 Found 47 hits with Last Name = 'krause' and Initial = 'sm'
Found 47 hits with Last Name = 'krause' and Initial = 'sm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Metabotropic glutamate receptor 5
(Rattus norvegicus (Rat)) | BDBM50084137
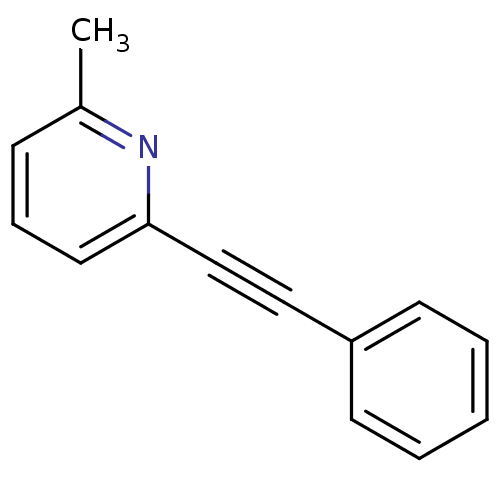
(2-Methyl-6-(phenylethynyl)pyridine | 2-Methyl-6-ph...)Show InChI InChI=1S/C14H11N/c1-12-6-5-9-14(15-12)11-10-13-7-3-2-4-8-13/h2-9H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Life Sci 73: 371-9 (2003)
Article DOI: 10.1016/s0024-3205(03)00272-8
BindingDB Entry DOI: 10.7270/Q2KD1WHW |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50277511

(3-[(3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromet...)Show SMILES C[C@@H](O[C@H]1CC[C@@H]2CN(C[C@H]2[C@@H]1c1ccc(F)cc1)C1=CC(=O)CC1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r,t:22| Show InChI InChI=1S/C29H28F7NO2/c1-16(19-10-20(28(31,32)33)12-21(11-19)29(34,35)36)39-26-9-4-18-14-37(23-7-8-24(38)13-23)15-25(18)27(26)17-2-5-22(30)6-3-17/h2-3,5-6,10-13,16,18,25-27H,4,7-9,14-15H2,1H3/t16-,18-,25-,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human NK2 receptor |
J Med Chem 52: 3039-46 (2009)
Article DOI: 10.1021/jm8016514
BindingDB Entry DOI: 10.7270/Q2FX79BT |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50277511

(3-[(3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromet...)Show SMILES C[C@@H](O[C@H]1CC[C@@H]2CN(C[C@H]2[C@@H]1c1ccc(F)cc1)C1=CC(=O)CC1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r,t:22| Show InChI InChI=1S/C29H28F7NO2/c1-16(19-10-20(28(31,32)33)12-21(11-19)29(34,35)36)39-26-9-4-18-14-37(23-7-8-24(38)13-23)15-25(18)27(26)17-2-5-22(30)6-3-17/h2-3,5-6,10-13,16,18,25-27H,4,7-9,14-15H2,1H3/t16-,18-,25-,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human NK3 receptor |
J Med Chem 52: 3039-46 (2009)
Article DOI: 10.1021/jm8016514
BindingDB Entry DOI: 10.7270/Q2FX79BT |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50030629
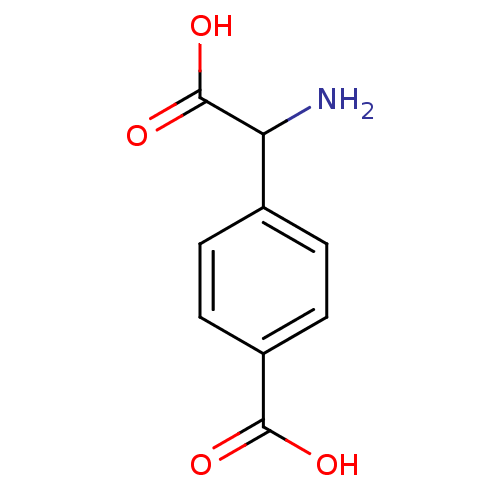
((R,S)-4-Carboxy-3-hydroxyphenylglycine | (S)-4CPG ...)Show InChI InChI=1S/C9H9NO4/c10-7(9(13)14)5-1-3-6(4-2-5)8(11)12/h1-4,7H,10H2,(H,11,12)(H,13,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Life Sci 73: 371-9 (2003)
Article DOI: 10.1016/s0024-3205(03)00272-8
BindingDB Entry DOI: 10.7270/Q2KD1WHW |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50030630
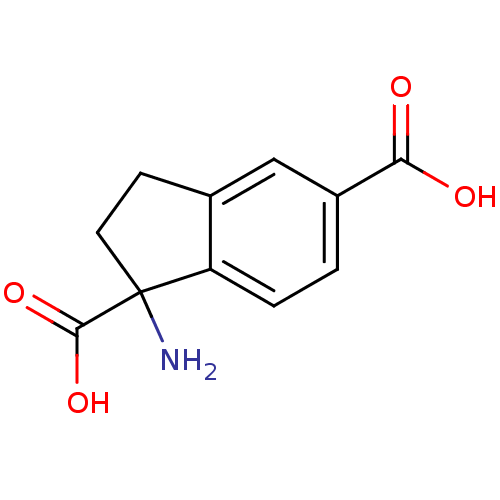
((RS)-1-aminoindan-1,5-dicarboxylic acid | 1-Amino-...)Show InChI InChI=1S/C11H11NO4/c12-11(10(15)16)4-3-6-5-7(9(13)14)1-2-8(6)11/h1-2,5H,3-4,12H2,(H,13,14)(H,15,16) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Life Sci 73: 371-9 (2003)
Article DOI: 10.1016/s0024-3205(03)00272-8
BindingDB Entry DOI: 10.7270/Q2KD1WHW |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(RAT) | BDBM86153
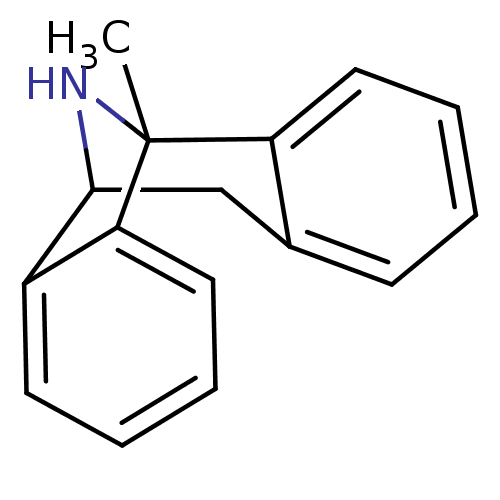
(CAS_180081 | MK-801 | NSC_180081)Show SMILES CC12NC(Cc3ccccc13)c1ccccc21 |TLB:9:10:2:11.16,6:5:2:11.16,THB:12:11:10.5.4:2,15:16:10.5.4:2| Show InChI InChI=1S/C16H15N/c1-16-13-8-4-2-6-11(13)10-15(17-16)12-7-3-5-9-14(12)16/h2-9,15,17H,10H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Life Sci 73: 371-9 (2003)
Article DOI: 10.1016/s0024-3205(03)00272-8
BindingDB Entry DOI: 10.7270/Q2KD1WHW |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50032651
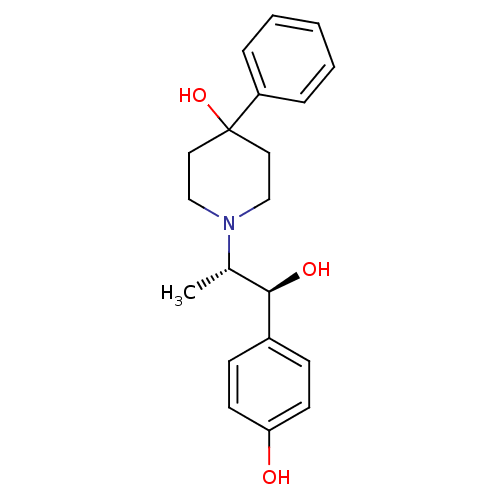
(1-((1S,2S)-1-hydroxy-1-(4-hydroxyphenyl)propan-2-y...)Show SMILES C[C@@H]([C@@H](O)c1ccc(O)cc1)N1CCC(O)(CC1)c1ccccc1 Show InChI InChI=1S/C20H25NO3/c1-15(19(23)16-7-9-18(22)10-8-16)21-13-11-20(24,12-14-21)17-5-3-2-4-6-17/h2-10,15,19,22-24H,11-14H2,1H3/t15-,19+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Life Sci 73: 371-9 (2003)
Article DOI: 10.1016/s0024-3205(03)00272-8
BindingDB Entry DOI: 10.7270/Q2KD1WHW |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM86230
(CAS_146-48-5 | NSC_2866 | YOHIMBINE)Show SMILES COC(=O)C1C(O)CCC2CN3CCc4c([nH]c5ccccc45)C3CC12 Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Life Sci 73: 371-9 (2003)
Article DOI: 10.1016/s0024-3205(03)00272-8
BindingDB Entry DOI: 10.7270/Q2KD1WHW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM86231

(ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...)Show SMILES CN1C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Life Sci 73: 371-9 (2003)
Article DOI: 10.1016/s0024-3205(03)00272-8
BindingDB Entry DOI: 10.7270/Q2KD1WHW |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(RAT) | BDBM86232
(Benoxathian | CAS_2325 | NSC_2325)Show InChI InChI=1S/C19H23NO4S/c1-21-16-7-5-8-17(22-2)19(16)23-11-10-20-12-14-13-25-18-9-4-3-6-15(18)24-14/h3-9,14,20H,10-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Life Sci 73: 371-9 (2003)
Article DOI: 10.1016/s0024-3205(03)00272-8
BindingDB Entry DOI: 10.7270/Q2KD1WHW |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM22869

(6-chloro-10-(4-methylpiperazin-1-yl)-2,9-diazatric...)Show SMILES CN1CCN(CC1)C1=c2ccccc2=Nc2ccc(Cl)cc2N1 |c:8,15| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,21H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Life Sci 73: 371-9 (2003)
Article DOI: 10.1016/s0024-3205(03)00272-8
BindingDB Entry DOI: 10.7270/Q2KD1WHW |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50277511

(3-[(3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromet...)Show SMILES C[C@@H](O[C@H]1CC[C@@H]2CN(C[C@H]2[C@@H]1c1ccc(F)cc1)C1=CC(=O)CC1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r,t:22| Show InChI InChI=1S/C29H28F7NO2/c1-16(19-10-20(28(31,32)33)12-21(11-19)29(34,35)36)39-26-9-4-18-14-37(23-7-8-24(38)13-23)15-25(18)27(26)17-2-5-22(30)6-3-17/h2-3,5-6,10-13,16,18,25-27H,4,7-9,14-15H2,1H3/t16-,18-,25-,26+,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]substance P human recombinant NK1 receptor expressed in CHO cells in absence of human serum albumin |
J Med Chem 52: 3039-46 (2009)
Article DOI: 10.1021/jm8016514
BindingDB Entry DOI: 10.7270/Q2FX79BT |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50277568

((3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromethyl...)Show SMILES CNC(=O)N1C[C@H]2CC[C@H](O[C@H](C)c3cc(cc(c3)C(F)(F)F)C(F)(F)F)[C@H]([C@@H]2C1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H27F7N2O2/c1-14(17-9-18(25(28,29)30)11-19(10-17)26(31,32)33)37-22-8-5-16-12-35(24(36)34-2)13-21(16)23(22)15-3-6-20(27)7-4-15/h3-4,6-7,9-11,14,16,21-23H,5,8,12-13H2,1-2H3,(H,34,36)/t14-,16-,21-,22+,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]substance P human recombinant NK1 receptor expressed in CHO cells in absence of human serum albumin |
J Med Chem 52: 3039-46 (2009)
Article DOI: 10.1021/jm8016514
BindingDB Entry DOI: 10.7270/Q2FX79BT |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50277571

(3-[(4R,5S)-5-{(1R)-1-[3,5-Bis(trifluoromethyl)phen...)Show SMILES C[C@@H](O[C@H]1CC[C@@H]2CN(C[C@H]2[C@@H]1c1ccc(F)cc1)C1=C(C)C(=O)CC1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r,c:22| Show InChI InChI=1S/C30H30F7NO2/c1-16-25(8-9-26(16)39)38-14-19-5-10-27(28(24(19)15-38)18-3-6-23(31)7-4-18)40-17(2)20-11-21(29(32,33)34)13-22(12-20)30(35,36)37/h3-4,6-7,11-13,17,19,24,27-28H,5,8-10,14-15H2,1-2H3/t17-,19-,24-,27+,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]substance P human recombinant NK1 receptor expressed in CHO cells in absence of human serum albumin |
J Med Chem 52: 3039-46 (2009)
Article DOI: 10.1021/jm8016514
BindingDB Entry DOI: 10.7270/Q2FX79BT |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50277569

((4R,5S)-2-Acetyl-5-{(1R)-1-[3,5-bis(trifluoromethy...)Show SMILES C[C@@H](O[C@H]1CC[C@@H]2CN(C[C@H]2[C@@H]1c1ccc(F)cc1)C(C)=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C26H26F7NO2/c1-14(18-9-19(25(28,29)30)11-20(10-18)26(31,32)33)36-23-8-5-17-12-34(15(2)35)13-22(17)24(23)16-3-6-21(27)7-4-16/h3-4,6-7,9-11,14,17,22-24H,5,8,12-13H2,1-2H3/t14-,17-,22-,23+,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]substance P human recombinant NK1 receptor expressed in CHO cells in absence of human serum albumin |
J Med Chem 52: 3039-46 (2009)
Article DOI: 10.1021/jm8016514
BindingDB Entry DOI: 10.7270/Q2FX79BT |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50277572
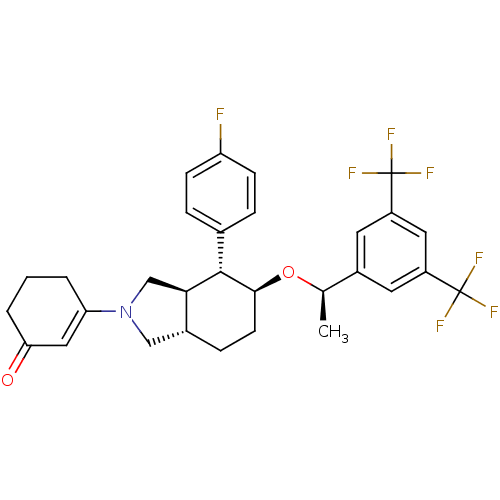
(3-[(4R,5S)-5-{(1R)-1-[3,5-Bis(trifluoromethyl)phen...)Show SMILES C[C@@H](O[C@H]1CC[C@@H]2CN(C[C@H]2[C@@H]1c1ccc(F)cc1)C1=CC(=O)CCC1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r,t:22| Show InChI InChI=1S/C30H30F7NO2/c1-17(20-11-21(29(32,33)34)13-22(12-20)30(35,36)37)40-27-10-7-19-15-38(24-3-2-4-25(39)14-24)16-26(19)28(27)18-5-8-23(31)9-6-18/h5-6,8-9,11-14,17,19,26-28H,2-4,7,10,15-16H2,1H3/t17-,19-,26-,27+,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]substance P human recombinant NK1 receptor expressed in CHO cells in absence of human serum albumin |
J Med Chem 52: 3039-46 (2009)
Article DOI: 10.1021/jm8016514
BindingDB Entry DOI: 10.7270/Q2FX79BT |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50277510
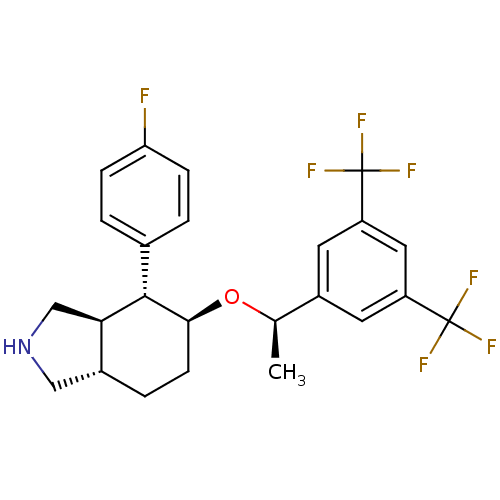
((3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromethyl...)Show SMILES C[C@@H](O[C@H]1CC[C@@H]2CNC[C@H]2[C@@H]1c1ccc(F)cc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C24H24F7NO/c1-13(16-8-17(23(26,27)28)10-18(9-16)24(29,30)31)33-21-7-4-15-11-32-12-20(15)22(21)14-2-5-19(25)6-3-14/h2-3,5-6,8-10,13,15,20-22,32H,4,7,11-12H2,1H3/t13-,15-,20-,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]substance P human recombinant NK1 receptor expressed in CHO cells in absence of human serum albumin |
J Med Chem 52: 3039-46 (2009)
Article DOI: 10.1021/jm8016514
BindingDB Entry DOI: 10.7270/Q2FX79BT |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50277567

((4R,5S)-5-{(1R)-1-[3,5-Bis(trifluoromethyl)phenyl]...)Show SMILES C[C@@H](O[C@H]1CC[C@@H]2CN(C)C[C@H]2[C@@H]1c1ccc(F)cc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C25H26F7NO/c1-14(17-9-18(24(27,28)29)11-19(10-17)25(30,31)32)34-22-8-5-16-12-33(2)13-21(16)23(22)15-3-6-20(26)7-4-15/h3-4,6-7,9-11,14,16,21-23H,5,8,12-13H2,1-2H3/t14-,16-,21-,22+,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.255 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]substance P human recombinant NK1 receptor expressed in CHO cells in absence of human serum albumin |
J Med Chem 52: 3039-46 (2009)
Article DOI: 10.1021/jm8016514
BindingDB Entry DOI: 10.7270/Q2FX79BT |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50200841

(CHEMBL220360 | MK-0364 | MK-0634 | N-((2S,3S)-4-(4...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1ccc(cn1)C(F)(F)F)[C@@H](Cc1ccc(Cl)cc1)c1cccc(c1)C#N |r| Show InChI InChI=1S/C27H25ClF3N3O2/c1-17(34-25(35)26(2,3)36-24-12-9-21(16-33-24)27(29,30)31)23(14-18-7-10-22(28)11-8-18)20-6-4-5-19(13-20)15-32/h4-13,16-17,23H,14H2,1-3H3,(H,34,35)/t17-,23+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells |
J Med Chem 50: 3427-30 (2007)
Article DOI: 10.1021/jm070131b
BindingDB Entry DOI: 10.7270/Q2Z037VJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50217220

(CHEMBL226590 | N-{(1S,2S)-2-(3-cyanophenyl)-3-[4-(...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1cc(C)ccn1)[C@@H](Cc1ccc(OCCF)cc1)c1cccc(c1)C#N Show InChI InChI=1S/C29H32FN3O3/c1-20-12-14-32-27(16-20)36-29(3,4)28(34)33-21(2)26(24-7-5-6-23(17-24)19-31)18-22-8-10-25(11-9-22)35-15-13-30/h5-12,14,16-17,21,26H,13,15,18H2,1-4H3,(H,33,34)/t21-,26+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells |
J Med Chem 50: 3427-30 (2007)
Article DOI: 10.1021/jm070131b
BindingDB Entry DOI: 10.7270/Q2Z037VJ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50277570

((4R,5S)-5-{(1R)-1-[3,5-Bis(trifluoromethyl)phenyl]...)Show SMILES C[C@@H](O[C@H]1CC[C@@H]2CN(C[C@H]2[C@@H]1c1ccc(F)cc1)c1cnccn1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C28H26F7N3O/c1-16(19-10-20(27(30,31)32)12-21(11-19)28(33,34)35)39-24-7-4-18-14-38(25-13-36-8-9-37-25)15-23(18)26(24)17-2-5-22(29)6-3-17/h2-3,5-6,8-13,16,18,23-24,26H,4,7,14-15H2,1H3/t16-,18-,23-,24+,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.525 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]substance P human recombinant NK1 receptor expressed in CHO cells in absence of human serum albumin |
J Med Chem 52: 3039-46 (2009)
Article DOI: 10.1021/jm8016514
BindingDB Entry DOI: 10.7270/Q2FX79BT |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50217216
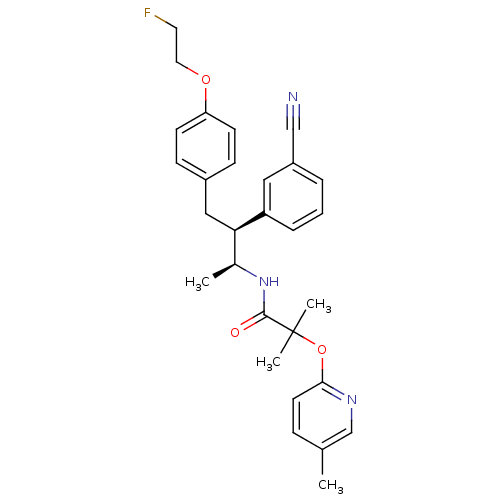
(CHEMBL226591 | CHEMBL226642 | N-{(1S,2S)-2-(3-cyan...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1ccc(C)cn1)[C@@H](Cc1ccc(OCCF)cc1)c1cccc(c1)C#N Show InChI InChI=1S/C29H32FN3O3/c1-20-8-13-27(32-19-20)36-29(3,4)28(34)33-21(2)26(24-7-5-6-23(16-24)18-31)17-22-9-11-25(12-10-22)35-15-14-30/h5-13,16,19,21,26H,14-15,17H2,1-4H3,(H,33,34)/t21-,26+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells |
J Med Chem 50: 3427-30 (2007)
Article DOI: 10.1021/jm070131b
BindingDB Entry DOI: 10.7270/Q2Z037VJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50217216

(CHEMBL226591 | CHEMBL226642 | N-{(1S,2S)-2-(3-cyan...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1ccc(C)cn1)[C@@H](Cc1ccc(OCCF)cc1)c1cccc(c1)C#N Show InChI InChI=1S/C29H32FN3O3/c1-20-8-13-27(32-19-20)36-29(3,4)28(34)33-21(2)26(24-7-5-6-23(16-24)18-31)17-22-9-11-25(12-10-22)35-15-14-30/h5-13,16,19,21,26H,14-15,17H2,1-4H3,(H,33,34)/t21-,26+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells |
J Med Chem 50: 3427-30 (2007)
Article DOI: 10.1021/jm070131b
BindingDB Entry DOI: 10.7270/Q2Z037VJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50217217
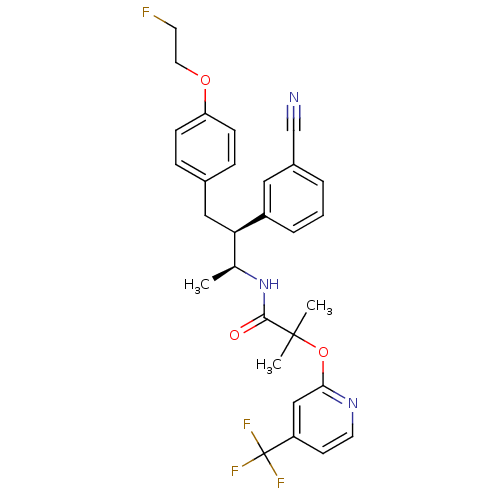
(CHEMBL376794 | N-{(1S,2S)-2-(3-cyanophenyl)-3-[4-(...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1cc(ccn1)C(F)(F)F)[C@@H](Cc1ccc(OCCF)cc1)c1cccc(c1)C#N Show InChI InChI=1S/C29H29F4N3O3/c1-19(36-27(37)28(2,3)39-26-17-23(11-13-35-26)29(31,32)33)25(22-6-4-5-21(15-22)18-34)16-20-7-9-24(10-8-20)38-14-12-30/h4-11,13,15,17,19,25H,12,14,16H2,1-3H3,(H,36,37)/t19-,25+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells |
J Med Chem 50: 3427-30 (2007)
Article DOI: 10.1021/jm070131b
BindingDB Entry DOI: 10.7270/Q2Z037VJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50217213
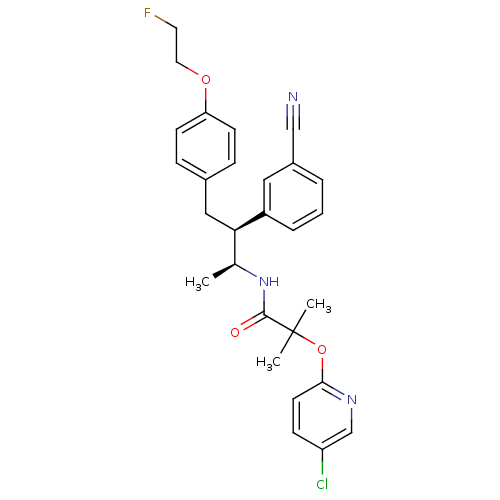
(CHEMBL226589 | N-{(1S,2S)-2-(3-cyanophenyl)-3-[4-(...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1ccc(Cl)cn1)[C@@H](Cc1ccc(OCCF)cc1)c1cccc(c1)C#N Show InChI InChI=1S/C28H29ClFN3O3/c1-19(33-27(34)28(2,3)36-26-12-9-23(29)18-32-26)25(22-6-4-5-21(15-22)17-31)16-20-7-10-24(11-8-20)35-14-13-30/h4-12,15,18-19,25H,13-14,16H2,1-3H3,(H,33,34)/t19-,25+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells |
J Med Chem 50: 3427-30 (2007)
Article DOI: 10.1021/jm070131b
BindingDB Entry DOI: 10.7270/Q2Z037VJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50217223
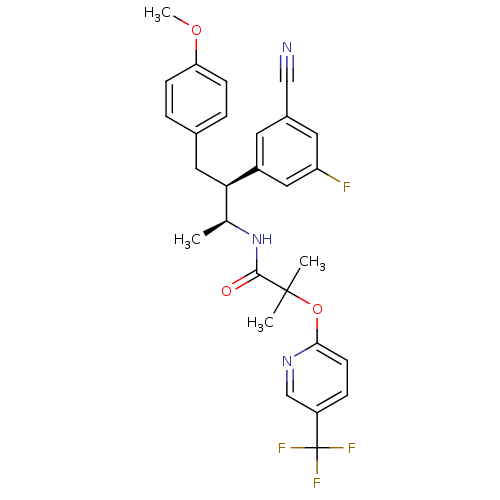
(CHEMBL226684 | N-[(1S,2S)-2-(3-cyano-5-fluoropheny...)Show SMILES COc1ccc(C[C@H]([C@H](C)NC(=O)C(C)(C)Oc2ccc(cn2)C(F)(F)F)c2cc(F)cc(c2)C#N)cc1 Show InChI InChI=1S/C28H27F4N3O3/c1-17(35-26(36)27(2,3)38-25-10-7-21(16-34-25)28(30,31)32)24(13-18-5-8-23(37-4)9-6-18)20-11-19(15-33)12-22(29)14-20/h5-12,14,16-17,24H,13H2,1-4H3,(H,35,36)/t17-,24+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells |
J Med Chem 50: 3427-30 (2007)
Article DOI: 10.1021/jm070131b
BindingDB Entry DOI: 10.7270/Q2Z037VJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50217222

(CHEMBL226534 | N-{(1S,2S)-2-(3-cyanophenyl)-3-[4-(...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1ccc(cn1)C(F)(F)F)[C@@H](Cc1ccc(OCCF)cc1)c1cccc(c1)C#N Show InChI InChI=1S/C29H29F4N3O3/c1-19(36-27(37)28(2,3)39-26-12-9-23(18-35-26)29(31,32)33)25(22-6-4-5-21(15-22)17-34)16-20-7-10-24(11-8-20)38-14-13-30/h4-12,15,18-19,25H,13-14,16H2,1-3H3,(H,36,37)/t19-,25+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells |
J Med Chem 50: 3427-30 (2007)
Article DOI: 10.1021/jm070131b
BindingDB Entry DOI: 10.7270/Q2Z037VJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50217221
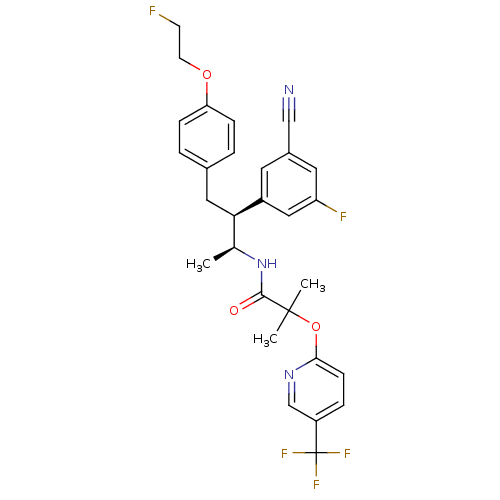
(CHEMBL376767 | N-{(1S,2S)-2-(3-cyano-5-fluoropheny...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1ccc(cn1)C(F)(F)F)[C@@H](Cc1ccc(OCCF)cc1)c1cc(F)cc(c1)C#N Show InChI InChI=1S/C29H28F5N3O3/c1-18(37-27(38)28(2,3)40-26-9-6-22(17-36-26)29(32,33)34)25(21-12-20(16-35)13-23(31)15-21)14-19-4-7-24(8-5-19)39-11-10-30/h4-9,12-13,15,17-18,25H,10-11,14H2,1-3H3,(H,37,38)/t18-,25+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells |
J Med Chem 50: 3427-30 (2007)
Article DOI: 10.1021/jm070131b
BindingDB Entry DOI: 10.7270/Q2Z037VJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50217218

(CHEMBL227329 | N-{(1S,2S)-2-(3-cyanophenyl)-3-[4-(...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1ccccn1)[C@@H](Cc1ccc(OCCF)cc1)c1cccc(c1)C#N Show InChI InChI=1S/C28H30FN3O3/c1-20(32-27(33)28(2,3)35-26-9-4-5-15-31-26)25(23-8-6-7-22(17-23)19-30)18-21-10-12-24(13-11-21)34-16-14-29/h4-13,15,17,20,25H,14,16,18H2,1-3H3,(H,32,33)/t20-,25+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells |
J Med Chem 50: 3427-30 (2007)
Article DOI: 10.1021/jm070131b
BindingDB Entry DOI: 10.7270/Q2Z037VJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50217224
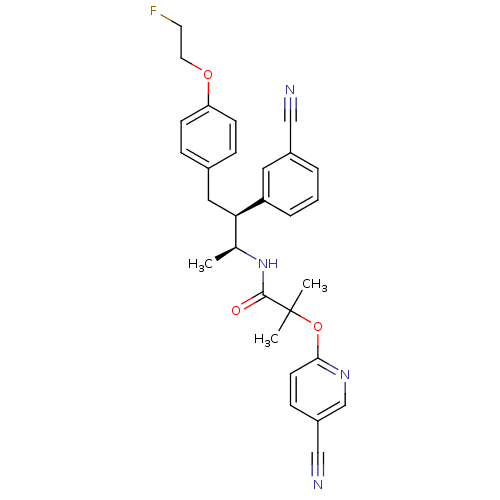
(CHEMBL387526 | N-{(1S,2S)-2-(3-cyanophenyl)-3-[4-(...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1ccc(cn1)C#N)[C@@H](Cc1ccc(OCCF)cc1)c1cccc(c1)C#N Show InChI InChI=1S/C29H29FN4O3/c1-20(34-28(35)29(2,3)37-27-12-9-23(18-32)19-33-27)26(24-6-4-5-22(15-24)17-31)16-21-7-10-25(11-8-21)36-14-13-30/h4-12,15,19-20,26H,13-14,16H2,1-3H3,(H,34,35)/t20-,26+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells |
J Med Chem 50: 3427-30 (2007)
Article DOI: 10.1021/jm070131b
BindingDB Entry DOI: 10.7270/Q2Z037VJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50217214

(CHEMBL226640 | N-{(1S,2S)-2-(3-cyanophenyl)-3-[4-(...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1cc(ncn1)C(F)(F)F)[C@@H](Cc1ccc(OCCF)cc1)c1cccc(c1)C#N Show InChI InChI=1S/C28H28F4N4O3/c1-18(36-26(37)27(2,3)39-25-15-24(28(30,31)32)34-17-35-25)23(21-6-4-5-20(13-21)16-33)14-19-7-9-22(10-8-19)38-12-11-29/h4-10,13,15,17-18,23H,11-12,14H2,1-3H3,(H,36,37)/t18-,23+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells |
J Med Chem 50: 3427-30 (2007)
Article DOI: 10.1021/jm070131b
BindingDB Entry DOI: 10.7270/Q2Z037VJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50217215
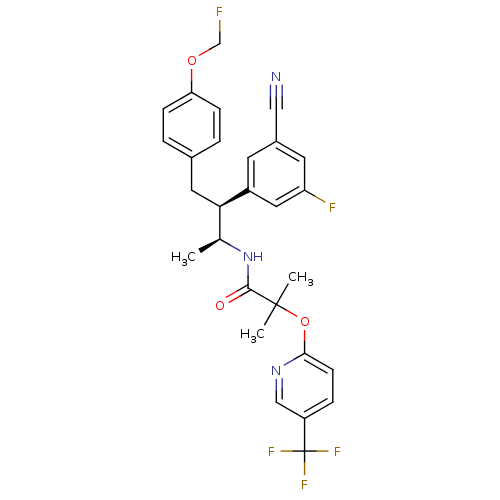
(CHEMBL226685 | N-[(1S,2S)-2-(3-cyano-5-fluoropheny...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1ccc(cn1)C(F)(F)F)[C@@H](Cc1ccc(OCF)cc1)c1cc(F)cc(c1)C#N Show InChI InChI=1S/C28H26F5N3O3/c1-17(36-26(37)27(2,3)39-25-9-6-21(15-35-25)28(31,32)33)24(20-10-19(14-34)11-22(30)13-20)12-18-4-7-23(8-5-18)38-16-29/h4-11,13,15,17,24H,12,16H2,1-3H3,(H,36,37)/t17-,24+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells |
J Med Chem 50: 3427-30 (2007)
Article DOI: 10.1021/jm070131b
BindingDB Entry DOI: 10.7270/Q2Z037VJ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50277510

((3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromethyl...)Show SMILES C[C@@H](O[C@H]1CC[C@@H]2CNC[C@H]2[C@@H]1c1ccc(F)cc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C24H24F7NO/c1-13(16-8-17(23(26,27)28)10-18(9-16)24(29,30)31)33-21-7-4-15-11-32-12-20(15)22(21)14-2-5-19(25)6-3-14/h2-3,5-6,8-10,13,15,20-22,32H,4,7,11-12H2,1H3/t13-,15-,20-,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]substance P human recombinant NK1 receptor expressed in CHO cells in presence of human serum albumin |
J Med Chem 52: 3039-46 (2009)
Article DOI: 10.1021/jm8016514
BindingDB Entry DOI: 10.7270/Q2FX79BT |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50277569

((4R,5S)-2-Acetyl-5-{(1R)-1-[3,5-bis(trifluoromethy...)Show SMILES C[C@@H](O[C@H]1CC[C@@H]2CN(C[C@H]2[C@@H]1c1ccc(F)cc1)C(C)=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C26H26F7NO2/c1-14(18-9-19(25(28,29)30)11-20(10-18)26(31,32)33)36-23-8-5-17-12-34(15(2)35)13-22(17)24(23)16-3-6-21(27)7-4-16/h3-4,6-7,9-11,14,17,22-24H,5,8,12-13H2,1-2H3/t14-,17-,22-,23+,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]substance P human recombinant NK1 receptor expressed in CHO cells in presence of human serum albumin |
J Med Chem 52: 3039-46 (2009)
Article DOI: 10.1021/jm8016514
BindingDB Entry DOI: 10.7270/Q2FX79BT |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50277511

(3-[(3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromet...)Show SMILES C[C@@H](O[C@H]1CC[C@@H]2CN(C[C@H]2[C@@H]1c1ccc(F)cc1)C1=CC(=O)CC1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r,t:22| Show InChI InChI=1S/C29H28F7NO2/c1-16(19-10-20(28(31,32)33)12-21(11-19)29(34,35)36)39-26-9-4-18-14-37(23-7-8-24(38)13-23)15-25(18)27(26)17-2-5-22(30)6-3-17/h2-3,5-6,10-13,16,18,25-27H,4,7-9,14-15H2,1H3/t16-,18-,25-,26+,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]substance P human recombinant NK1 receptor expressed in CHO cells in presence of human serum albumin |
J Med Chem 52: 3039-46 (2009)
Article DOI: 10.1021/jm8016514
BindingDB Entry DOI: 10.7270/Q2FX79BT |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50277568

((3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromethyl...)Show SMILES CNC(=O)N1C[C@H]2CC[C@H](O[C@H](C)c3cc(cc(c3)C(F)(F)F)C(F)(F)F)[C@H]([C@@H]2C1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H27F7N2O2/c1-14(17-9-18(25(28,29)30)11-19(10-17)26(31,32)33)37-22-8-5-16-12-35(24(36)34-2)13-21(16)23(22)15-3-6-20(27)7-4-15/h3-4,6-7,9-11,14,16,21-23H,5,8,12-13H2,1-2H3,(H,34,36)/t14-,16-,21-,22+,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]substance P human recombinant NK1 receptor expressed in CHO cells in presence of human serum albumin |
J Med Chem 52: 3039-46 (2009)
Article DOI: 10.1021/jm8016514
BindingDB Entry DOI: 10.7270/Q2FX79BT |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50277571

(3-[(4R,5S)-5-{(1R)-1-[3,5-Bis(trifluoromethyl)phen...)Show SMILES C[C@@H](O[C@H]1CC[C@@H]2CN(C[C@H]2[C@@H]1c1ccc(F)cc1)C1=C(C)C(=O)CC1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r,c:22| Show InChI InChI=1S/C30H30F7NO2/c1-16-25(8-9-26(16)39)38-14-19-5-10-27(28(24(19)15-38)18-3-6-23(31)7-4-18)40-17(2)20-11-21(29(32,33)34)13-22(12-20)30(35,36)37/h3-4,6-7,11-13,17,19,24,27-28H,5,8-10,14-15H2,1-2H3/t17-,19-,24-,27+,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]substance P human recombinant NK1 receptor expressed in CHO cells in presence of human serum albumin |
J Med Chem 52: 3039-46 (2009)
Article DOI: 10.1021/jm8016514
BindingDB Entry DOI: 10.7270/Q2FX79BT |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50277567

((4R,5S)-5-{(1R)-1-[3,5-Bis(trifluoromethyl)phenyl]...)Show SMILES C[C@@H](O[C@H]1CC[C@@H]2CN(C)C[C@H]2[C@@H]1c1ccc(F)cc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C25H26F7NO/c1-14(17-9-18(24(27,28)29)11-19(10-17)25(30,31)32)34-22-8-5-16-12-33(2)13-21(16)23(22)15-3-6-20(26)7-4-15/h3-4,6-7,9-11,14,16,21-23H,5,8,12-13H2,1-2H3/t14-,16-,21-,22+,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]substance P human recombinant NK1 receptor expressed in CHO cells in presence of human serum albumin |
J Med Chem 52: 3039-46 (2009)
Article DOI: 10.1021/jm8016514
BindingDB Entry DOI: 10.7270/Q2FX79BT |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50277572

(3-[(4R,5S)-5-{(1R)-1-[3,5-Bis(trifluoromethyl)phen...)Show SMILES C[C@@H](O[C@H]1CC[C@@H]2CN(C[C@H]2[C@@H]1c1ccc(F)cc1)C1=CC(=O)CCC1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r,t:22| Show InChI InChI=1S/C30H30F7NO2/c1-17(20-11-21(29(32,33)34)13-22(12-20)30(35,36)37)40-27-10-7-19-15-38(24-3-2-4-25(39)14-24)16-26(19)28(27)18-5-8-23(31)9-6-18/h5-6,8-9,11-14,17,19,26-28H,2-4,7,10,15-16H2,1H3/t17-,19-,26-,27+,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]substance P human recombinant NK1 receptor expressed in CHO cells in presence of human serum albumin |
J Med Chem 52: 3039-46 (2009)
Article DOI: 10.1021/jm8016514
BindingDB Entry DOI: 10.7270/Q2FX79BT |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50217216

(CHEMBL226591 | CHEMBL226642 | N-{(1S,2S)-2-(3-cyan...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1ccc(C)cn1)[C@@H](Cc1ccc(OCCF)cc1)c1cccc(c1)C#N Show InChI InChI=1S/C29H32FN3O3/c1-20-8-13-27(32-19-20)36-29(3,4)28(34)33-21(2)26(24-7-5-6-23(16-24)18-31)17-22-9-11-25(12-10-22)35-15-14-30/h5-13,16,19,21,26H,14-15,17H2,1-4H3,(H,33,34)/t21-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2R |
J Med Chem 50: 3427-30 (2007)
Article DOI: 10.1021/jm070131b
BindingDB Entry DOI: 10.7270/Q2Z037VJ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50277570

((4R,5S)-5-{(1R)-1-[3,5-Bis(trifluoromethyl)phenyl]...)Show SMILES C[C@@H](O[C@H]1CC[C@@H]2CN(C[C@H]2[C@@H]1c1ccc(F)cc1)c1cnccn1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C28H26F7N3O/c1-16(19-10-20(27(30,31)32)12-21(11-19)28(33,34)35)39-24-7-4-18-14-38(25-13-36-8-9-37-25)15-23(18)26(24)17-2-5-22(29)6-3-17/h2-3,5-6,8-13,16,18,23-24,26H,4,7,14-15H2,1H3/t16-,18-,23-,24+,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]substance P human recombinant NK1 receptor expressed in CHO cells in presence of human serum albumin |
J Med Chem 52: 3039-46 (2009)
Article DOI: 10.1021/jm8016514
BindingDB Entry DOI: 10.7270/Q2FX79BT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50277511

(3-[(3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromet...)Show SMILES C[C@@H](O[C@H]1CC[C@@H]2CN(C[C@H]2[C@@H]1c1ccc(F)cc1)C1=CC(=O)CC1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r,t:22| Show InChI InChI=1S/C29H28F7NO2/c1-16(19-10-20(28(31,32)33)12-21(11-19)29(34,35)36)39-26-9-4-18-14-37(23-7-8-24(38)13-23)15-25(18)27(26)17-2-5-22(30)6-3-17/h2-3,5-6,10-13,16,18,25-27H,4,7-9,14-15H2,1H3/t16-,18-,25-,26+,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
J Med Chem 52: 3039-46 (2009)
Article DOI: 10.1021/jm8016514
BindingDB Entry DOI: 10.7270/Q2FX79BT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50277511

(3-[(3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromet...)Show SMILES C[C@@H](O[C@H]1CC[C@@H]2CN(C[C@H]2[C@@H]1c1ccc(F)cc1)C1=CC(=O)CC1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r,t:22| Show InChI InChI=1S/C29H28F7NO2/c1-16(19-10-20(28(31,32)33)12-21(11-19)29(34,35)36)39-26-9-4-18-14-37(23-7-8-24(38)13-23)15-25(18)27(26)17-2-5-22(30)6-3-17/h2-3,5-6,10-13,16,18,25-27H,4,7-9,14-15H2,1H3/t16-,18-,25-,26+,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 |
J Med Chem 52: 3039-46 (2009)
Article DOI: 10.1021/jm8016514
BindingDB Entry DOI: 10.7270/Q2FX79BT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50277511

(3-[(3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromet...)Show SMILES C[C@@H](O[C@H]1CC[C@@H]2CN(C[C@H]2[C@@H]1c1ccc(F)cc1)C1=CC(=O)CC1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r,t:22| Show InChI InChI=1S/C29H28F7NO2/c1-16(19-10-20(28(31,32)33)12-21(11-19)29(34,35)36)39-26-9-4-18-14-37(23-7-8-24(38)13-23)15-25(18)27(26)17-2-5-22(30)6-3-17/h2-3,5-6,10-13,16,18,25-27H,4,7-9,14-15H2,1H3/t16-,18-,25-,26+,27+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
J Med Chem 52: 3039-46 (2009)
Article DOI: 10.1021/jm8016514
BindingDB Entry DOI: 10.7270/Q2FX79BT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50277511

(3-[(3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromet...)Show SMILES C[C@@H](O[C@H]1CC[C@@H]2CN(C[C@H]2[C@@H]1c1ccc(F)cc1)C1=CC(=O)CC1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r,t:22| Show InChI InChI=1S/C29H28F7NO2/c1-16(19-10-20(28(31,32)33)12-21(11-19)29(34,35)36)39-26-9-4-18-14-37(23-7-8-24(38)13-23)15-25(18)27(26)17-2-5-22(30)6-3-17/h2-3,5-6,10-13,16,18,25-27H,4,7-9,14-15H2,1H3/t16-,18-,25-,26+,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
J Med Chem 52: 3039-46 (2009)
Article DOI: 10.1021/jm8016514
BindingDB Entry DOI: 10.7270/Q2FX79BT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50277511

(3-[(3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromet...)Show SMILES C[C@@H](O[C@H]1CC[C@@H]2CN(C[C@H]2[C@@H]1c1ccc(F)cc1)C1=CC(=O)CC1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r,t:22| Show InChI InChI=1S/C29H28F7NO2/c1-16(19-10-20(28(31,32)33)12-21(11-19)29(34,35)36)39-26-9-4-18-14-37(23-7-8-24(38)13-23)15-25(18)27(26)17-2-5-22(30)6-3-17/h2-3,5-6,10-13,16,18,25-27H,4,7-9,14-15H2,1H3/t16-,18-,25-,26+,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C8 |
J Med Chem 52: 3039-46 (2009)
Article DOI: 10.1021/jm8016514
BindingDB Entry DOI: 10.7270/Q2FX79BT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50277511

(3-[(3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromet...)Show SMILES C[C@@H](O[C@H]1CC[C@@H]2CN(C[C@H]2[C@@H]1c1ccc(F)cc1)C1=CC(=O)CC1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r,t:22| Show InChI InChI=1S/C29H28F7NO2/c1-16(19-10-20(28(31,32)33)12-21(11-19)29(34,35)36)39-26-9-4-18-14-37(23-7-8-24(38)13-23)15-25(18)27(26)17-2-5-22(30)6-3-17/h2-3,5-6,10-13,16,18,25-27H,4,7-9,14-15H2,1H3/t16-,18-,25-,26+,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 |
J Med Chem 52: 3039-46 (2009)
Article DOI: 10.1021/jm8016514
BindingDB Entry DOI: 10.7270/Q2FX79BT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data