

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Lactobacillus casei) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition constant of compound against L. casei Dihydrofolate reductase | J Med Chem 27: 1672-6 (1985) BindingDB Entry DOI: 10.7270/Q2G73CQG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50225252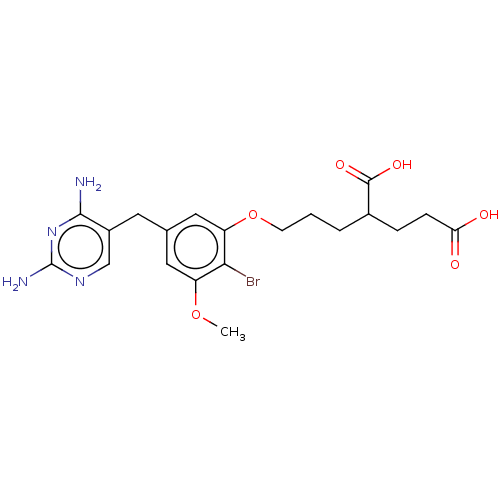 (CHEMBL296525) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition constant of compound against Lactobacillus casei Dihydrofolate reductase | J Med Chem 27: 1672-6 (1985) BindingDB Entry DOI: 10.7270/Q2G73CQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50027975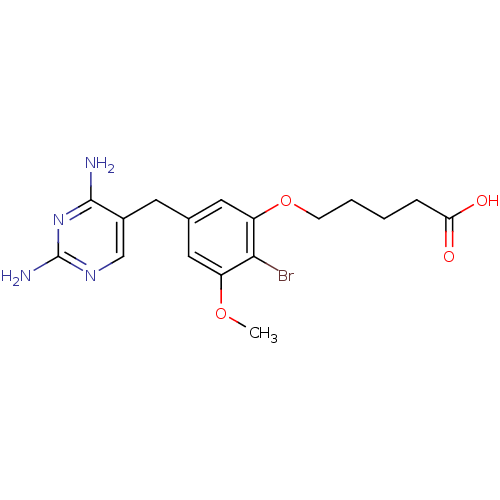 (5-[2-Bromo-5-(2,4-diamino-pyrimidin-5-ylmethyl)-3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition constant of compound against L. casei Dihydrofolate reductase | J Med Chem 27: 1672-6 (1985) BindingDB Entry DOI: 10.7270/Q2G73CQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50027973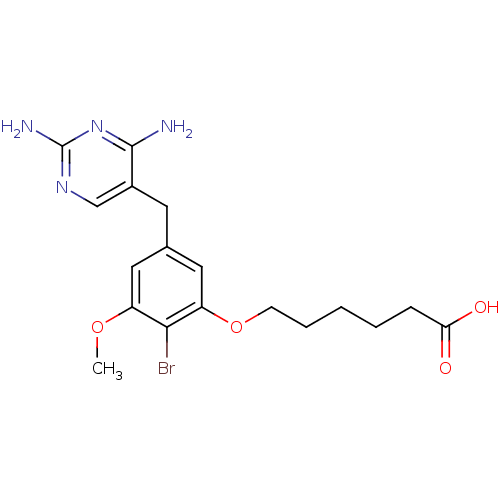 (6-[2-Bromo-5-(2,4-diamino-pyrimidin-5-ylmethyl)-3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition constant of compound against L. casei Dihydrofolate reductase | J Med Chem 27: 1672-6 (1985) BindingDB Entry DOI: 10.7270/Q2G73CQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50027972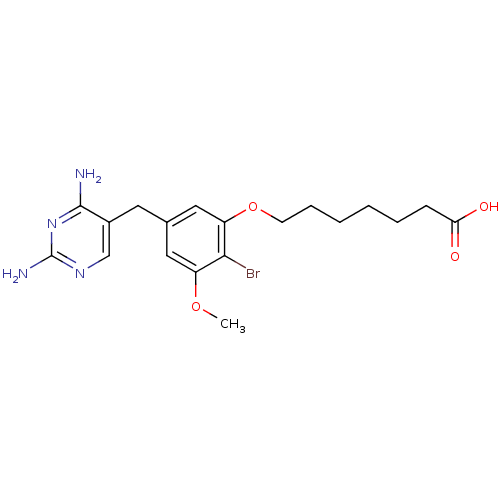 (7-[2-Bromo-5-(2,4-diamino-pyrimidin-5-ylmethyl)-3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition constant of compound against L. casei Dihydrofolate reductase | J Med Chem 27: 1672-6 (1985) BindingDB Entry DOI: 10.7270/Q2G73CQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50027971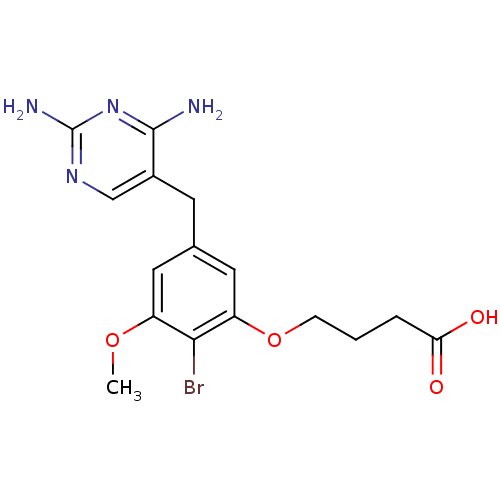 (4-[2-Bromo-5-(2,4-diamino-pyrimidin-5-ylmethyl)-3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition constant of compound against L. casei Dihydrofolate reductase | J Med Chem 27: 1672-6 (1985) BindingDB Entry DOI: 10.7270/Q2G73CQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50027977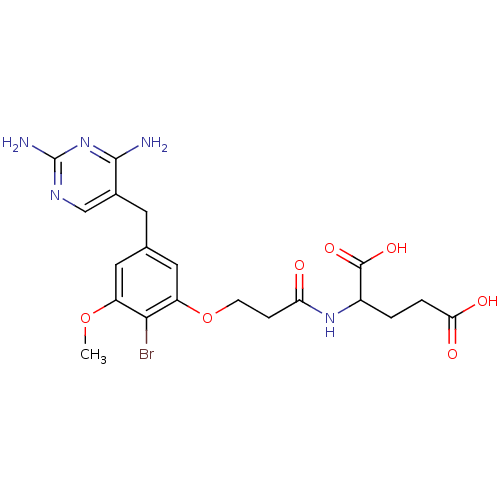 (2-{3-[2-Bromo-5-(2,4-diamino-pyrimidin-5-ylmethyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition constant of compound against L. casei Dihydrofolate reductase | J Med Chem 27: 1672-6 (1985) BindingDB Entry DOI: 10.7270/Q2G73CQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50027976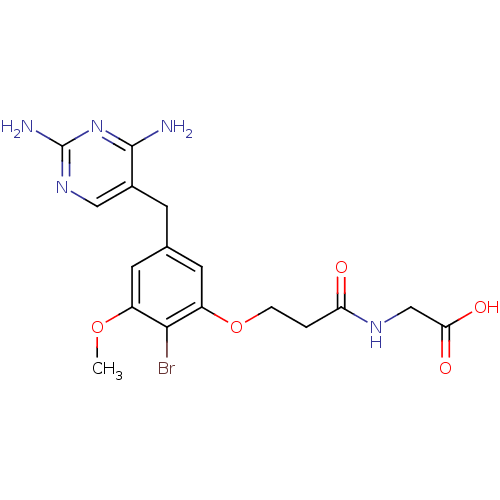 (CHEMBL45202 | {3-[2-Bromo-5-(2,4-diamino-pyrimidin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition constant of compound against L. casei Dihydrofolate reductase | J Med Chem 27: 1672-6 (1985) BindingDB Entry DOI: 10.7270/Q2G73CQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50027974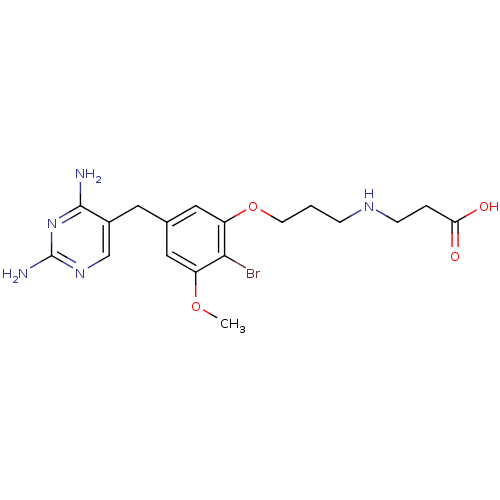 (3-{3-[2-Bromo-5-(2,4-diamino-pyrimidin-5-ylmethyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition constant of compound against L. casei dihydrofolate reductase | J Med Chem 27: 1672-6 (1985) BindingDB Entry DOI: 10.7270/Q2G73CQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50027970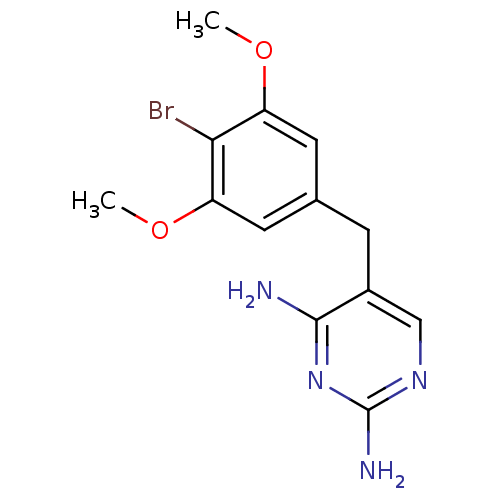 (5-(4-Bromo-3,5-dimethoxy-benzyl)-pyrimidine-2,4-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition constant of compound against L. casei dihydrofolate reductase | J Med Chem 27: 1672-6 (1985) BindingDB Entry DOI: 10.7270/Q2G73CQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50112960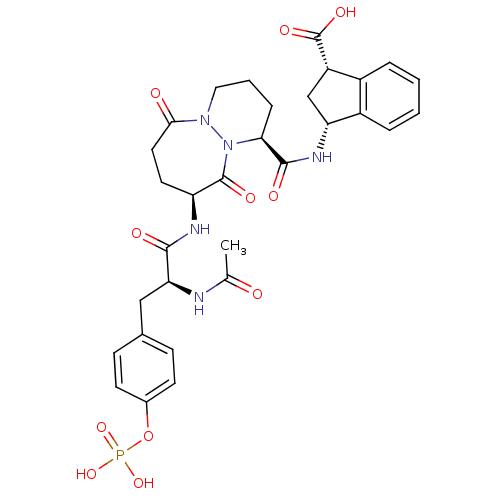 ((1S,3R)-3-({(1S,9S)-9-[(S)-2-Acetylamino-3-(4-phos...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited Curated by ChEMBL | Assay Description Inhibitory activity towards p56 Lck tyrosine kinase SH2 domain using scintillation proximity assay (SPA) | Bioorg Med Chem Lett 12: 1365-9 (2002) BindingDB Entry DOI: 10.7270/Q20002MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50112980 ((1R,3R)-3-({(1S,9S)-9-[(S)-2-Acetylamino-3-(4-phos...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited Curated by ChEMBL | Assay Description Inhibitory activity towards p56 Lck tyrosine kinase SH2 domain using scintillation proximity assay (SPA) | Bioorg Med Chem Lett 12: 1365-9 (2002) BindingDB Entry DOI: 10.7270/Q20002MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50112966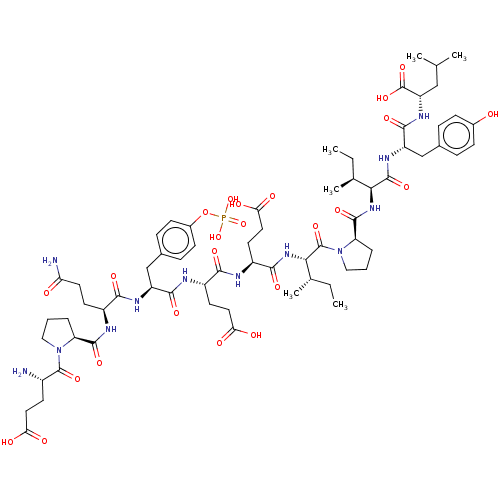 (CHEMBL2372049 | EPQpYEEIPIYL) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited Curated by ChEMBL | Assay Description Inhibitory activity towards p56 Lck tyrosine kinase SH2 domain using scintillation proximity assay (SPA) | Bioorg Med Chem Lett 12: 1365-9 (2002) BindingDB Entry DOI: 10.7270/Q20002MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50112959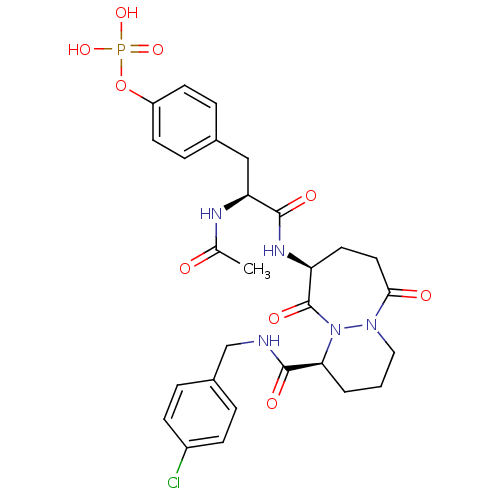 (CHEMBL29298 | Phosphoric acid mono-(4-{(S)-2-acety...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited Curated by ChEMBL | Assay Description Inhibitory activity towards p56 Lck tyrosine kinase SH2 domain using scintillation proximity assay (SPA) | Bioorg Med Chem Lett 12: 1365-9 (2002) BindingDB Entry DOI: 10.7270/Q20002MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50112974 (CHEMBL29820 | Phosphoric acid mono-(4-{(S)-2-acety...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited Curated by ChEMBL | Assay Description Inhibitory activity towards p56 Lck tyrosine kinase SH2 domain using scintillation proximity assay (SPA) | Bioorg Med Chem Lett 12: 1365-9 (2002) BindingDB Entry DOI: 10.7270/Q20002MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50112973 (CHEMBL418579 | Phosphoric acid mono-[4-((S)-2-acet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited Curated by ChEMBL | Assay Description Inhibitory activity towards p56 Lck tyrosine kinase SH2 domain using scintillation proximity assay (SPA) | Bioorg Med Chem Lett 12: 1365-9 (2002) BindingDB Entry DOI: 10.7270/Q20002MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50112975 (CHEMBL282111 | Phosphoric acid mono-(4-{(S)-2-acet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited Curated by ChEMBL | Assay Description Inhibitory activity towards p56 Lck tyrosine kinase SH2 domain using scintillation proximity assay (SPA) | Bioorg Med Chem Lett 12: 1365-9 (2002) BindingDB Entry DOI: 10.7270/Q20002MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50112971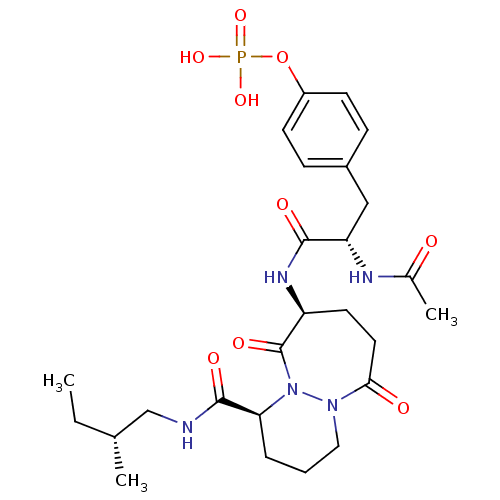 (CHEMBL418027 | Phosphoric acid mono-(4-{(S)-2-acet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited Curated by ChEMBL | Assay Description Inhibitory activity towards p56 Lck tyrosine kinase SH2 domain using scintillation proximity assay (SPA) | Bioorg Med Chem Lett 12: 1365-9 (2002) BindingDB Entry DOI: 10.7270/Q20002MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50112968 (CHEMBL413761 | Phosphoric acid mono-{4-[(S)-2-acet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited Curated by ChEMBL | Assay Description Inhibitory activity towards p56 Lck tyrosine kinase SH2 domain using scintillation proximity assay (SPA) | Bioorg Med Chem Lett 12: 1365-9 (2002) BindingDB Entry DOI: 10.7270/Q20002MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50112964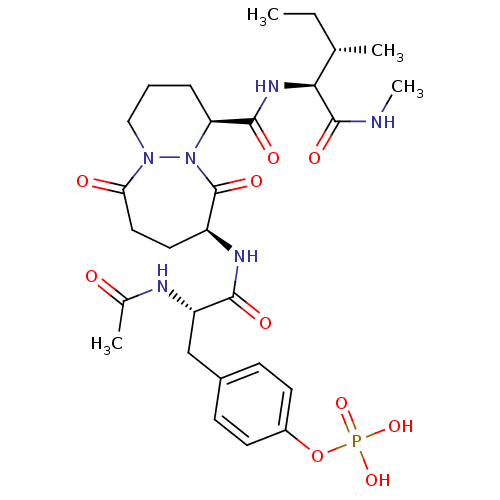 (CHEMBL29564 | Phosphoric acid mono-(4-{(S)-2-acety...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited Curated by ChEMBL | Assay Description Inhibitory activity towards p56 Lck tyrosine kinase SH2 domain using scintillation proximity assay (SPA) | Bioorg Med Chem Lett 12: 1365-9 (2002) BindingDB Entry DOI: 10.7270/Q20002MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50112958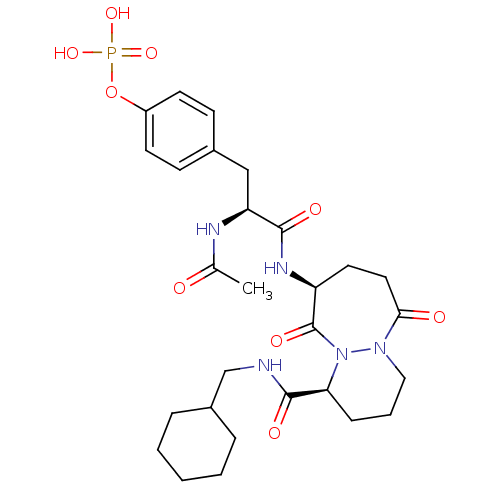 (CHEMBL281685 | Phosphoric acid mono-(4-{(S)-2-acet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited Curated by ChEMBL | Assay Description Inhibitory activity towards p56 Lck tyrosine kinase SH2 domain using scintillation proximity assay (SPA) | Bioorg Med Chem Lett 12: 1365-9 (2002) BindingDB Entry DOI: 10.7270/Q20002MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50112972 ((S)-4-{(S)-2-[(S)-2-Acetylamino-3-(4-phosphonooxy-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited Curated by ChEMBL | Assay Description Inhibitory activity towards p56 Lck tyrosine kinase SH2 domain using scintillation proximity assay (SPA) | Bioorg Med Chem Lett 12: 1365-9 (2002) BindingDB Entry DOI: 10.7270/Q20002MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50112969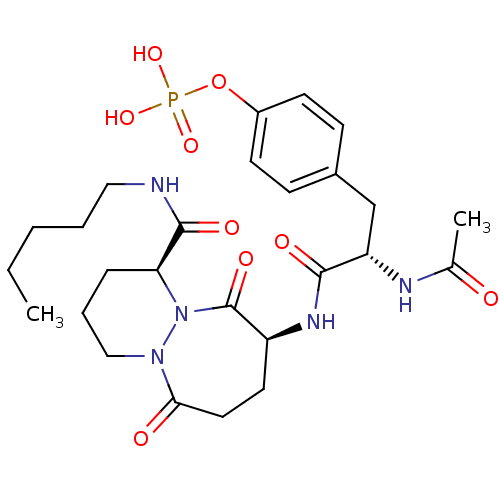 (CHEMBL286989 | Phosphoric acid mono-{4-[(S)-2-acet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited Curated by ChEMBL | Assay Description Inhibitory activity towards p56 Lck tyrosine kinase SH2 domain using scintillation proximity assay (SPA) | Bioorg Med Chem Lett 12: 1365-9 (2002) BindingDB Entry DOI: 10.7270/Q20002MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50112970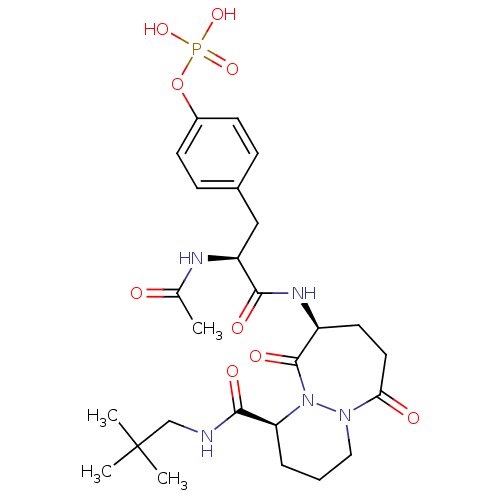 (CHEMBL30157 | Phosphoric acid mono-(4-{(S)-2-acety...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited Curated by ChEMBL | Assay Description Inhibitory activity towards p56 Lck tyrosine kinase SH2 domain using scintillation proximity assay (SPA) | Bioorg Med Chem Lett 12: 1365-9 (2002) BindingDB Entry DOI: 10.7270/Q20002MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50112961 (CHEMBL29139 | Phosphoric acid mono-[4-((S)-2-acety...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited Curated by ChEMBL | Assay Description Inhibitory activity towards p56 Lck tyrosine kinase SH2 domain using scintillation proximity assay (SPA) | Bioorg Med Chem Lett 12: 1365-9 (2002) BindingDB Entry DOI: 10.7270/Q20002MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50112981 (CHEMBL28638 | Phosphoric acid mono-{4-[(S)-2-acety...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited Curated by ChEMBL | Assay Description Inhibitory activity towards p56 Lck tyrosine kinase SH2 domain using scintillation proximity assay (SPA) | Bioorg Med Chem Lett 12: 1365-9 (2002) BindingDB Entry DOI: 10.7270/Q20002MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50112979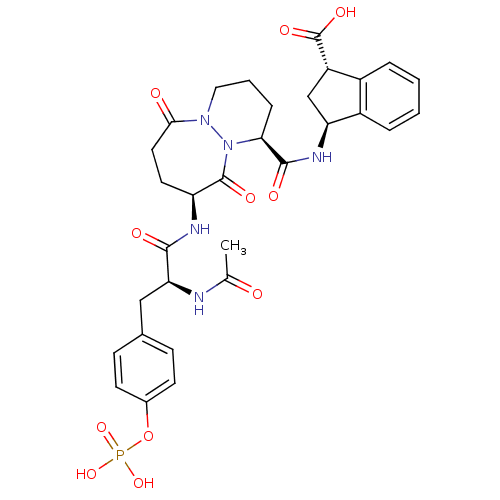 ((1S,3S)-3-({(1S,9S)-9-[(S)-2-Acetylamino-3-(4-phos...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited Curated by ChEMBL | Assay Description Inhibitory activity towards p56 Lck tyrosine kinase SH2 domain using scintillation proximity assay (SPA) | Bioorg Med Chem Lett 12: 1365-9 (2002) BindingDB Entry DOI: 10.7270/Q20002MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50366781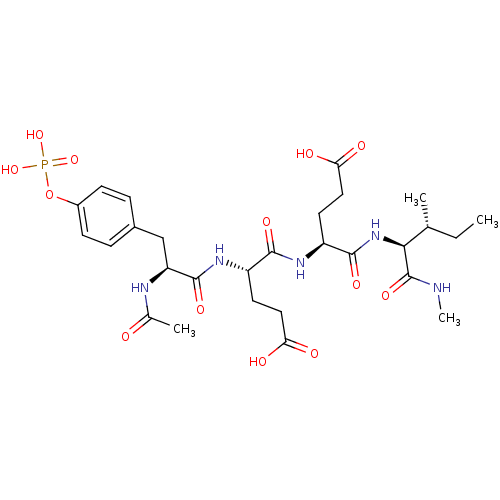 (CHEMBL1790100) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited Curated by ChEMBL | Assay Description Inhibitory activity towards p56 Lck tyrosine kinase SH2 domain using scintillation proximity assay (SPA) | Bioorg Med Chem Lett 12: 1365-9 (2002) BindingDB Entry DOI: 10.7270/Q20002MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50112962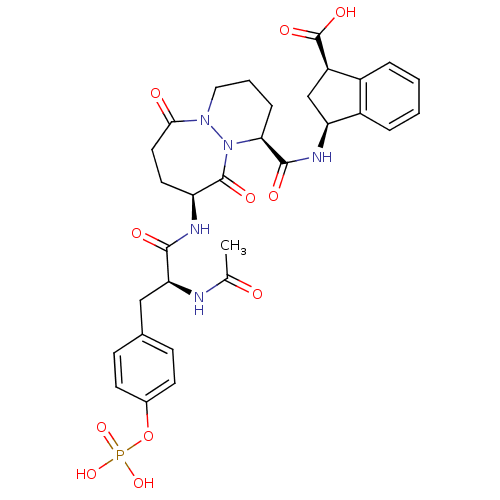 ((1R,3S)-3-({(1S,9S)-9-[(S)-2-Acetylamino-3-(4-phos...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited Curated by ChEMBL | Assay Description Inhibitory activity towards p56 Lck tyrosine kinase SH2 domain using scintillation proximity assay (SPA) | Bioorg Med Chem Lett 12: 1365-9 (2002) BindingDB Entry DOI: 10.7270/Q20002MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50112976 (CHEMBL416938 | Phosphoric acid mono-{4-[(S)-2-acet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited Curated by ChEMBL | Assay Description Inhibitory activity towards p56 Lck tyrosine kinase SH2 domain using scintillation proximity assay (SPA) | Bioorg Med Chem Lett 12: 1365-9 (2002) BindingDB Entry DOI: 10.7270/Q20002MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50112977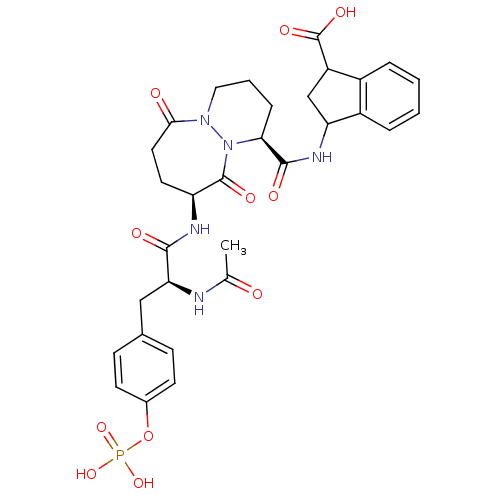 (3-({(1S,9S)-9-[(S)-2-Acetylamino-3-(4-phosphonooxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited Curated by ChEMBL | Assay Description Inhibitory activity towards p56 Lck tyrosine kinase SH2 domain using scintillation proximity assay (SPA) | Bioorg Med Chem Lett 12: 1365-9 (2002) BindingDB Entry DOI: 10.7270/Q20002MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50112977 (3-({(1S,9S)-9-[(S)-2-Acetylamino-3-(4-phosphonooxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited Curated by ChEMBL | Assay Description Inhibitory activity towards p56 Lck tyrosine kinase SH2 domain using scintillation proximity assay (SPA) | Bioorg Med Chem Lett 12: 1365-9 (2002) BindingDB Entry DOI: 10.7270/Q20002MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50112965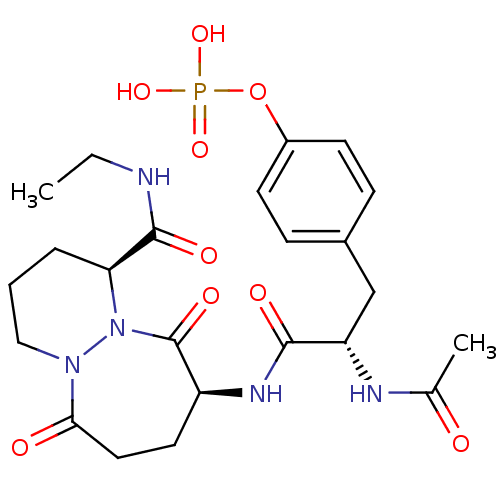 (CHEMBL441774 | Phosphoric acid mono-{4-[(S)-2-acet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited Curated by ChEMBL | Assay Description Inhibitory activity towards p56 Lck tyrosine kinase SH2 domain using scintillation proximity assay (SPA) | Bioorg Med Chem Lett 12: 1365-9 (2002) BindingDB Entry DOI: 10.7270/Q20002MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50112963 (CHEMBL29723 | Phosphoric acid mono-(4-{(S)-2-acety...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited Curated by ChEMBL | Assay Description Inhibitory activity towards p56 Lck tyrosine kinase SH2 domain using scintillation proximity assay (SPA) | Bioorg Med Chem Lett 12: 1365-9 (2002) BindingDB Entry DOI: 10.7270/Q20002MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50112957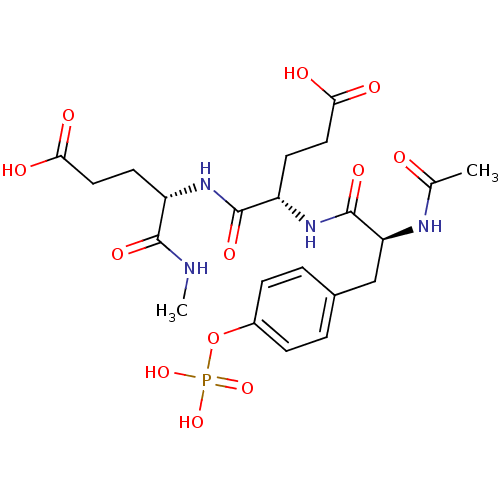 ((S)-4-[(S)-2-Acetylamino-3-(4-phosphonooxy-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited Curated by ChEMBL | Assay Description Inhibitory activity towards p56 Lck tyrosine kinase SH2 domain using scintillation proximity assay (SPA) | Bioorg Med Chem Lett 12: 1365-9 (2002) BindingDB Entry DOI: 10.7270/Q20002MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50112967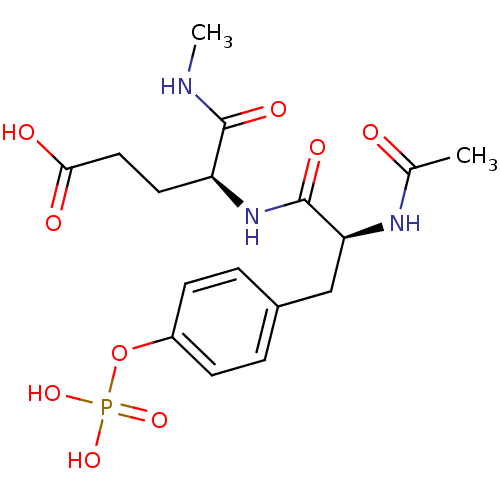 ((S)-4-[(S)-2-Acetylamino-3-(4-phosphonooxy-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited Curated by ChEMBL | Assay Description Inhibitory activity towards p56 Lck tyrosine kinase SH2 domain using scintillation proximity assay (SPA) | Bioorg Med Chem Lett 12: 1365-9 (2002) BindingDB Entry DOI: 10.7270/Q20002MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||