

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50442991 (CHEMBL432537 | GNF-Pf-3777 | US10669273, Compound ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of human recombinant IDO1 using L-tryptophan as substrate | J Med Chem 56: 8321-31 (2013) Article DOI: 10.1021/jm401195n BindingDB Entry DOI: 10.7270/Q2X34ZWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50514041 (CHEMBL4452150) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Mixed uncompetitive inhibition of recombinant human TDO expressed in Escherichia coli BL21 (DE3) assessed as inhibition constant using varying concen... | J Med Chem 62: 9161-9174 (2019) Article DOI: 10.1021/acs.jmedchem.9b01079 BindingDB Entry DOI: 10.7270/Q27W6GJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50514040 (CHEMBL4436480) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of recombinant human TDO expressed in Escherichia coli BL21 (DE3) assessed as assessed as inhibition constant using varying ... | J Med Chem 62: 9161-9174 (2019) Article DOI: 10.1021/acs.jmedchem.9b01079 BindingDB Entry DOI: 10.7270/Q27W6GJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50514038 (CHEMBL4577825) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Mixed uncompetitive inhibition of recombinant human TDO expressed in Escherichia coli BL21 (DE3) assessed as inhibition constant using varying concen... | J Med Chem 62: 9161-9174 (2019) Article DOI: 10.1021/acs.jmedchem.9b01079 BindingDB Entry DOI: 10.7270/Q27W6GJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM48009 (8-fluoranylindolo[2,1-b]quinazoline-6,12-dione | 8...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 161 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of human recombinant IDO1 using L-tryptophan as substrate | J Med Chem 56: 8321-31 (2013) Article DOI: 10.1021/jm401195n BindingDB Entry DOI: 10.7270/Q2X34ZWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50514037 (CHEMBL4536439) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Mixed uncompetitive inhibition of recombinant human TDO expressed in Escherichia coli BL21 (DE3) assessed as inhibition constant using varying concen... | J Med Chem 62: 9161-9174 (2019) Article DOI: 10.1021/acs.jmedchem.9b01079 BindingDB Entry DOI: 10.7270/Q27W6GJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50442991 (CHEMBL432537 | GNF-Pf-3777 | US10669273, Compound ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 215 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of recombinant full length C-terminal His-tagged human TDO expressed in Escherichia coli using L-Trp as substrate after 30 m... | Eur J Med Chem 160: 133-145 (2018) Article DOI: 10.1016/j.ejmech.2018.10.017 BindingDB Entry DOI: 10.7270/Q2GB27GB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50514035 (CHEMBL4437046) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of recombinant human TDO expressed in Escherichia coli BL21 (DE3) assessed as assessed as inhibition constant using varying ... | J Med Chem 62: 9161-9174 (2019) Article DOI: 10.1021/acs.jmedchem.9b01079 BindingDB Entry DOI: 10.7270/Q27W6GJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514041 (CHEMBL4452150) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of recombinant human IDO1 expressed in Escherichia coli BL21 cells assessed inhibition constant using varying concentration ... | J Med Chem 62: 9161-9174 (2019) Article DOI: 10.1021/acs.jmedchem.9b01079 BindingDB Entry DOI: 10.7270/Q27W6GJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM48009 (8-fluoranylindolo[2,1-b]quinazoline-6,12-dione | 8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 336 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of recombinant full length C-terminal His-tagged human TDO expressed in Escherichia coli using L-Trp as substrate after 30 m... | Eur J Med Chem 160: 133-145 (2018) Article DOI: 10.1016/j.ejmech.2018.10.017 BindingDB Entry DOI: 10.7270/Q2GB27GB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50514034 (CHEMBL4588805) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of recombinant human TDO expressed in Escherichia coli BL21 (DE3) assessed as assessed as inhibition constant using varying ... | J Med Chem 62: 9161-9174 (2019) Article DOI: 10.1021/acs.jmedchem.9b01079 BindingDB Entry DOI: 10.7270/Q27W6GJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50442987 (8-Bromotryptanthrin | CHEMBL72165) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 356 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of recombinant full length C-terminal His-tagged human TDO expressed in Escherichia coli using L-Trp as substrate after 30 m... | Eur J Med Chem 160: 133-145 (2018) Article DOI: 10.1016/j.ejmech.2018.10.017 BindingDB Entry DOI: 10.7270/Q2GB27GB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50442987 (8-Bromotryptanthrin | CHEMBL72165) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 389 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of human recombinant IDO1 using L-tryptophan as substrate | J Med Chem 56: 8321-31 (2013) Article DOI: 10.1021/jm401195n BindingDB Entry DOI: 10.7270/Q2X34ZWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50514043 (CHEMBL4545004) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Mixed uncompetitive inhibition of recombinant human TDO expressed in Escherichia coli BL21 (DE3) assessed as inhibition constant using varying concen... | J Med Chem 62: 9161-9174 (2019) Article DOI: 10.1021/acs.jmedchem.9b01079 BindingDB Entry DOI: 10.7270/Q27W6GJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514034 (CHEMBL4588805) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of recombinant human IDO1 expressed in Escherichia coli BL21 cells assessed inhibition constant using varying concentration ... | J Med Chem 62: 9161-9174 (2019) Article DOI: 10.1021/acs.jmedchem.9b01079 BindingDB Entry DOI: 10.7270/Q27W6GJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50240612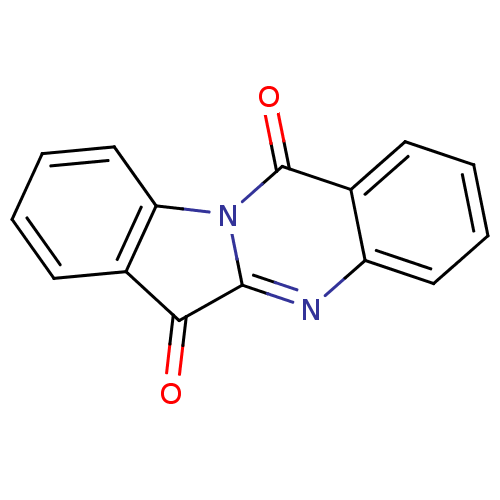 (CHEMBL306946 | GNF-PF-2691 | Indolo[2,1-b]quinazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 581 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of recombinant full length C-terminal His-tagged human TDO expressed in Escherichia coli using L-Trp as substrate after 30 m... | Eur J Med Chem 160: 133-145 (2018) Article DOI: 10.1016/j.ejmech.2018.10.017 BindingDB Entry DOI: 10.7270/Q2GB27GB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50514032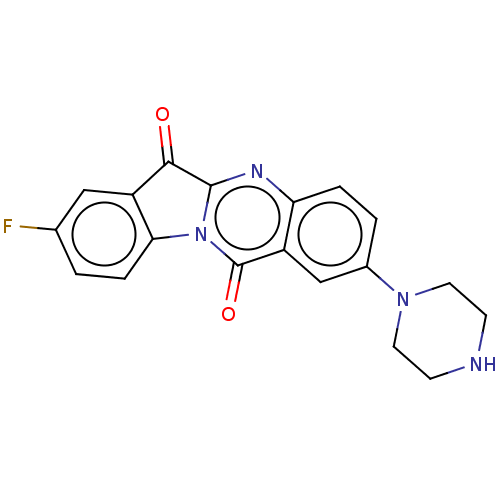 (CHEMBL4471831) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Mixed uncompetitive inhibition of recombinant human TDO expressed in Escherichia coli BL21 (DE3) assessed as inhibition constant using varying concen... | J Med Chem 62: 9161-9174 (2019) Article DOI: 10.1021/acs.jmedchem.9b01079 BindingDB Entry DOI: 10.7270/Q27W6GJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50442994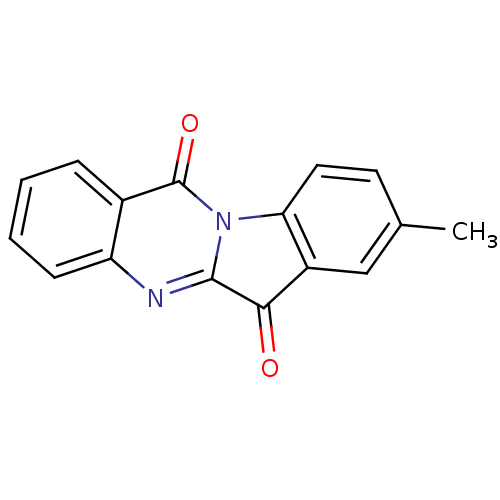 (CHEMBL312537 | US10669273, Compound 5b) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 834 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Mixed type uncompetitive inhibition of recombinant full length C-terminal His-tagged human TDO expressed in Escherichia coli using L-Trp as substrate... | Eur J Med Chem 160: 133-145 (2018) Article DOI: 10.1016/j.ejmech.2018.10.017 BindingDB Entry DOI: 10.7270/Q2GB27GB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50442995 (CHEMBL3086870) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 876 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Mixed type uncompetitive inhibition of recombinant full length C-terminal His-tagged human TDO expressed in Escherichia coli using L-Trp as substrate... | Eur J Med Chem 160: 133-145 (2018) Article DOI: 10.1016/j.ejmech.2018.10.017 BindingDB Entry DOI: 10.7270/Q2GB27GB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50442991 (CHEMBL432537 | GNF-Pf-3777 | US10669273, Compound ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of recombinant human C-terminal His6-tagged IDO2 (14-420 residues) expressed in Escherichia coli BL21(DE3) in presence of va... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50442988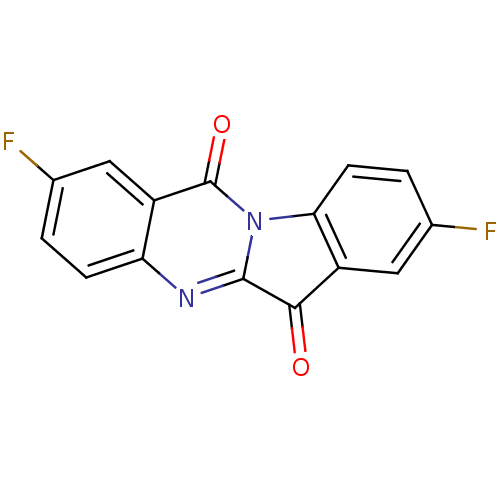 (CHEMBL3087009) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Mixed type uncompetitive inhibition of recombinant full length C-terminal His-tagged human TDO expressed in Escherichia coli using L-Trp as substrate... | Eur J Med Chem 160: 133-145 (2018) Article DOI: 10.1016/j.ejmech.2018.10.017 BindingDB Entry DOI: 10.7270/Q2GB27GB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50514036 (CHEMBL4468741) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of recombinant human TDO expressed in Escherichia coli BL21 (DE3) assessed as assessed as inhibition constant using varying ... | J Med Chem 62: 9161-9174 (2019) Article DOI: 10.1021/acs.jmedchem.9b01079 BindingDB Entry DOI: 10.7270/Q27W6GJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50514042 (CHEMBL4578338) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of recombinant human TDO expressed in Escherichia coli BL21 (DE3) assessed as assessed as inhibition constant using varying ... | J Med Chem 62: 9161-9174 (2019) Article DOI: 10.1021/acs.jmedchem.9b01079 BindingDB Entry DOI: 10.7270/Q27W6GJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50514040 (CHEMBL4436480) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of C-terminal His6-tagged human IDO2 (14 to 420 residues) expressed in Escherichia coli BL21 (DE3) assessed as inhibition co... | J Med Chem 62: 9161-9174 (2019) Article DOI: 10.1021/acs.jmedchem.9b01079 BindingDB Entry DOI: 10.7270/Q27W6GJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50514039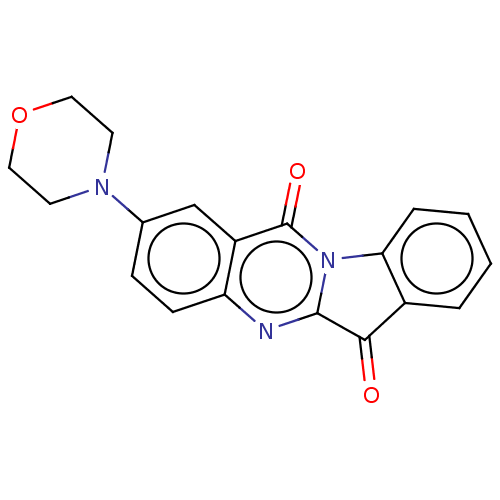 (CHEMBL4584081) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of recombinant human TDO expressed in Escherichia coli BL21 (DE3) assessed as assessed as inhibition constant using varying ... | J Med Chem 62: 9161-9174 (2019) Article DOI: 10.1021/acs.jmedchem.9b01079 BindingDB Entry DOI: 10.7270/Q27W6GJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514035 (CHEMBL4437046) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of recombinant human IDO1 expressed in Escherichia coli BL21 cells assessed inhibition constant using varying concentration ... | J Med Chem 62: 9161-9174 (2019) Article DOI: 10.1021/acs.jmedchem.9b01079 BindingDB Entry DOI: 10.7270/Q27W6GJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM48009 (8-fluoranylindolo[2,1-b]quinazoline-6,12-dione | 8...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of recombinant human C-terminal His6-tagged IDO2 (14-420 residues) expressed in Escherichia coli BL21(DE3) in presence of va... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514040 (CHEMBL4436480) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Noncompetitive inhibition of recombinant human IDO1 expressed in Escherichia coli BL21 cells assessed inhibition constant using varying concentration... | J Med Chem 62: 9161-9174 (2019) Article DOI: 10.1021/acs.jmedchem.9b01079 BindingDB Entry DOI: 10.7270/Q27W6GJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50514041 (CHEMBL4452150) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of C-terminal His6-tagged human IDO2 (14 to 420 residues) expressed in Escherichia coli BL21 (DE3) assessed as inhibition co... | J Med Chem 62: 9161-9174 (2019) Article DOI: 10.1021/acs.jmedchem.9b01079 BindingDB Entry DOI: 10.7270/Q27W6GJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514042 (CHEMBL4578338) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of recombinant human IDO1 expressed in Escherichia coli BL21 cells assessed inhibition constant using varying concentration ... | J Med Chem 62: 9161-9174 (2019) Article DOI: 10.1021/acs.jmedchem.9b01079 BindingDB Entry DOI: 10.7270/Q27W6GJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50442993 (CHEMBL3087012) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of human recombinant IDO1 using L-tryptophan as substrate | J Med Chem 56: 8321-31 (2013) Article DOI: 10.1021/jm401195n BindingDB Entry DOI: 10.7270/Q2X34ZWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50240612 (CHEMBL306946 | GNF-PF-2691 | Indolo[2,1-b]quinazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 4.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Mixed competitive inhibition of human recombinant IDO1 using L-tryptophan as substrate | J Med Chem 56: 8321-31 (2013) Article DOI: 10.1021/jm401195n BindingDB Entry DOI: 10.7270/Q2X34ZWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50442988 (CHEMBL3087009) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of human recombinant IDO1 using L-tryptophan as substrate | J Med Chem 56: 8321-31 (2013) Article DOI: 10.1021/jm401195n BindingDB Entry DOI: 10.7270/Q2X34ZWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50350248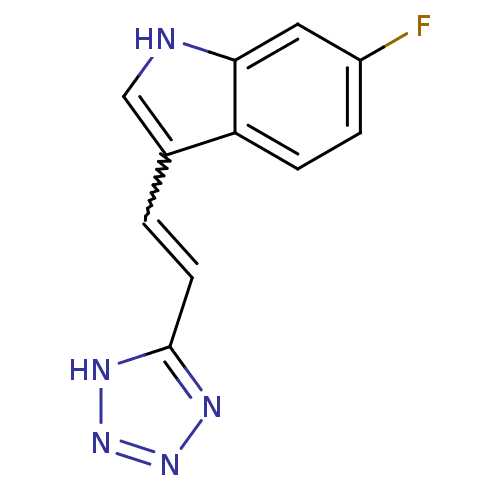 (CHEMBL1812545) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant human TDO expressed in Escherichia coli using L-Trp as substrate after 10 to 60 mins | Eur J Med Chem 160: 133-145 (2018) Article DOI: 10.1016/j.ejmech.2018.10.017 BindingDB Entry DOI: 10.7270/Q2GB27GB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50442987 (8-Bromotryptanthrin | CHEMBL72165) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of recombinant human C-terminal His6-tagged IDO2 (14-420 residues) expressed in Escherichia coli BL21(DE3) in presence of va... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514043 (CHEMBL4545004) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of recombinant human IDO1 expressed in Escherichia coli BL21 cells assessed inhibition constant using varying concentration ... | J Med Chem 62: 9161-9174 (2019) Article DOI: 10.1021/acs.jmedchem.9b01079 BindingDB Entry DOI: 10.7270/Q27W6GJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514032 (CHEMBL4471831) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of recombinant human IDO1 expressed in Escherichia coli BL21 cells assessed inhibition constant using varying concentration ... | J Med Chem 62: 9161-9174 (2019) Article DOI: 10.1021/acs.jmedchem.9b01079 BindingDB Entry DOI: 10.7270/Q27W6GJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50514038 (CHEMBL4577825) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of C-terminal His6-tagged human IDO2 (14 to 420 residues) expressed in Escherichia coli BL21 (DE3) assessed as inhibition co... | J Med Chem 62: 9161-9174 (2019) Article DOI: 10.1021/acs.jmedchem.9b01079 BindingDB Entry DOI: 10.7270/Q27W6GJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50514035 (CHEMBL4437046) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Mixed competitive inhibition of C-terminal His6-tagged human IDO2 (14 to 420 residues) expressed in Escherichia coli BL21 (DE3) assessed as inhibitio... | J Med Chem 62: 9161-9174 (2019) Article DOI: 10.1021/acs.jmedchem.9b01079 BindingDB Entry DOI: 10.7270/Q27W6GJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514037 (CHEMBL4536439) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Noncompetitive inhibition of recombinant human IDO1 expressed in Escherichia coli BL21 cells assessed inhibition constant using varying concentration... | J Med Chem 62: 9161-9174 (2019) Article DOI: 10.1021/acs.jmedchem.9b01079 BindingDB Entry DOI: 10.7270/Q27W6GJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514038 (CHEMBL4577825) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of recombinant human IDO1 expressed in Escherichia coli BL21 cells assessed inhibition constant using varying concentration ... | J Med Chem 62: 9161-9174 (2019) Article DOI: 10.1021/acs.jmedchem.9b01079 BindingDB Entry DOI: 10.7270/Q27W6GJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50350248 (CHEMBL1812545) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of recombinant full length C-terminal His-tagged human TDO expressed in Escherichia coli using L-Trp as substrate after 30 m... | Eur J Med Chem 160: 133-145 (2018) Article DOI: 10.1016/j.ejmech.2018.10.017 BindingDB Entry DOI: 10.7270/Q2GB27GB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514033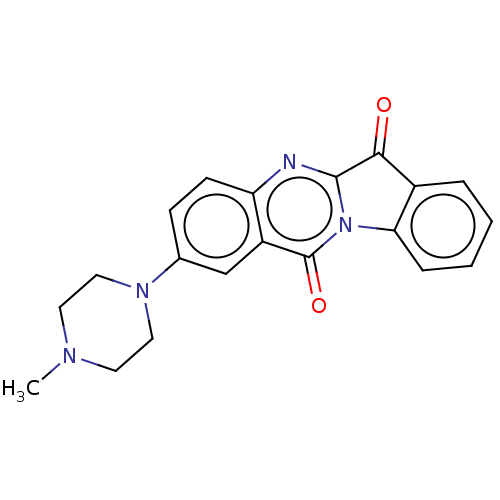 (CHEMBL4471895) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of recombinant human IDO1 expressed in Escherichia coli BL21 cells assessed inhibition constant using varying concentration ... | J Med Chem 62: 9161-9174 (2019) Article DOI: 10.1021/acs.jmedchem.9b01079 BindingDB Entry DOI: 10.7270/Q27W6GJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50442989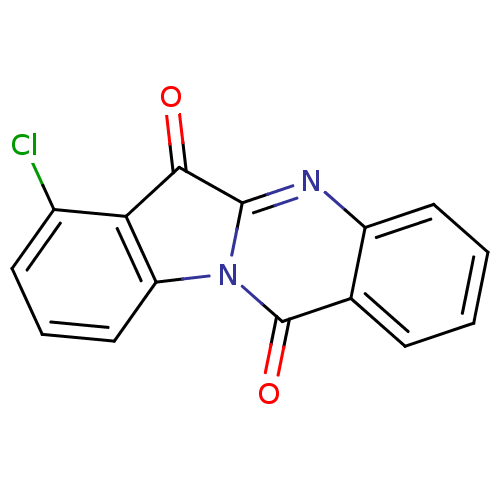 (CHEMBL3087010) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of recombinant full length C-terminal His-tagged human TDO expressed in Escherichia coli using L-Trp as substrate after 30 m... | Eur J Med Chem 160: 133-145 (2018) Article DOI: 10.1016/j.ejmech.2018.10.017 BindingDB Entry DOI: 10.7270/Q2GB27GB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50442992 (CHEMBL2314972) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of human recombinant IDO1 using L-tryptophan as substrate | J Med Chem 56: 8321-31 (2013) Article DOI: 10.1021/jm401195n BindingDB Entry DOI: 10.7270/Q2X34ZWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514036 (CHEMBL4468741) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of recombinant human IDO1 expressed in Escherichia coli BL21 cells assessed inhibition constant using varying concentration ... | J Med Chem 62: 9161-9174 (2019) Article DOI: 10.1021/acs.jmedchem.9b01079 BindingDB Entry DOI: 10.7270/Q27W6GJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50514043 (CHEMBL4545004) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Mixed uncompetitive inhibition of C-terminal His6-tagged human IDO2 (14 to 420 residues) expressed in Escherichia coli BL21 (DE3) assessed as inhibit... | J Med Chem 62: 9161-9174 (2019) Article DOI: 10.1021/acs.jmedchem.9b01079 BindingDB Entry DOI: 10.7270/Q27W6GJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50442989 (CHEMBL3087010) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of human recombinant IDO1 using L-tryptophan as substrate | J Med Chem 56: 8321-31 (2013) Article DOI: 10.1021/jm401195n BindingDB Entry DOI: 10.7270/Q2X34ZWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50442988 (CHEMBL3087009) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Mixed competitive inhibition of recombinant human C-terminal His6-tagged IDO2 (14-420 residues) expressed in Escherichia coli BL21(DE3) in presence o... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50355862 (CHEMBL1909734) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of human recombinant indoleamine-2,3-dioxygenase expressed in Escherichia coli BL21 using L-tryptophan as substrate by Dixon... | Eur J Med Chem 46: 5680-7 (2011) Article DOI: 10.1016/j.ejmech.2011.08.044 BindingDB Entry DOI: 10.7270/Q2KH0NRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 307 total ) | Next | Last >> |