 Found 6309 hits with Last Name = 'kreutter' and Initial = 'kd'
Found 6309 hits with Last Name = 'kreutter' and Initial = 'kd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prothrombin
(Homo sapiens (Human)) | BDBM50123504
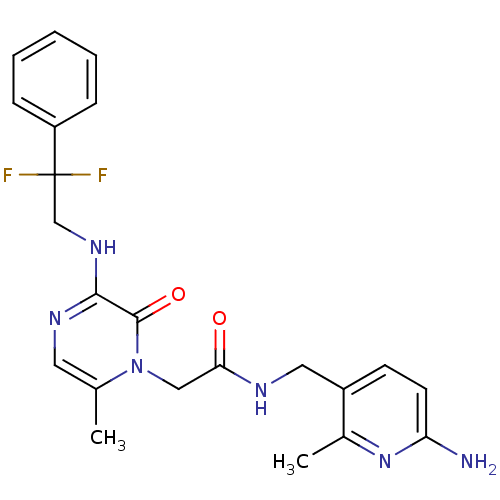
(CHEMBL142546 | N-((6-amino-2-methylpyridin-3-yl)me...)Show SMILES Cc1cnc(NCC(F)(F)c2ccccc2)c(=O)n1CC(=O)NCc1ccc(N)nc1C Show InChI InChI=1S/C22H24F2N6O2/c1-14-10-27-20(28-13-22(23,24)17-6-4-3-5-7-17)21(32)30(14)12-19(31)26-11-16-8-9-18(25)29-15(16)2/h3-10H,11-13H2,1-2H3,(H2,25,29)(H,26,31)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377618
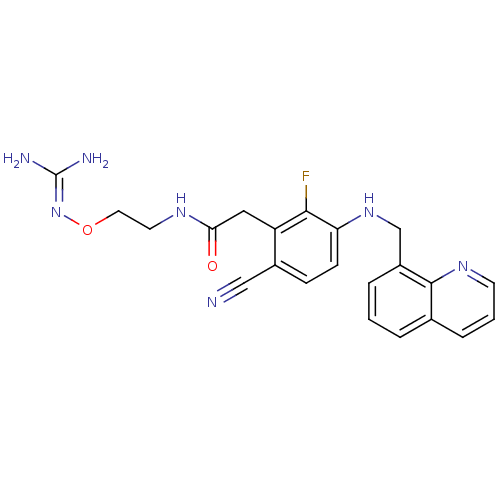
(CHEMBL254353)Show SMILES [#7]\[#6](-[#7])=[#7]\[#8]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-c1c(F)c(-[#7]-[#6]-c2cccc3cccnc23)ccc1C#N Show InChI InChI=1S/C22H22FN7O2/c23-20-17(11-19(31)27-9-10-32-30-22(25)26)15(12-24)6-7-18(20)29-13-16-4-1-3-14-5-2-8-28-21(14)16/h1-8,29H,9-11,13H2,(H,27,31)(H4,25,26,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377625
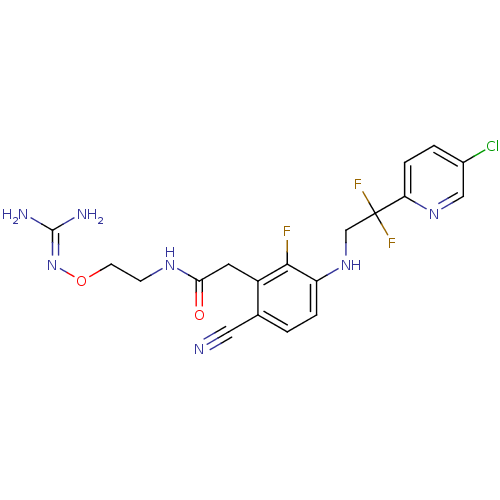
(CHEMBL254557)Show SMILES [#7]\[#6](-[#7])=[#7]\[#8]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-c1c(F)c(-[#7]-[#6]C(F)(F)c2ccc(Cl)cn2)ccc1C#N Show InChI InChI=1S/C19H19ClF3N7O2/c20-12-2-4-15(28-9-12)19(22,23)10-29-14-3-1-11(8-24)13(17(14)21)7-16(31)27-5-6-32-30-18(25)26/h1-4,9,29H,5-7,10H2,(H,27,31)(H4,25,26,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377615
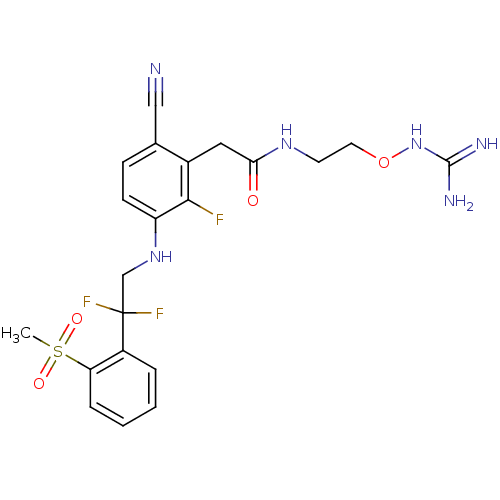
(CHEMBL254962)Show SMILES CS(=O)(=O)c1ccccc1C(F)(F)CNc1ccc(C#N)c(CC(=O)NCCONC(N)=N)c1F Show InChI InChI=1S/C21H23F3N6O4S/c1-35(32,33)17-5-3-2-4-15(17)21(23,24)12-29-16-7-6-13(11-25)14(19(16)22)10-18(31)28-8-9-34-30-20(26)27/h2-7,29H,8-10,12H2,1H3,(H,28,31)(H4,26,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377623
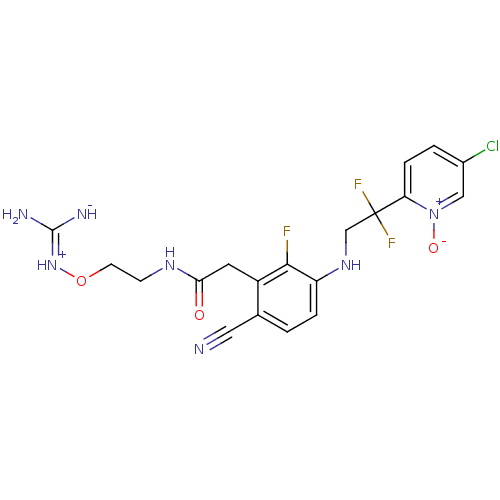
(CHEMBL254759)Show SMILES N\C([NH-])=[NH+]\OCCNC(=O)Cc1c(F)c(NCC(F)(F)c2ccc(Cl)c[n+]2[O-])ccc1C#N Show InChI InChI=1S/C19H19ClF3N7O3/c20-12-2-4-15(30(32)9-12)19(22,23)10-28-14-3-1-11(8-24)13(17(14)21)7-16(31)27-5-6-33-29-18(25)26/h1-4,9,28H,5-7,10H2,(H5,25,26,27,29,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50223074
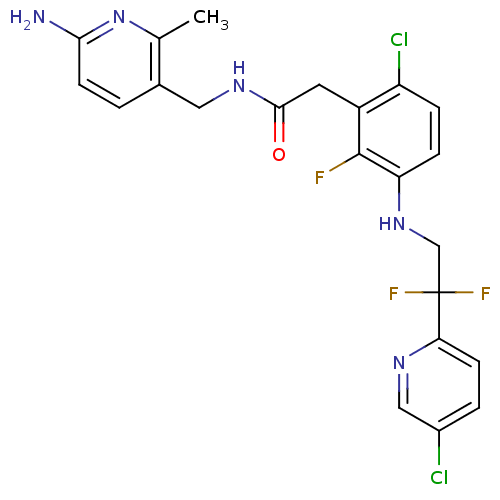
(CHEMBL250466 | N-((6-amino-2-methylpyridin-3-yl)me...)Show SMILES Cc1nc(N)ccc1CNC(=O)Cc1c(Cl)ccc(NCC(F)(F)c2ccc(Cl)cn2)c1F Show InChI InChI=1S/C22H20Cl2F3N5O/c1-12-13(2-7-19(28)32-12)9-30-20(33)8-15-16(24)4-5-17(21(15)25)31-11-22(26,27)18-6-3-14(23)10-29-18/h2-7,10,31H,8-9,11H2,1H3,(H2,28,32)(H,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin after 15 mins by standard chromogenic assay |
Bioorg Med Chem Lett 17: 6266-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.013
BindingDB Entry DOI: 10.7270/Q29G5MH9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377611
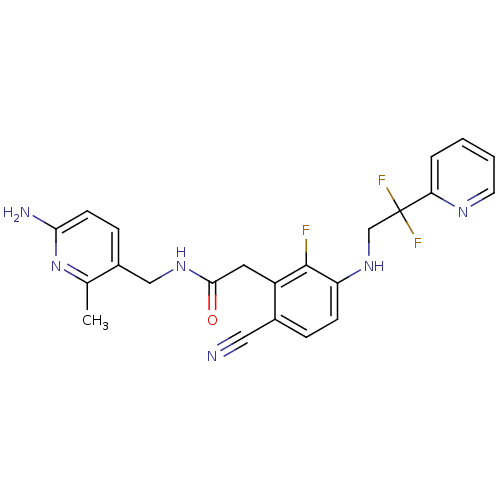
(CHEMBL258018)Show SMILES Cc1nc(N)ccc1CNC(=O)Cc1c(F)c(NCC(F)(F)c2ccccn2)ccc1C#N Show InChI InChI=1S/C23H21F3N6O/c1-14-16(6-8-20(28)32-14)12-30-21(33)10-17-15(11-27)5-7-18(22(17)24)31-13-23(25,26)19-4-2-3-9-29-19/h2-9,31H,10,12-13H2,1H3,(H2,28,32)(H,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377619
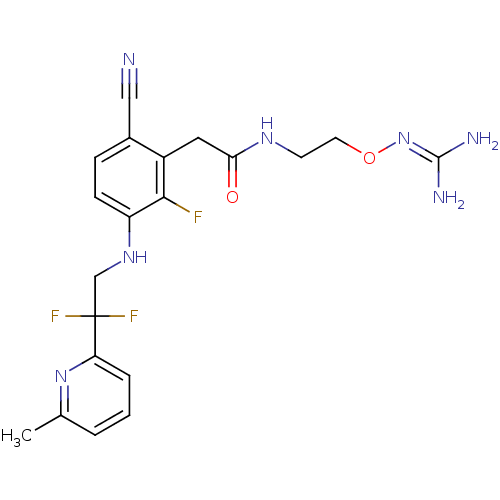
(CHEMBL402758)Show SMILES [#6]-c1cccc(n1)C(F)(F)[#6]-[#7]-c1ccc(C#N)c(-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#8]\[#7]=[#6](\[#7])-[#7])c1F Show InChI InChI=1S/C20H22F3N7O2/c1-12-3-2-4-16(29-12)20(22,23)11-28-15-6-5-13(10-24)14(18(15)21)9-17(31)27-7-8-32-30-19(25)26/h2-6,28H,7-9,11H2,1H3,(H,27,31)(H4,25,26,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50223067

(CHEMBL250651 | N-(6-amino-2-methyl-pyridin-3-ylmet...)Show SMILES Cc1nc(N)ccc1CNC(=O)Cc1c(Cl)ccc(NCC(F)(F)c2ccc(Cl)c[n+]2[O-])c1F Show InChI InChI=1S/C22H20Cl2F3N5O2/c1-12-13(2-7-19(28)31-12)9-29-20(33)8-15-16(24)4-5-17(21(15)25)30-11-22(26,27)18-6-3-14(23)10-32(18)34/h2-7,10,30H,8-9,11H2,1H3,(H2,28,31)(H,29,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin after 15 mins by standard chromogenic assay |
Bioorg Med Chem Lett 17: 6266-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.013
BindingDB Entry DOI: 10.7270/Q29G5MH9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377622
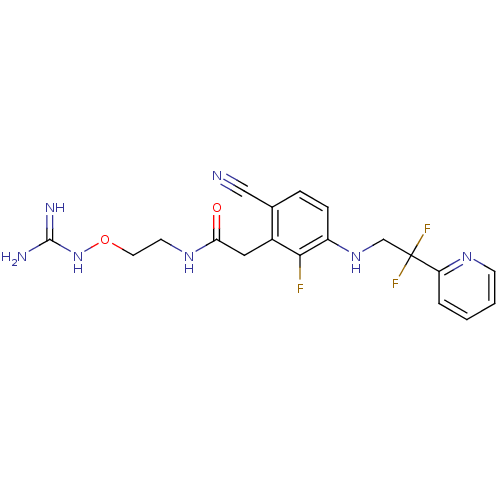
(CHEMBL257543)Show SMILES NC(=N)NOCCNC(=O)Cc1c(F)c(NCC(F)(F)c2ccccn2)ccc1C#N Show InChI InChI=1S/C19H20F3N7O2/c20-17-13(9-16(30)27-7-8-31-29-18(24)25)12(10-23)4-5-14(17)28-11-19(21,22)15-3-1-2-6-26-15/h1-6,28H,7-9,11H2,(H,27,30)(H4,24,25,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50377620
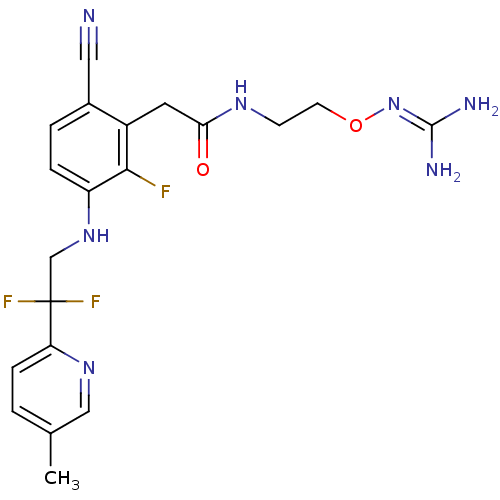
(CHEMBL254784)Show SMILES [#6]-c1ccc(nc1)C(F)(F)[#6]-[#7]-c1ccc(C#N)c(-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#8]\[#7]=[#6](\[#7])-[#7])c1F Show InChI InChI=1S/C20H22F3N7O2/c1-12-2-5-16(28-10-12)20(22,23)11-29-15-4-3-13(9-24)14(18(15)21)8-17(31)27-6-7-32-30-19(25)26/h2-5,10,29H,6-8,11H2,1H3,(H,27,31)(H4,25,26,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377614
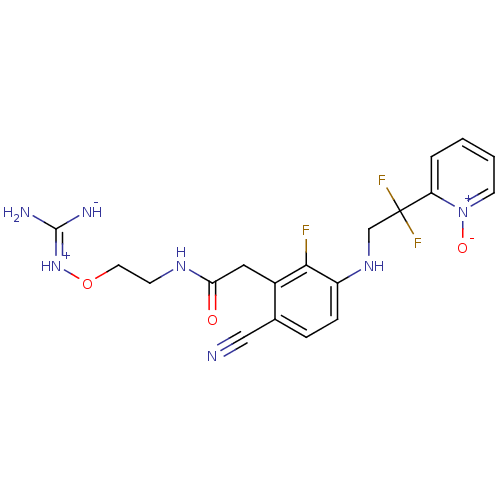
(CHEMBL401655)Show SMILES N\C([NH-])=[NH+]\OCCNC(=O)Cc1c(F)c(NCC(F)(F)c2cccc[n+]2[O-])ccc1C#N Show InChI InChI=1S/C19H20F3N7O3/c20-17-13(9-16(30)26-6-8-32-28-18(24)25)12(10-23)4-5-14(17)27-11-19(21,22)15-3-1-2-7-29(15)31/h1-5,7,27H,6,8-9,11H2,(H5,24,25,26,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377617
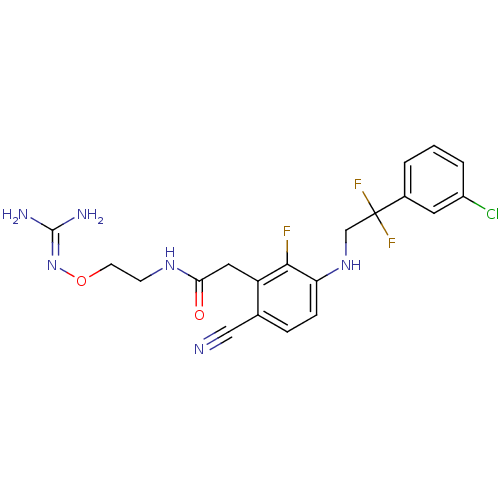
(CHEMBL403359)Show SMILES [#7]\[#6](-[#7])=[#7]\[#8]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-c1c(F)c(-[#7]-[#6]C(F)(F)c2cccc(Cl)c2)ccc1C#N Show InChI InChI=1S/C20H20ClF3N6O2/c21-14-3-1-2-13(8-14)20(23,24)11-29-16-5-4-12(10-25)15(18(16)22)9-17(31)28-6-7-32-30-19(26)27/h1-5,8,29H,6-7,9,11H2,(H,28,31)(H4,26,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377624
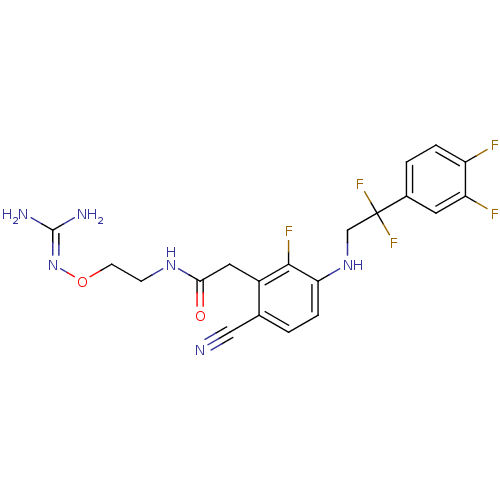
(CHEMBL403310)Show SMILES [#7]\[#6](-[#7])=[#7]\[#8]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-c1c(F)c(-[#7]-[#6]C(F)(F)c2ccc(F)c(F)c2)ccc1C#N Show InChI InChI=1S/C20H19F5N6O2/c21-14-3-2-12(7-15(14)22)20(24,25)10-30-16-4-1-11(9-26)13(18(16)23)8-17(32)29-5-6-33-31-19(27)28/h1-4,7,30H,5-6,8,10H2,(H,29,32)(H4,27,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50223066

(CHEMBL250650 | N-[2-({[amino(imino)methyl]amino}ox...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#8]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-c1c(Cl)ccc(-[#7]-[#6]C(F)(F)c2ccc(Cl)cn2)c1F Show InChI InChI=1S/C18H19Cl2F3N6O2/c19-10-1-4-14(27-8-10)18(22,23)9-28-13-3-2-12(20)11(16(13)21)7-15(30)26-5-6-31-29-17(24)25/h1-4,8,28H,5-7,9H2,(H,26,30)(H4,24,25,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin after 15 mins by standard chromogenic assay |
Bioorg Med Chem Lett 17: 6266-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.013
BindingDB Entry DOI: 10.7270/Q29G5MH9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377607
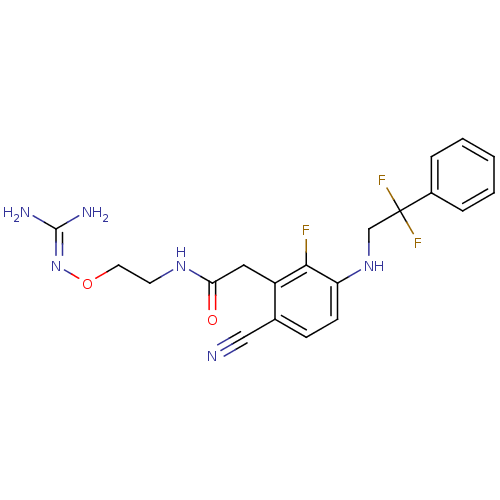
(CHEMBL404025)Show SMILES [#7]\[#6](-[#7])=[#7]\[#8]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-c1c(F)c(-[#7]-[#6]C(F)(F)c2ccccc2)ccc1C#N Show InChI InChI=1S/C20H21F3N6O2/c21-18-15(10-17(30)27-8-9-31-29-19(25)26)13(11-24)6-7-16(18)28-12-20(22,23)14-4-2-1-3-5-14/h1-7,28H,8-10,12H2,(H,27,30)(H4,25,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50223081
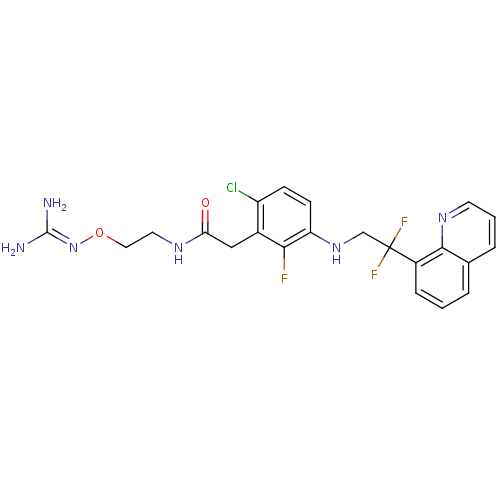
(CHEMBL250273 | N-[2-(carbamimidamidooxy)ethyl]-2-(...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#8]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-c1c(Cl)ccc(-[#7]-[#6]C(F)(F)c2cccc3cccnc23)c1F Show InChI InChI=1S/C22H22ClF3N6O2/c23-16-6-7-17(19(24)14(16)11-18(33)29-9-10-34-32-21(27)28)31-12-22(25,26)15-5-1-3-13-4-2-8-30-20(13)15/h1-8,31H,9-12H2,(H,29,33)(H4,27,28,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin after 15 mins by standard chromogenic assay |
Bioorg Med Chem Lett 17: 6266-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.013
BindingDB Entry DOI: 10.7270/Q29G5MH9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50223071
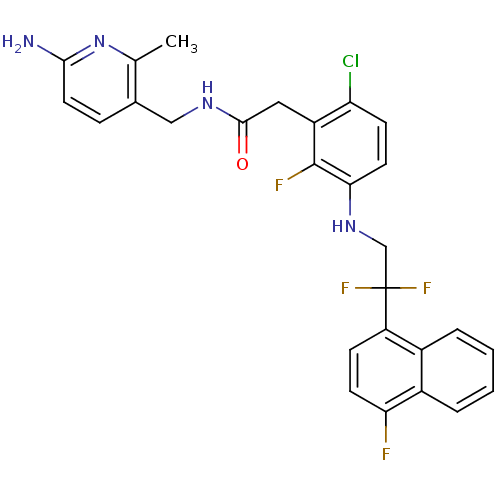
(CHEMBL399868 | N-((6-amino-2-methylpyridin-3-yl)me...)Show SMILES Cc1nc(N)ccc1CNC(=O)Cc1c(Cl)ccc(NCC(F)(F)c2ccc(F)c3ccccc23)c1F Show InChI InChI=1S/C27H23ClF4N4O/c1-15-16(6-11-24(33)36-15)13-34-25(37)12-19-21(28)8-10-23(26(19)30)35-14-27(31,32)20-7-9-22(29)18-5-3-2-4-17(18)20/h2-11,35H,12-14H2,1H3,(H2,33,36)(H,34,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin after 15 mins by standard chromogenic assay |
Bioorg Med Chem Lett 17: 6266-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.013
BindingDB Entry DOI: 10.7270/Q29G5MH9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377626
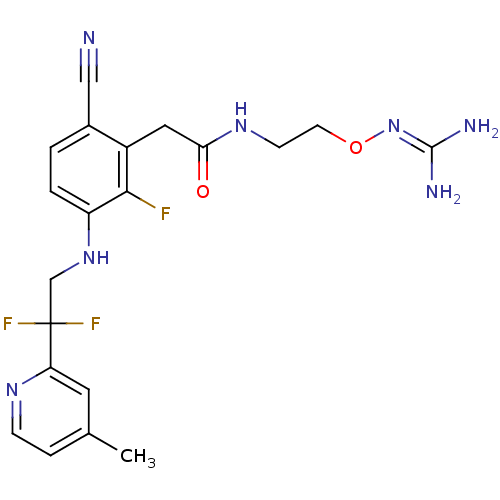
(CHEMBL254786)Show SMILES [#6]-c1ccnc(c1)C(F)(F)[#6]-[#7]-c1ccc(C#N)c(-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#8]\[#7]=[#6](\[#7])-[#7])c1F Show InChI InChI=1S/C20H22F3N7O2/c1-12-4-5-27-16(8-12)20(22,23)11-29-15-3-2-13(10-24)14(18(15)21)9-17(31)28-6-7-32-30-19(25)26/h2-5,8,29H,6-7,9,11H2,1H3,(H,28,31)(H4,25,26,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50223069

(CHEMBL250461 | N-[2-(carbamimidamidooxy)ethyl]-2-(...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#8]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-c1c(Cl)ccc(-[#7]-[#6]C(F)(F)c2ccc(F)c3ccccc23)c1F Show InChI InChI=1S/C23H22ClF4N5O2/c24-17-6-8-19(21(26)15(17)11-20(34)31-9-10-35-33-22(29)30)32-12-23(27,28)16-5-7-18(25)14-4-2-1-3-13(14)16/h1-8,32H,9-12H2,(H,31,34)(H4,29,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin after 15 mins by standard chromogenic assay |
Bioorg Med Chem Lett 17: 6266-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.013
BindingDB Entry DOI: 10.7270/Q29G5MH9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377616
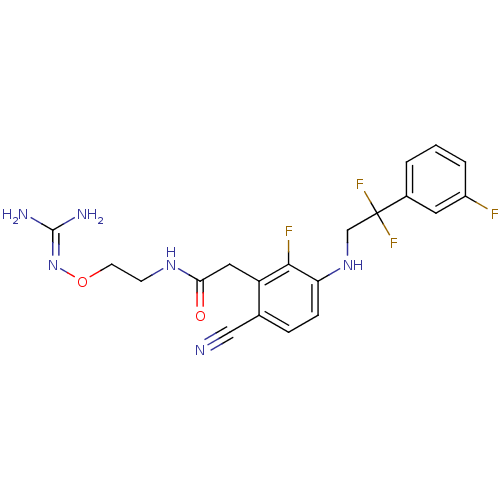
(CHEMBL258198)Show SMILES [#7]\[#6](-[#7])=[#7]\[#8]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-c1c(F)c(-[#7]-[#6]C(F)(F)c2cccc(F)c2)ccc1C#N Show InChI InChI=1S/C20H20F4N6O2/c21-14-3-1-2-13(8-14)20(23,24)11-29-16-5-4-12(10-25)15(18(16)22)9-17(31)28-6-7-32-30-19(26)27/h1-5,8,29H,6-7,9,11H2,(H,28,31)(H4,26,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50223078
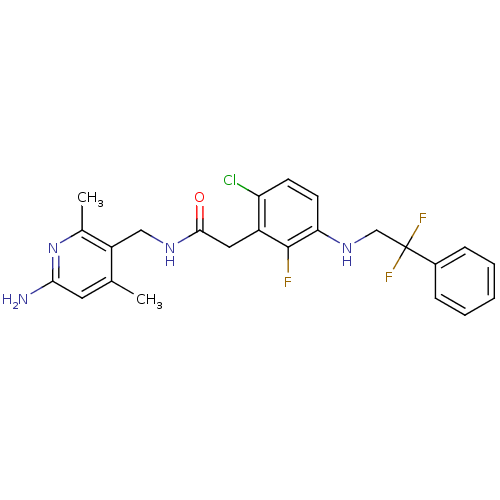
(CHEMBL250465 | N-((6-amino-2,4-dimethylpyridin-3-y...)Show SMILES Cc1cc(N)nc(C)c1CNC(=O)Cc1c(Cl)ccc(NCC(F)(F)c2ccccc2)c1F Show InChI InChI=1S/C24H24ClF3N4O/c1-14-10-21(29)32-15(2)18(14)12-30-22(33)11-17-19(25)8-9-20(23(17)26)31-13-24(27,28)16-6-4-3-5-7-16/h3-10,31H,11-13H2,1-2H3,(H2,29,32)(H,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin after 15 mins by standard chromogenic assay |
Bioorg Med Chem Lett 17: 6266-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.013
BindingDB Entry DOI: 10.7270/Q29G5MH9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50149023
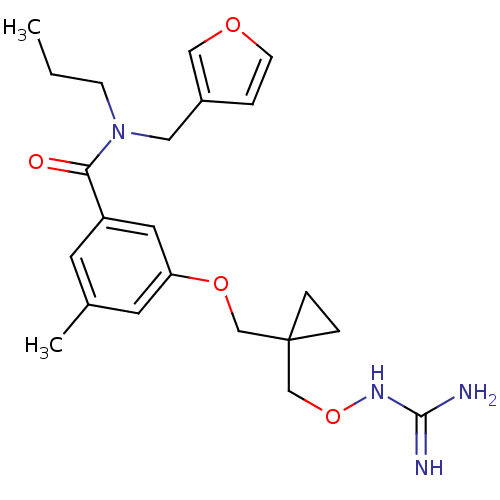
(3-{[1-({[(diaminomethylidene)amino]oxy}methyl)cycl...)Show SMILES CCCN(Cc1ccoc1)C(=O)c1cc(C)cc(OCC2(CONC(N)=N)CC2)c1 Show InChI InChI=1S/C22H30N4O4/c1-3-7-26(12-17-4-8-28-13-17)20(27)18-9-16(2)10-19(11-18)29-14-22(5-6-22)15-30-25-21(23)24/h4,8-11,13H,3,5-7,12,14-15H2,1-2H3,(H4,23,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377608
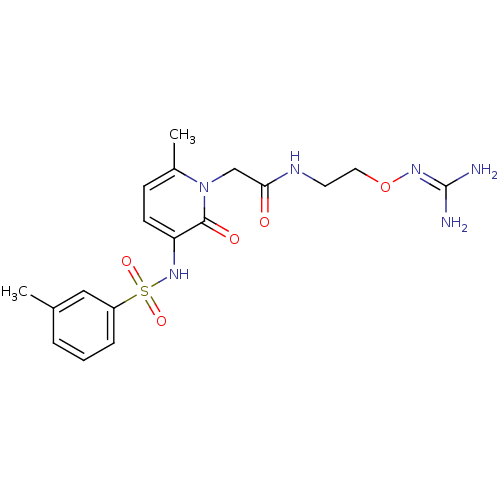
(CHEMBL256941)Show SMILES [#6]-c1cccc(c1)S(=O)(=O)[#7]-c1ccc(-[#6])n(-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#8]\[#7]=[#6](\[#7])-[#7])c1=O Show InChI InChI=1S/C18H24N6O5S/c1-12-4-3-5-14(10-12)30(27,28)23-15-7-6-13(2)24(17(15)26)11-16(25)21-8-9-29-22-18(19)20/h3-7,10,23H,8-9,11H2,1-2H3,(H,21,25)(H4,19,20,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50149023

(3-{[1-({[(diaminomethylidene)amino]oxy}methyl)cycl...)Show SMILES CCCN(Cc1ccoc1)C(=O)c1cc(C)cc(OCC2(CONC(N)=N)CC2)c1 Show InChI InChI=1S/C22H30N4O4/c1-3-7-26(12-17-4-8-28-13-17)20(27)18-9-16(2)10-19(11-18)29-14-22(5-6-22)15-30-25-21(23)24/h4,8-11,13H,3,5-7,12,14-15H2,1-2H3,(H4,23,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50223065
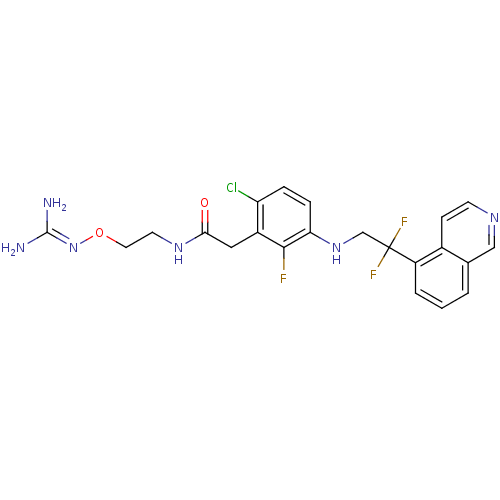
(CHEMBL250460 | N-[2-(carbamimidamidooxy)ethyl]-2-(...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#8]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-c1c(Cl)ccc(-[#7]-[#6]C(F)(F)c2cccc3cnccc23)c1F Show InChI InChI=1S/C22H22ClF3N6O2/c23-17-4-5-18(20(24)15(17)10-19(33)30-8-9-34-32-21(27)28)31-12-22(25,26)16-3-1-2-13-11-29-7-6-14(13)16/h1-7,11,31H,8-10,12H2,(H,30,33)(H4,27,28,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin after 15 mins by standard chromogenic assay |
Bioorg Med Chem Lett 17: 6266-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.013
BindingDB Entry DOI: 10.7270/Q29G5MH9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50223075
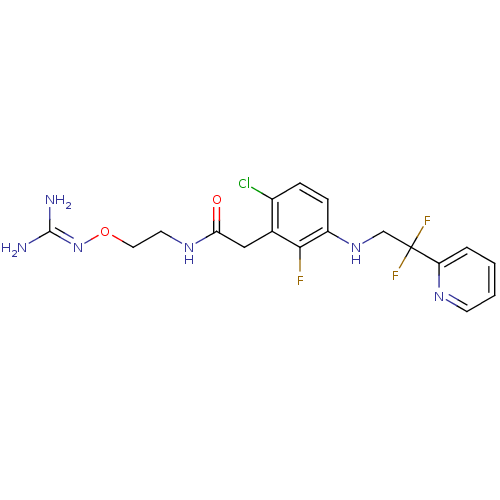
(CHEMBL250463 | N-[2-({[amino(imino)methyl]amino}ox...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#8]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-c1c(Cl)ccc(-[#7]-[#6]C(F)(F)c2ccccn2)c1F Show InChI InChI=1S/C18H20ClF3N6O2/c19-12-4-5-13(27-10-18(21,22)14-3-1-2-6-25-14)16(20)11(12)9-15(29)26-7-8-30-28-17(23)24/h1-6,27H,7-10H2,(H,26,29)(H4,23,24,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin after 15 mins by standard chromogenic assay |
Bioorg Med Chem Lett 17: 6266-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.013
BindingDB Entry DOI: 10.7270/Q29G5MH9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377613
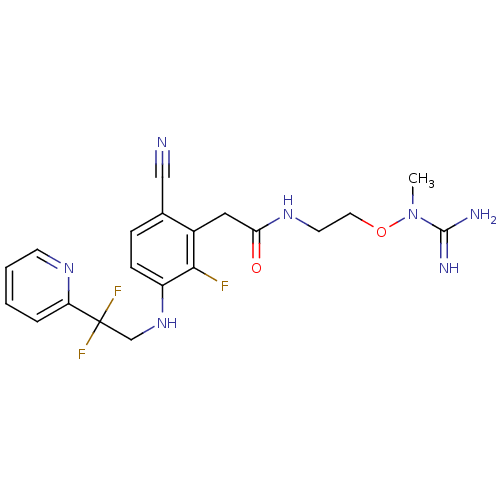
(CHEMBL258225)Show SMILES CN(OCCNC(=O)Cc1c(F)c(NCC(F)(F)c2ccccn2)ccc1C#N)C(N)=N Show InChI InChI=1S/C20H22F3N7O2/c1-30(19(25)26)32-9-8-28-17(31)10-14-13(11-24)5-6-15(18(14)21)29-12-20(22,23)16-4-2-3-7-27-16/h2-7,29H,8-10,12H2,1H3,(H3,25,26)(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM471715
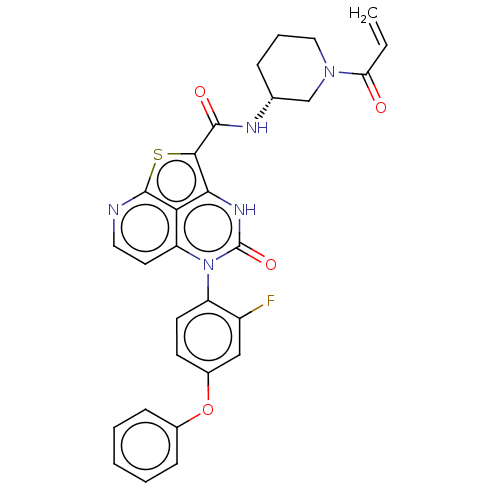
((R)-N-(1-Acryloylpiperidin-3-yl)-5-(2-fluoro-4-phe...)Show SMILES Fc1cc(Oc2ccccc2)ccc1-n1c2ccnc3sc(C(=O)N[C@@H]4CCCN(C4)C(=O)C=C)c([nH]c1=O)c23 |r,wU:25.26,(-1.78,4.35,;-3.12,3.58,;-4.45,4.35,;-5.78,3.58,;-7.12,4.35,;-8.45,3.58,;-9.78,4.35,;-11.12,3.58,;-11.12,2.04,;-9.78,1.27,;-8.45,2.04,;-5.78,2.04,;-4.45,1.27,;-3.12,2.04,;-1.78,1.27,;-1.78,-.27,;-3.12,-1.04,;-3.12,-2.58,;-1.78,-3.35,;-.45,-2.58,;1.04,-2.98,;1.88,-1.68,;3.42,-1.68,;4.19,-.35,;4.19,-3.02,;5.73,-3.02,;6.5,-4.35,;8.04,-4.35,;8.81,-3.02,;8.04,-1.68,;6.5,-1.68,;8.81,-.35,;8.04,.98,;10.35,-.35,;11.12,-1.68,;.89,-.27,;.89,1.27,;-.45,2.04,;-.45,3.58,;-.45,-1.04,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00044
BindingDB Entry DOI: 10.7270/Q25D8WM5 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377621
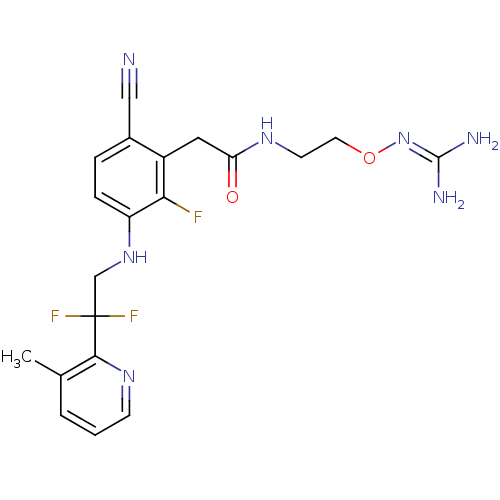
(CHEMBL254785)Show SMILES [#6]-c1cccnc1C(F)(F)[#6]-[#7]-c1ccc(C#N)c(-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#8]\[#7]=[#6](\[#7])-[#7])c1F Show InChI InChI=1S/C20H22F3N7O2/c1-12-3-2-6-28-18(12)20(22,23)11-29-15-5-4-13(10-24)14(17(15)21)9-16(31)27-7-8-32-30-19(25)26/h2-6,29H,7-9,11H2,1H3,(H,27,31)(H4,25,26,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50223076
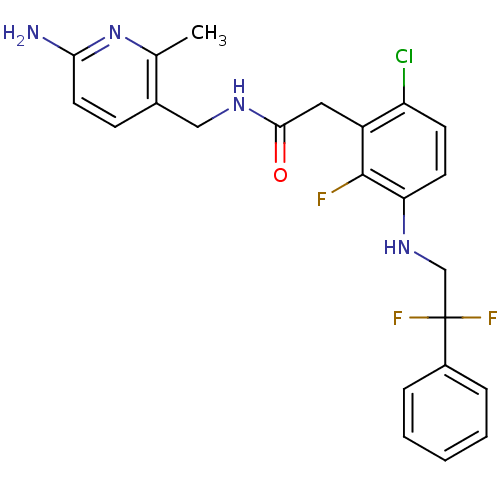
(CHEMBL399662 | N-((6-amino-2-methylpyridin-3-yl)me...)Show SMILES Cc1nc(N)ccc1CNC(=O)Cc1c(Cl)ccc(NCC(F)(F)c2ccccc2)c1F Show InChI InChI=1S/C23H22ClF3N4O/c1-14-15(7-10-20(28)31-14)12-29-21(32)11-17-18(24)8-9-19(22(17)25)30-13-23(26,27)16-5-3-2-4-6-16/h2-10,30H,11-13H2,1H3,(H2,28,31)(H,29,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin after 15 mins by standard chromogenic assay |
Bioorg Med Chem Lett 17: 6266-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.013
BindingDB Entry DOI: 10.7270/Q29G5MH9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377612
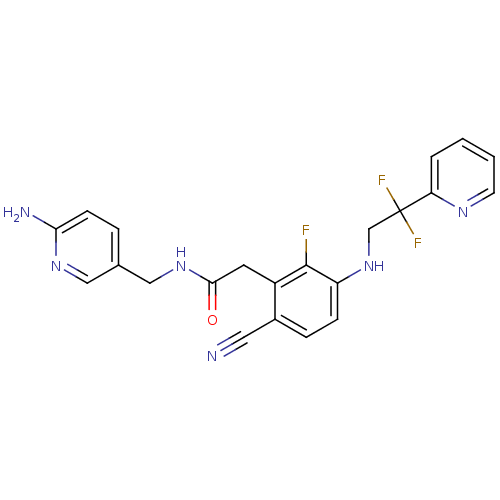
(CHEMBL255916)Show SMILES Nc1ccc(CNC(=O)Cc2c(F)c(NCC(F)(F)c3ccccn3)ccc2C#N)cn1 Show InChI InChI=1S/C22H19F3N6O/c23-21-16(9-20(32)30-12-14-4-7-19(27)29-11-14)15(10-26)5-6-17(21)31-13-22(24,25)18-3-1-2-8-28-18/h1-8,11,31H,9,12-13H2,(H2,27,29)(H,30,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM467364
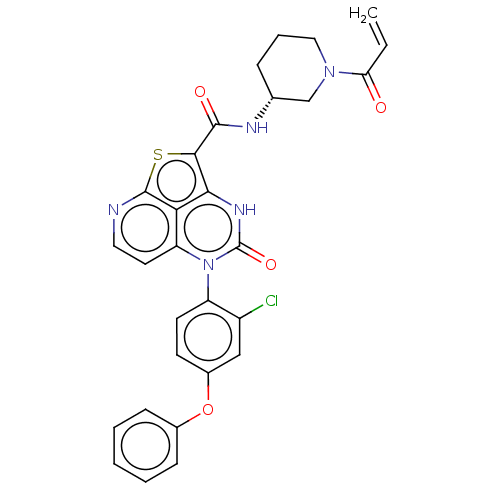
((R)-N-(1-Acryloylpiperidin-3-yl)-5-(2-chloro-4-phe...)Show SMILES Clc1cc(Oc2ccccc2)ccc1-n1c2ccnc3sc(C(=O)N[C@@H]4CCCN(C4)C(=O)C=C)c([nH]c1=O)c23 |r,wU:25.26,(-1.95,3.85,;-3.29,3.08,;-4.62,3.85,;-5.95,3.08,;-7.29,3.85,;-8.62,3.08,;-9.95,3.85,;-11.29,3.08,;-11.29,1.54,;-9.95,.77,;-8.62,1.54,;-5.95,1.54,;-4.62,.77,;-3.29,1.54,;-1.95,.77,;-1.95,-.77,;-3.29,-1.54,;-3.29,-3.08,;-1.95,-3.85,;-.62,-3.08,;.72,-3.85,;2.05,-2.07,;3.59,-2.07,;4.36,-3.41,;4.36,-.74,;5.9,-.74,;6.67,-2.07,;8.21,-2.07,;8.98,-.74,;8.21,.59,;6.67,.59,;8.98,1.93,;8.21,3.26,;10.52,1.93,;11.29,.59,;.72,-.77,;.72,.77,;-.62,1.54,;-.62,3.08,;-.62,-1.54,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00044
BindingDB Entry DOI: 10.7270/Q25D8WM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50569791

(CHEMBL4846828)Show SMILES Cc1cc(Oc2ccccc2)ccc1-n1c2ccnc3sc(C(=O)N[C@@H]4CC[C@@H](CC(=O)C=C)C4)c([nH]c1=O)c23 |r,wU:28.30,wD:25.26,(55.67,-29.88,;55.67,-28.34,;54.33,-27.57,;54.33,-26.03,;53,-25.26,;53,-23.72,;54.34,-22.95,;54.34,-21.41,;53,-20.64,;51.67,-21.42,;51.67,-22.95,;55.66,-25.25,;57,-26.02,;57,-27.57,;58.33,-28.34,;58.34,-29.88,;57.02,-30.64,;57.01,-32.16,;58.34,-32.93,;59.66,-32.16,;61.52,-32.32,;62.15,-30.91,;63.65,-30.59,;64.68,-31.74,;64.13,-29.13,;65.64,-28.81,;66.26,-27.4,;67.79,-27.56,;68.11,-29.07,;69.52,-29.7,;70.76,-28.79,;70.61,-27.26,;72.17,-29.42,;73.42,-28.52,;66.78,-29.84,;61,-29.88,;61,-28.34,;59.66,-27.56,;59.66,-26.02,;59.67,-30.65,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00044
BindingDB Entry DOI: 10.7270/Q25D8WM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM485273
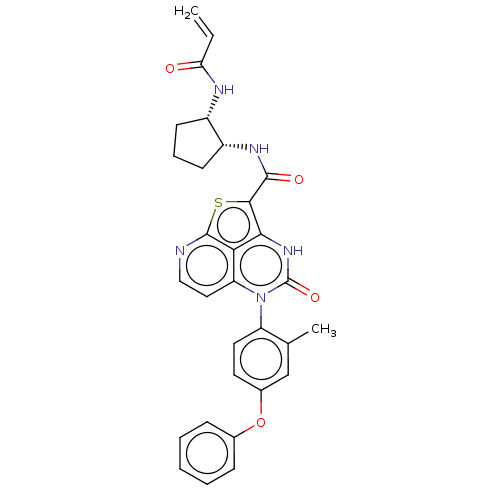
(N-((1R,2S)-2-Acrylamidocyclopentyl)-5-(*S)-(2-meth...)Show SMILES Cc1cc(Oc2ccccc2)ccc1-n1c2ccnc3sc(C(=O)N[C@@H]4CCC[C@@H]4NC(=O)C=C)c([nH]c1=O)c23 |r,wU:25.26,29.32,(-.48,2.9,;-1.82,2.13,;-3.15,2.9,;-4.48,2.13,;-5.82,2.9,;-7.15,2.13,;-8.49,2.9,;-9.82,2.13,;-9.82,.59,;-8.49,-.18,;-7.15,.59,;-4.48,.59,;-3.15,-.18,;-1.82,.59,;-.48,-.18,;-.48,-1.72,;-1.82,-2.49,;-1.82,-4.03,;-.48,-4.8,;.85,-4.03,;2.3,-4.48,;3,-3,;4.54,-3,;5.31,-4.33,;5.31,-1.66,;6.85,-1.66,;7.76,-2.91,;9.22,-2.43,;9.22,-.89,;7.76,-.42,;7.28,1.05,;8.31,2.19,;9.82,1.87,;7.84,3.66,;8.87,4.8,;2.18,-1.72,;2.18,-.18,;.85,.59,;.85,2.13,;.85,-2.49,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00044
BindingDB Entry DOI: 10.7270/Q25D8WM5 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377609
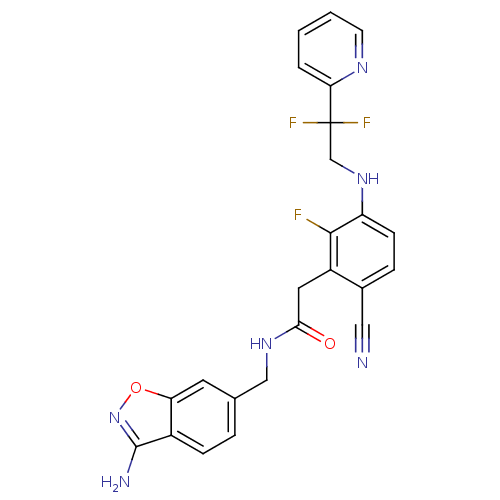
(CHEMBL256091)Show SMILES Nc1noc2cc(CNC(=O)Cc3c(F)c(NCC(F)(F)c4ccccn4)ccc3C#N)ccc12 Show InChI InChI=1S/C24H19F3N6O2/c25-22-17(10-21(34)31-12-14-4-6-16-19(9-14)35-33-23(16)29)15(11-28)5-7-18(22)32-13-24(26,27)20-3-1-2-8-30-20/h1-9,32H,10,12-13H2,(H2,29,33)(H,31,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM468103
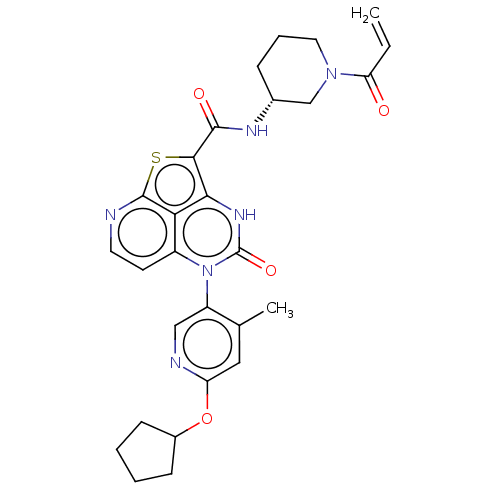
((R)-N-(1-Acryloylpiperidin-3-yl)-5-(*S)-(6-(cyclop...)Show SMILES Cc1cc(OC2CCCC2)ncc1-n1c2ccnc3sc(C(=O)N[C@@H]4CCCN(C4)C(=O)C=C)c([nH]c1=O)c23 |r,wD:24.25,(-1.92,5.75,;-3.25,4.98,;-4.59,5.75,;-5.92,4.98,;-7.25,5.75,;-8.59,4.98,;-9.99,5.6,;-11.02,4.46,;-10.25,3.13,;-8.75,3.45,;-5.92,3.44,;-4.59,2.67,;-3.25,3.44,;-1.92,2.67,;-1.92,1.13,;-3.25,.36,;-3.25,-1.18,;-1.92,-1.95,;-.59,-1.18,;.88,-1.66,;1.78,-.41,;3.32,-.41,;4.09,.92,;4.09,-1.75,;5.63,-1.75,;6.4,-.41,;7.94,-.41,;8.71,-1.75,;7.94,-3.08,;6.4,-3.08,;8.71,-4.41,;7.94,-5.75,;10.25,-4.41,;11.02,-5.75,;.75,1.13,;.75,2.67,;-.59,3.44,;-.59,4.98,;-.59,.36,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01026
BindingDB Entry DOI: 10.7270/Q2MK6HZ3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM468000
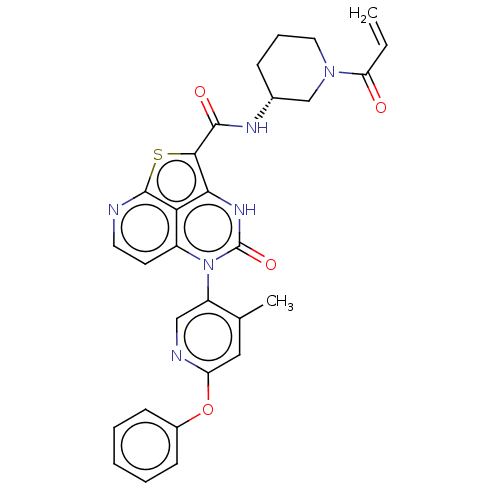
((R)-N-(1-Acryloylpiperidin-3-yl)-5-(*S)-(4-methyl-...)Show SMILES Cc1cc(Oc2ccccc2)ncc1-n1c2ccnc3sc(C(=O)N[C@@H]4CCCN(C4)C(=O)C=C)c([nH]c1=O)c23 |r,wU:25.26,(-4.48,-.77,;-4.48,.77,;-5.82,1.54,;-5.82,3.08,;-7.15,3.85,;-8.48,3.08,;-9.82,3.85,;-11.15,3.08,;-11.15,1.54,;-9.82,.77,;-8.48,1.54,;-4.48,3.85,;-3.15,3.08,;-3.15,1.54,;-1.82,.77,;-1.82,-.77,;-3.15,-1.54,;-3.15,-3.08,;-1.82,-3.85,;-.48,-3.08,;1.01,-3.48,;1.94,-1.86,;3.48,-1.86,;4.25,-3.19,;4.25,-.53,;5.79,-.53,;6.56,-1.86,;8.1,-1.86,;8.87,-.53,;8.1,.81,;6.56,.81,;8.87,2.14,;8.1,3.48,;10.41,2.14,;11.15,3.43,;.85,-.77,;.85,.77,;-.48,1.54,;-.48,3.08,;-.48,-1.54,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01026
BindingDB Entry DOI: 10.7270/Q2MK6HZ3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM467718
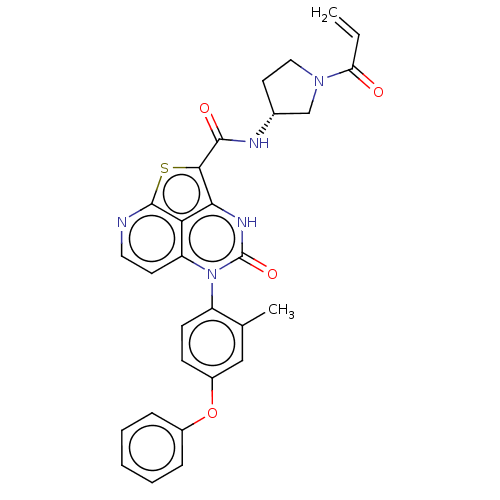
((R)-N-(1-Acryloylpyrrolidin-3-yl)-5-(2-methyl-4-(2...)Show SMILES Cc1cc(Oc2ccccc2)ccc1-n1c2ccnc3sc(C(=O)N[C@@H]4CCN(C4)C(=O)C=C)c([nH]c1=O)c23 |r,wD:25.26,(-1,4.5,;-2.28,3.77,;-3.62,4.54,;-4.95,3.77,;-6.28,4.54,;-7.62,3.77,;-8.95,4.54,;-10.28,3.77,;-10.28,2.23,;-8.95,1.46,;-7.62,2.23,;-4.95,2.23,;-3.62,1.46,;-2.28,2.23,;-.95,1.46,;-.95,-.08,;-2.28,-.85,;-2.28,-2.39,;-.95,-3.16,;.39,-2.39,;1.85,-2.87,;2.76,-1.62,;4.29,-1.65,;5.09,-.33,;5.04,-3,;6.58,-3,;7.49,-1.75,;8.95,-2.23,;8.95,-3.77,;7.49,-4.24,;10.28,-4.54,;10.28,-6.08,;11.62,-3.77,;11.62,-2.23,;1.72,-.08,;1.72,1.46,;.39,2.23,;.39,3.77,;.39,-.85,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01026
BindingDB Entry DOI: 10.7270/Q2MK6HZ3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM467718

((R)-N-(1-Acryloylpyrrolidin-3-yl)-5-(2-methyl-4-(2...)Show SMILES Cc1cc(Oc2ccccc2)ccc1-n1c2ccnc3sc(C(=O)N[C@@H]4CCN(C4)C(=O)C=C)c([nH]c1=O)c23 |r,wD:25.26,(-1,4.5,;-2.28,3.77,;-3.62,4.54,;-4.95,3.77,;-6.28,4.54,;-7.62,3.77,;-8.95,4.54,;-10.28,3.77,;-10.28,2.23,;-8.95,1.46,;-7.62,2.23,;-4.95,2.23,;-3.62,1.46,;-2.28,2.23,;-.95,1.46,;-.95,-.08,;-2.28,-.85,;-2.28,-2.39,;-.95,-3.16,;.39,-2.39,;1.85,-2.87,;2.76,-1.62,;4.29,-1.65,;5.09,-.33,;5.04,-3,;6.58,-3,;7.49,-1.75,;8.95,-2.23,;8.95,-3.77,;7.49,-4.24,;10.28,-4.54,;10.28,-6.08,;11.62,-3.77,;11.62,-2.23,;1.72,-.08,;1.72,1.46,;.39,2.23,;.39,3.77,;.39,-.85,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00044
BindingDB Entry DOI: 10.7270/Q25D8WM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM467367
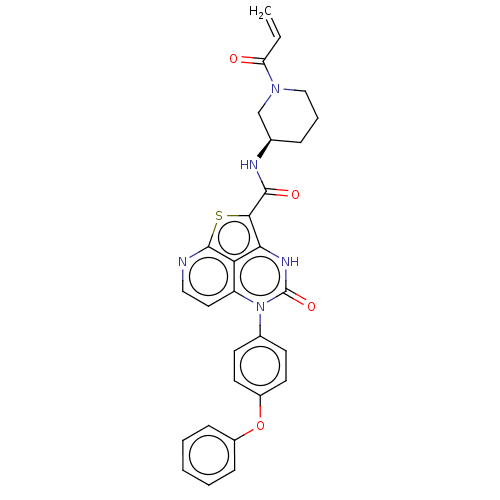
((R)-N-(1-Acryloylpiperidin-3-yl)-4-oxo-5-(4-phenox...)Show SMILES C=CC(=O)N1CCC[C@H](C1)NC(=O)c1sc2nccc3n(-c4ccc(Oc5ccccc5)cc4)c(=O)[nH]c1c23 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01026
BindingDB Entry DOI: 10.7270/Q2MK6HZ3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377610
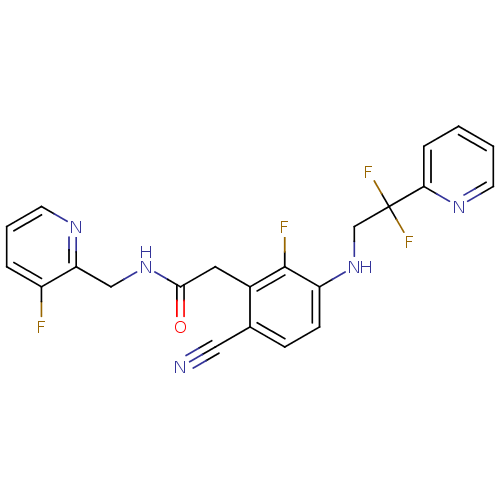
(CHEMBL250551)Show SMILES Fc1cccnc1CNC(=O)Cc1c(F)c(NCC(F)(F)c2ccccn2)ccc1C#N Show InChI InChI=1S/C22H17F4N5O/c23-16-4-3-9-28-18(16)12-30-20(32)10-15-14(11-27)6-7-17(21(15)24)31-13-22(25,26)19-5-1-2-8-29-19/h1-9,31H,10,12-13H2,(H,30,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50223077
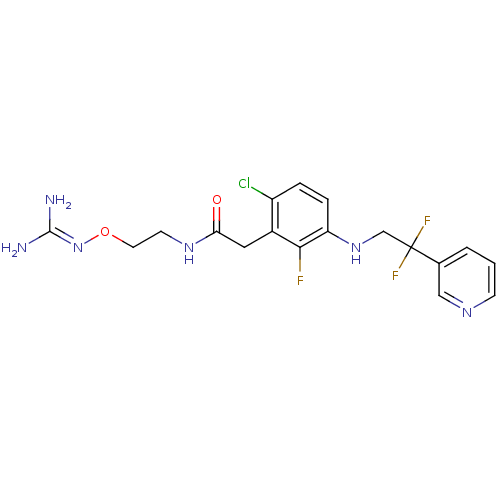
(CHEMBL250271 | N-[2-({[amino(imino)methyl]amino}ox...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#8]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-c1c(Cl)ccc(-[#7]-[#6]C(F)(F)c2cccnc2)c1F Show InChI InChI=1S/C18H20ClF3N6O2/c19-13-3-4-14(27-10-18(21,22)11-2-1-5-25-9-11)16(20)12(13)8-15(29)26-6-7-30-28-17(23)24/h1-5,9,27H,6-8,10H2,(H,26,29)(H4,23,24,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin after 15 mins by standard chromogenic assay |
Bioorg Med Chem Lett 17: 6266-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.013
BindingDB Entry DOI: 10.7270/Q29G5MH9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50223072
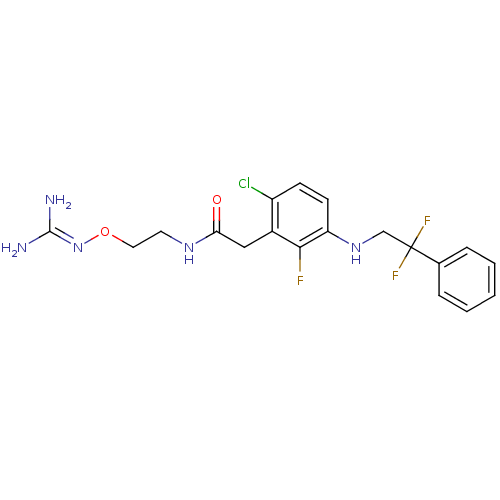
(CHEMBL401842 | N-[2-(carbamimidamidooxy)ethyl]-2-{...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#8]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-c1c(Cl)ccc(-[#7]-[#6]C(F)(F)c2ccccc2)c1F Show InChI InChI=1S/C19H21ClF3N5O2/c20-14-6-7-15(27-11-19(22,23)12-4-2-1-3-5-12)17(21)13(14)10-16(29)26-8-9-30-28-18(24)25/h1-7,27H,8-11H2,(H,26,29)(H4,24,25,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin after 15 mins by standard chromogenic assay |
Bioorg Med Chem Lett 17: 6266-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.013
BindingDB Entry DOI: 10.7270/Q29G5MH9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50223072

(CHEMBL401842 | N-[2-(carbamimidamidooxy)ethyl]-2-{...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#8]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-c1c(Cl)ccc(-[#7]-[#6]C(F)(F)c2ccccc2)c1F Show InChI InChI=1S/C19H21ClF3N5O2/c20-14-6-7-15(27-11-19(22,23)12-4-2-1-3-5-12)17(21)13(14)10-16(29)26-8-9-30-28-18(24)25/h1-7,27H,8-11H2,(H,26,29)(H4,24,25,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50601991
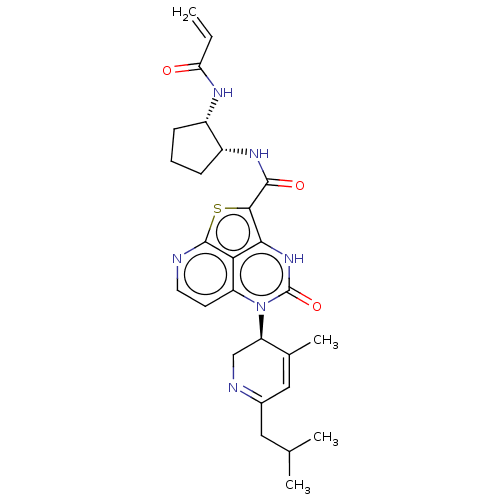
(CHEMBL5206366)Show SMILES CC(C)CC1=NC[C@H](C(C)=C1)n1c2ccnc3sc(C(=O)N[C@@H]4CCC[C@@H]4NC(=O)C=C)c([nH]c1=O)c23 |r,c:9,t:4| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01026
BindingDB Entry DOI: 10.7270/Q2MK6HZ3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50569790
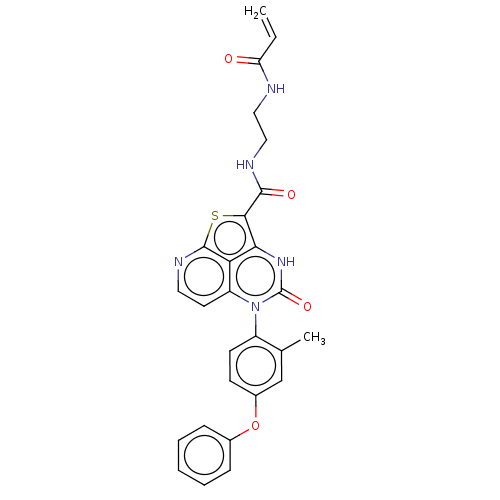
(CHEMBL4852459)Show SMILES Cc1cc(Oc2ccccc2)ccc1-n1c2ccnc3sc(C(=O)NCCNC(=O)C=C)c([nH]c1=O)c23 |(6.38,-11.63,;6.38,-10.09,;5.04,-9.32,;5.05,-7.78,;3.71,-7.01,;3.71,-5.47,;5.05,-4.7,;5.05,-3.16,;3.71,-2.39,;2.38,-3.17,;2.38,-4.71,;6.37,-7.01,;7.71,-7.77,;7.71,-9.32,;9.05,-10.09,;9.05,-11.63,;7.73,-12.39,;7.73,-13.92,;9.06,-14.68,;10.38,-13.91,;12.23,-14.07,;12.86,-12.67,;14.36,-12.35,;15.39,-13.49,;14.84,-10.88,;16.35,-10.57,;16.83,-9.1,;18.33,-8.78,;18.81,-7.32,;17.78,-6.17,;20.32,-7,;20.79,-5.54,;11.71,-11.63,;11.71,-10.09,;10.37,-9.31,;10.37,-7.77,;10.38,-12.4,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00044
BindingDB Entry DOI: 10.7270/Q25D8WM5 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50223073

(CHEMBL428413 | N-[2-(carbamimidamidooxy)ethyl]-2-[...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#8]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-c1c(Cl)ccc(-[#7]-[#6]C2([#6]-[#6]2)c2ccccn2)c1F Show InChI InChI=1S/C20H24ClFN6O2/c21-14-4-5-15(27-12-20(6-7-20)16-3-1-2-8-25-16)18(22)13(14)11-17(29)26-9-10-30-28-19(23)24/h1-5,8,27H,6-7,9-12H2,(H,26,29)(H4,23,24,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin after 15 mins by standard chromogenic assay |
Bioorg Med Chem Lett 17: 6266-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.013
BindingDB Entry DOI: 10.7270/Q29G5MH9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50223068

(CHEMBL250274 | N-[2-(carbamimidamidooxy)ethyl]-2-(...)Show SMILES [#6]C([#6])([#6]-[#7]-c1ccc(Cl)c(-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#8]\[#7]=[#6](\[#7])-[#7])c1F)c1ccccn1 Show InChI InChI=1S/C20H26ClFN6O2/c1-20(2,16-5-3-4-8-25-16)12-27-15-7-6-14(21)13(18(15)22)11-17(29)26-9-10-30-28-19(23)24/h3-8,27H,9-12H2,1-2H3,(H,26,29)(H4,23,24,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin after 15 mins by standard chromogenic assay |
Bioorg Med Chem Lett 17: 6266-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.013
BindingDB Entry DOI: 10.7270/Q29G5MH9 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM468010

((R)-N-(1-Acryloylpiperidin-3-yl)-5-(6-cyclobutoxy-...)Show SMILES Cc1cc(OC2CCC2)ncc1-n1c2ccnc3sc(C(=O)N[C@@H]4CCCN(C4)C(=O)C=C)c([nH]c1=O)c23 |r,wD:23.24,(-1.8,6.31,;-3.13,5.54,;-4.47,6.31,;-5.8,5.54,;-7.14,6.31,;-8.47,5.54,;-9.96,5.93,;-10.36,4.45,;-8.87,4.05,;-5.8,4,;-4.47,3.23,;-3.13,4,;-1.8,3.23,;-1.8,1.69,;-3.13,.92,;-3.13,-.62,;-1.8,-1.39,;-.47,-.62,;1.14,-.89,;1.88,.36,;3.42,.36,;4.19,1.7,;4.19,-.97,;5.73,-.97,;6.5,.36,;8.04,.36,;8.82,-.97,;8.04,-2.3,;6.5,-2.3,;8.82,-3.64,;10.36,-3.64,;8.04,-4.97,;8.82,-6.31,;.87,1.69,;.87,3.23,;-.47,4,;-.47,5.54,;-.47,.92,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01026
BindingDB Entry DOI: 10.7270/Q2MK6HZ3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data