION
US Patent
| Assay Description
To test the efficacy of caspase-3 inhibitors at the cellular level, the ability of selected compounds to inhibit the proteolytic cleavage of PARP (po... |
US Patent US9045524 (2015)
BindingDB Entry DOI: 10.7270/Q2GT5KX8 |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM160798
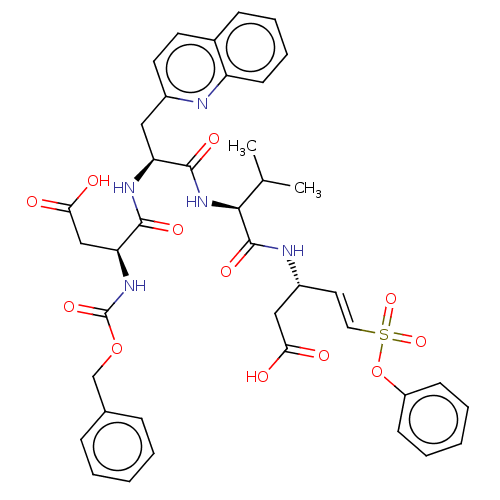
(US10167313, Compound 65 | US9045524, 65)Show SMILES CC(C)[C@H](NC(=O)[C@H](Cc1ccc2ccccc2n1)NC(=O)[C@H](CC(O)=O)NC(=O)OCc1ccccc1)C(=O)N[C@@H](CC(O)=O)\C=C\S(=O)(=O)Oc1ccccc1 |r| Show InChI InChI=1S/C40H43N5O12S/c1-25(2)36(39(52)42-29(22-34(46)47)19-20-58(54,55)57-30-14-7-4-8-15-30)45-38(51)32(21-28-18-17-27-13-9-10-16-31(27)41-28)43-37(50)33(23-35(48)49)44-40(53)56-24-26-11-5-3-6-12-26/h3-20,25,29,32-33,36H,21-24H2,1-2H3,(H,42,52)(H,43,50)(H,44,53)(H,45,51)(H,46,47)(H,48,49)/b20-19+/t29-,32+,33+,36+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVAGENESIS FOUNDATION
US Patent
| Assay Description
To test the efficacy of caspase-3 inhibitors at the cellular level, the ability of selected compounds to inhibit the proteolytic cleavage of PARP (po... |
US Patent US9045524 (2015)
BindingDB Entry DOI: 10.7270/Q2GT5KX8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data