 Found 1016 hits with Last Name = 'kuca' and Initial = 'k'
Found 1016 hits with Last Name = 'kuca' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606735
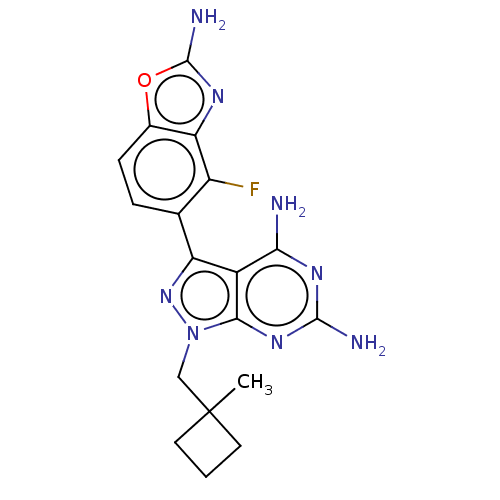
(CHEMBL5219718 | US11731973, Example 3)Show SMILES CC1(Cn2nc(-c3ccc4oc(N)nc4c3F)c3c(N)nc(N)nc23)CCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50389383

(CHEMBL2064464)Show SMILES CN(CCC(=O)N1CCN(CCNc2c3CCCCc3nc3ccccc23)CC1)C1CCCCC1 Show InChI InChI=1S/C29H43N5O/c1-32(23-9-3-2-4-10-23)17-15-28(35)34-21-19-33(20-22-34)18-16-30-29-24-11-5-7-13-26(24)31-27-14-8-6-12-25(27)29/h5,7,11,13,23H,2-4,6,8-10,12,14-22H2,1H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.318 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex using acetylthiocholine substrate prein... |
Eur J Med Chem 55: 23-31 (2012)
Article DOI: 10.1016/j.ejmech.2012.06.051
BindingDB Entry DOI: 10.7270/Q2HH6M4Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50389383

(CHEMBL2064464)Show SMILES CN(CCC(=O)N1CCN(CCNc2c3CCCCc3nc3ccccc23)CC1)C1CCCCC1 Show InChI InChI=1S/C29H43N5O/c1-32(23-9-3-2-4-10-23)17-15-28(35)34-21-19-33(20-22-34)18-16-30-29-24-11-5-7-13-26(24)31-27-14-8-6-12-25(27)29/h5,7,11,13,23H,2-4,6,8-10,12,14-22H2,1H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex using acetylthiocholine substrate preincubated fo... |
Eur J Med Chem 55: 23-31 (2012)
Article DOI: 10.1016/j.ejmech.2012.06.051
BindingDB Entry DOI: 10.7270/Q2HH6M4Z |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606737
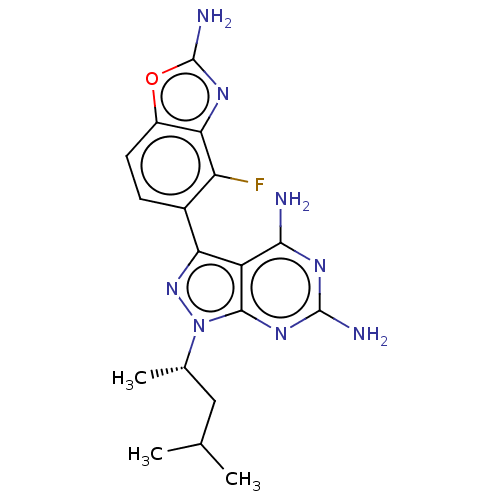
(CHEMBL5218727 | US11731973, Example 5)Show SMILES CC(C)C[C@H](C)n1nc(-c2ccc3oc(N)nc3c2F)c2c(N)nc(N)nc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606738
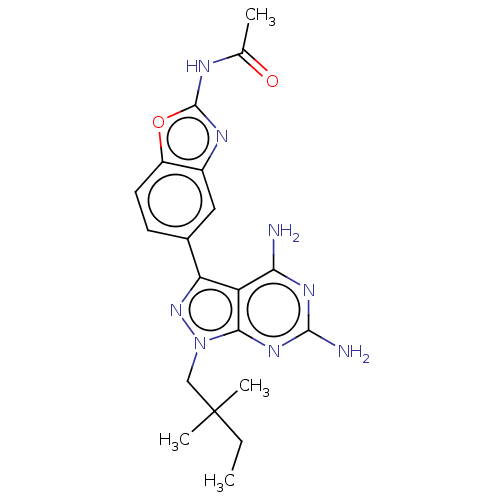
(CHEMBL5218590 | US11731973, Example 6)Show SMILES CCC(C)(C)Cn1nc(-c2ccc3oc(NC(C)=O)nc3c2)c2c(N)nc(N)nc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606740
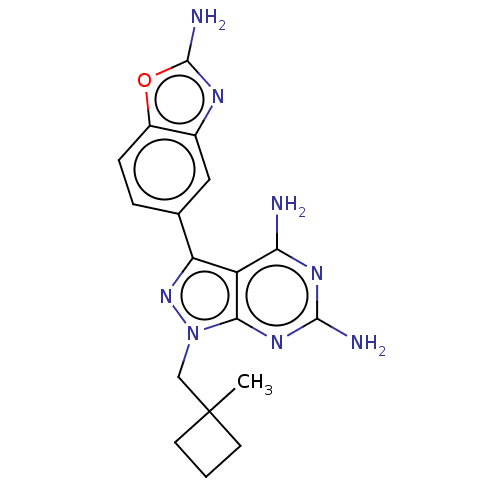
(CHEMBL5220536 | US11731973, Example 30)Show SMILES CC1(Cn2nc(-c3ccc4oc(N)nc4c3)c3c(N)nc(N)nc23)CCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606739
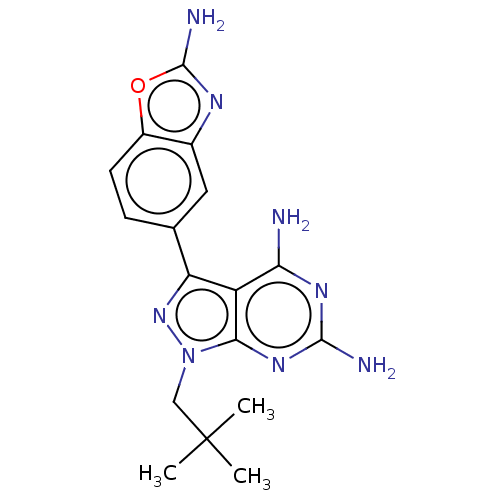
(CHEMBL5220152 | US11731973, Example 7)Show SMILES CC(C)(C)Cn1nc(-c2ccc3oc(N)nc3c2)c2c(N)nc(N)nc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606742
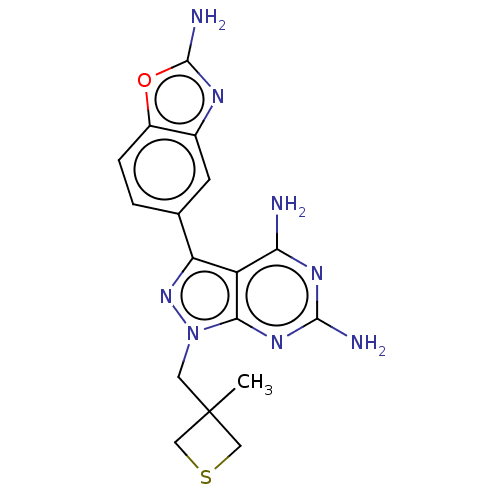
(CHEMBL5220948 | US11731973, Example 9)Show SMILES CC1(Cn2nc(-c3ccc4oc(N)nc4c3)c3c(N)nc(N)nc23)CSC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606741
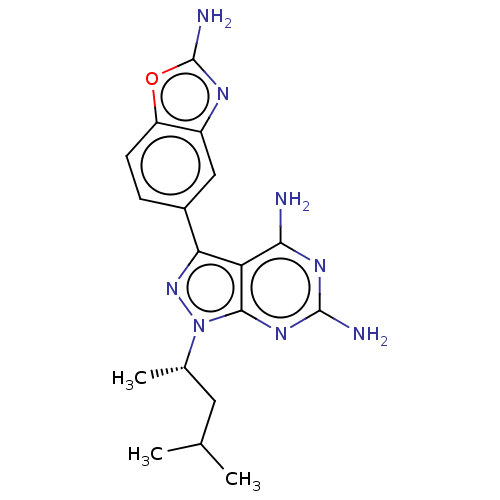
(CHEMBL5219710 | US11731973, Example 1)Show SMILES CC(C)C[C@H](C)n1nc(-c2ccc3oc(N)nc3c2)c2c(N)nc(N)nc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606736
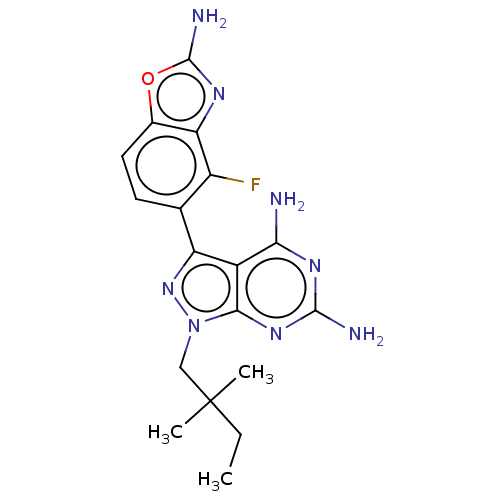
(CHEMBL5218916 | US11731973, Example 10)Show SMILES CCC(C)(C)Cn1nc(-c2ccc3oc(N)nc3c2F)c2c(N)nc(N)nc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50389382
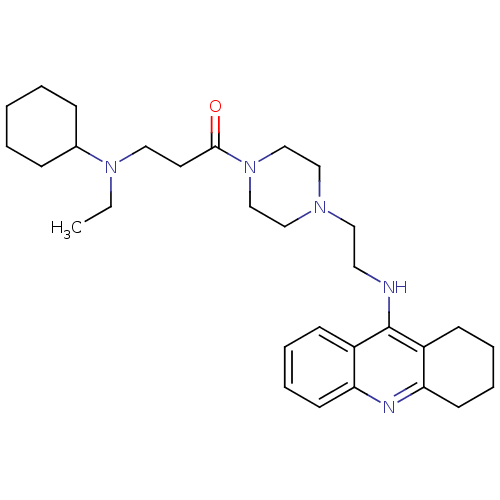
(CHEMBL2064465)Show SMILES CCN(CCC(=O)N1CCN(CCNc2c3CCCCc3nc3ccccc23)CC1)C1CCCCC1 Show InChI InChI=1S/C30H45N5O/c1-2-34(24-10-4-3-5-11-24)18-16-29(36)35-22-20-33(21-23-35)19-17-31-30-25-12-6-8-14-27(25)32-28-15-9-7-13-26(28)30/h6,8,12,14,24H,2-5,7,9-11,13,15-23H2,1H3,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex using acetylthiocholine substrate preincubated fo... |
Eur J Med Chem 55: 23-31 (2012)
Article DOI: 10.1016/j.ejmech.2012.06.051
BindingDB Entry DOI: 10.7270/Q2HH6M4Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50458458

(CHEMBL4210729)Show SMILES COc1ccc(Cn2cc(C(=O)NCCCNc3c4CCCCc4nc4cc(Cl)ccc34)c(=O)c3ccccc23)cc1 Show InChI InChI=1S/C34H33ClN4O3/c1-42-24-14-11-22(12-15-24)20-39-21-28(33(40)27-8-3-5-10-31(27)39)34(41)37-18-6-17-36-32-25-7-2-4-9-29(25)38-30-19-23(35)13-16-26(30)32/h3,5,8,10-16,19,21H,2,4,6-7,9,17-18,20H2,1H3,(H,36,38)(H,37,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human erythrocyte AChE assessed as enzyme-substrate-inhibitor complex using varying levels of acetylthiocholine iodide as su... |
Eur J Med Chem 150: 292-306 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.083
BindingDB Entry DOI: 10.7270/Q2W95CT0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606733

(CHEMBL5218988) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM582479

(5-(2-aminobenzoxazol-5-yl)-7-methyl-7-methylsulfan...) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50458458

(CHEMBL4210729)Show SMILES COc1ccc(Cn2cc(C(=O)NCCCNc3c4CCCCc4nc4cc(Cl)ccc34)c(=O)c3ccccc23)cc1 Show InChI InChI=1S/C34H33ClN4O3/c1-42-24-14-11-22(12-15-24)20-39-21-28(33(40)27-8-3-5-10-31(27)39)34(41)37-18-6-17-36-32-25-7-2-4-9-29(25)38-30-19-23(35)13-16-26(30)32/h3,5,8,10-16,19,21H,2,4,6-7,9,17-18,20H2,1H3,(H,36,38)(H,37,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human erythrocyte AChE assessed as enzyme-inhibitor complex using varying levels of acetylthiocholine iodide as substrate pr... |
Eur J Med Chem 150: 292-306 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.083
BindingDB Entry DOI: 10.7270/Q2W95CT0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50389382

(CHEMBL2064465)Show SMILES CCN(CCC(=O)N1CCN(CCNc2c3CCCCc3nc3ccccc23)CC1)C1CCCCC1 Show InChI InChI=1S/C30H45N5O/c1-2-34(24-10-4-3-5-11-24)18-16-29(36)35-22-20-33(21-23-35)19-17-31-30-25-12-6-8-14-27(25)32-28-15-9-7-13-26(28)30/h6,8,12,14,24H,2-5,7,9-11,13,15-23H2,1H3,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex using acetylthiocholine substrate prein... |
Eur J Med Chem 55: 23-31 (2012)
Article DOI: 10.1016/j.ejmech.2012.06.051
BindingDB Entry DOI: 10.7270/Q2HH6M4Z |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50606717
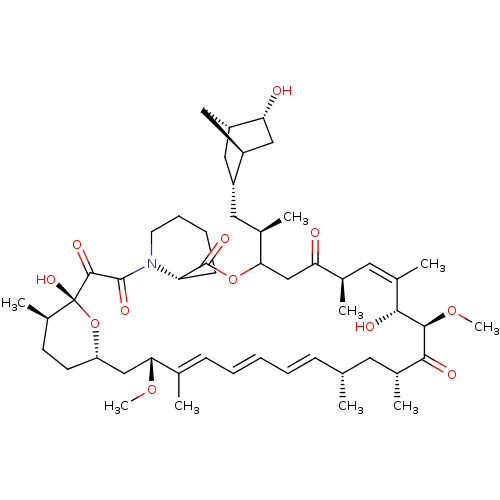
(CHEMBL5218737)Show SMILES [H][C@]12C[C@@H](C[C@@H](C)C3CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C[C@]4([H])CC[C@@H](C)[C@@](O)(O4)C(=O)C(=O)N4CCCC[C@H]4C(=O)O3)OC)[C@]([H])(C[C@H]1O)C2 |r,c:13,32,t:28,30| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056134
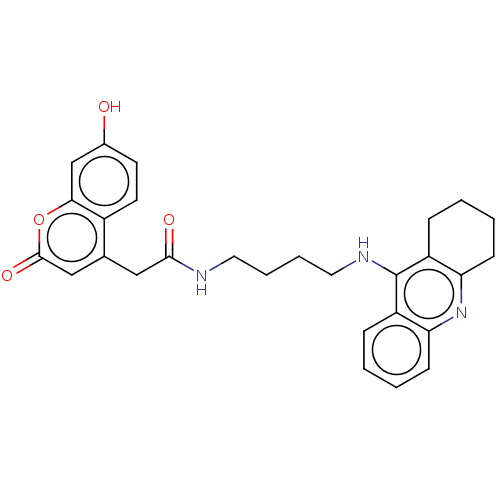
(CHEMBL3322160)Show SMILES Oc1ccc2c(CC(=O)NCCCCNc3c4CCCCc4nc4ccccc34)cc(=O)oc2c1 Show InChI InChI=1S/C28H29N3O4/c32-19-11-12-20-18(16-27(34)35-25(20)17-19)15-26(33)29-13-5-6-14-30-28-21-7-1-3-9-23(21)31-24-10-4-2-8-22(24)28/h1,3,7,9,11-12,16-17,32H,2,4-6,8,10,13-15H2,(H,29,33)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex by Lineweaver-Burk plot |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50606714
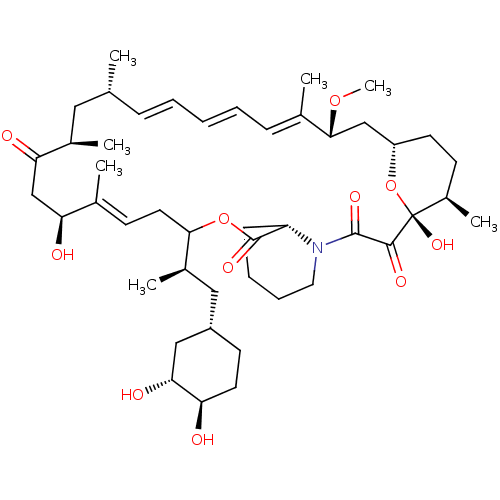
(CHEMBL5220794)Show SMILES [H][C@@]12CC[C@@H](C)[C@@](O)(O1)C(=O)C(=O)N1CCCC[C@H]1C(=O)OC(C\C=C(C)\[C@@H](O)CC(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C2)OC)[C@H](C)C[C@@H]1CC[C@@H](O)[C@H](O)C1 |r,c:26,43,t:39,41| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50336116

(Ambenonium dichloride | CHEMBL1669479)Show SMILES C[N+](C)(CCNC(=O)C(=O)NCC[N+](C)(C)Cc1ccccc1Cl)Cc1ccccc1Cl Show InChI InChI=1S/C24H32Cl2N4O2/c1-29(2,17-19-9-5-7-11-21(19)25)15-13-27-23(31)24(32)28-14-16-30(3,4)18-20-10-6-8-12-22(20)26/h5-12H,13-18H2,1-4H3/p+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Dissociation constant for enzyme-inhibitor complex of human recombinant AChE by Lineweaver-Burk plot analysis |
Eur J Med Chem 46: 811-8 (2011)
Article DOI: 10.1016/j.ejmech.2010.12.011
BindingDB Entry DOI: 10.7270/Q2PR7W8D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50262988
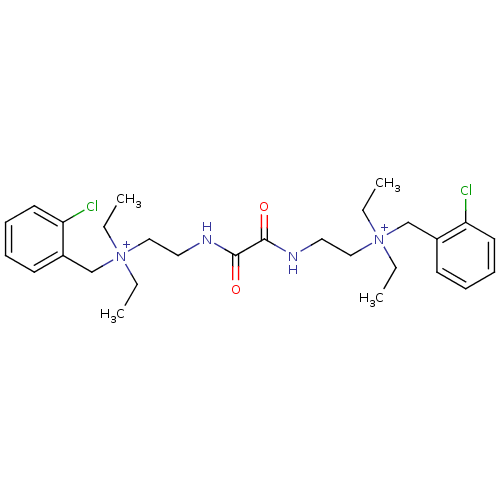
(CHEMBL1200541 | N-(2-chlorobenzyl)-2-(2-(2-((2-chl...)Show SMILES CC[N+](CC)(CCNC(=O)C(=O)NCC[N+](CC)(CC)Cc1ccccc1Cl)Cc1ccccc1Cl Show InChI InChI=1S/C28H40Cl2N4O2/c1-5-33(6-2,21-23-13-9-11-15-25(23)29)19-17-31-27(35)28(36)32-18-20-34(7-3,8-4)22-24-14-10-12-16-26(24)30/h9-16H,5-8,17-22H2,1-4H3/p+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex |
Bioorg Med Chem Lett 21: 2505-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.047
BindingDB Entry DOI: 10.7270/Q2JW8F6N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50336116

(Ambenonium dichloride | CHEMBL1669479)Show SMILES C[N+](C)(CCNC(=O)C(=O)NCC[N+](C)(C)Cc1ccccc1Cl)Cc1ccccc1Cl Show InChI InChI=1S/C24H32Cl2N4O2/c1-29(2,17-19-9-5-7-11-21(19)25)15-13-27-23(31)24(32)28-14-16-30(3,4)18-20-10-6-8-12-22(20)26/h5-12H,13-18H2,1-4H3/p+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50606713
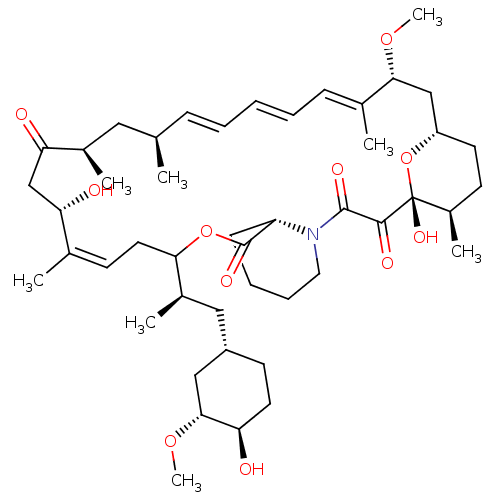
(CHEMBL5218786)Show SMILES [H][C@@]12CC[C@@H](C)[C@@](O)(O1)C(=O)C(=O)N1CCCC[C@H]1C(=O)OC(C\C=C(C)\[C@@H](O)CC(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C2)OC)[C@H](C)C[C@@H]1CC[C@@H](O)[C@@H](C1)OC |r,c:26,43,t:39,41| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50336116

(Ambenonium dichloride | CHEMBL1669479)Show SMILES C[N+](C)(CCNC(=O)C(=O)NCC[N+](C)(C)Cc1ccccc1Cl)Cc1ccccc1Cl Show InChI InChI=1S/C24H32Cl2N4O2/c1-29(2,17-19-9-5-7-11-21(19)25)15-13-27-23(31)24(32)28-14-16-30(3,4)18-20-10-6-8-12-22(20)26/h5-12H,13-18H2,1-4H3/p+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Dissociation constant for enzyme-inhibitor-substrate complex of human recombinant AChE by Lineweaver-Burk plot analysis |
Eur J Med Chem 46: 811-8 (2011)
Article DOI: 10.1016/j.ejmech.2010.12.011
BindingDB Entry DOI: 10.7270/Q2PR7W8D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50262988

(CHEMBL1200541 | N-(2-chlorobenzyl)-2-(2-(2-((2-chl...)Show SMILES CC[N+](CC)(CCNC(=O)C(=O)NCC[N+](CC)(CC)Cc1ccccc1Cl)Cc1ccccc1Cl Show InChI InChI=1S/C28H40Cl2N4O2/c1-5-33(6-2,21-23-13-9-11-15-25(23)29)19-17-31-27(35)28(36)32-18-20-34(7-3,8-4)22-24-14-10-12-16-26(24)30/h9-16H,5-8,17-22H2,1-4H3/p+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex |
Bioorg Med Chem Lett 21: 2505-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.047
BindingDB Entry DOI: 10.7270/Q2JW8F6N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50336116

(Ambenonium dichloride | CHEMBL1669479)Show SMILES C[N+](C)(CCNC(=O)C(=O)NCC[N+](C)(C)Cc1ccccc1Cl)Cc1ccccc1Cl Show InChI InChI=1S/C24H32Cl2N4O2/c1-29(2,17-19-9-5-7-11-21(19)25)15-13-27-23(31)24(32)28-14-16-30(3,4)18-20-10-6-8-12-22(20)26/h5-12H,13-18H2,1-4H3/p+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Non competitive inhibition of human recombinant AChE using ATChCl as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50606715
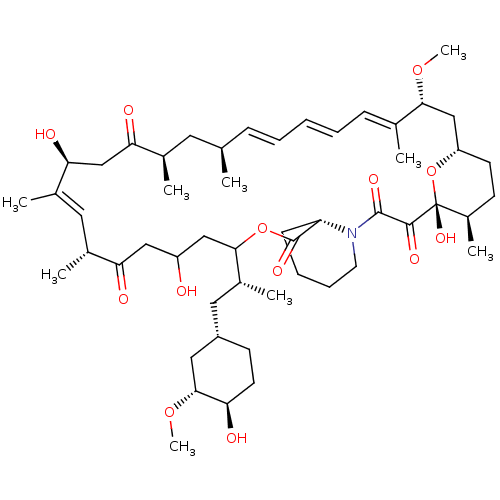
(CHEMBL5219513)Show SMILES [H][C@@]12CC[C@@H](C)[C@@](O)(O1)C(=O)C(=O)N1CCCC[C@H]1C(=O)OC(CC(O)CC(=O)[C@H](C)\C=C(C)\[C@@H](O)CC(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C2)OC)[C@H](C)C[C@@H]1CC[C@@H](O)[C@@H](C1)OC |r,c:33,50,t:46,48| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50606716

(CHEMBL5218757)Show SMILES [H][C@@]12CC[C@@H](C)[C@@](O)(O1)C(=O)C(=O)N1CCCC[C@H]1C(=O)OC(C\C=C(C)\[C@@H](O)CC(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](O)C2)[C@H](C)C[C@H]1CC[C@H](O)CC1 |r,wU:4.4,18.20,47.50,50.52,6.6,1.0,44.46,wD:53.56,27.29,32.34,35.37,c:26,43,t:39,41,(-3.43,-3.47,;-4.92,-3.86,;-6.38,-4.38,;-7.46,-3.39,;-7.18,-1.89,;-8.35,-.89,;-5.81,-1.37,;-6.9,-.28,;-4.64,-2.36,;-5.55,.05,;-6.64,1.03,;-4.09,.55,;-3.01,-.43,;-3.83,1.96,;-5,2.96,;-4.72,4.47,;-3.27,4.97,;-2.19,3.99,;-2.47,2.48,;-.98,2.88,;-.58,4.37,;.11,1.79,;1.59,2.19,;4.97,.24,;4.72,-1.11,;5.75,-2.25,;7.25,-1.94,;5.27,-3.72,;3.76,-4.04,;6.3,-4.86,;5.82,-6.33,;4.31,-6.65,;6.85,-7.47,;8.35,-7.16,;6.37,-8.94,;4.86,-9.26,;4.38,-10.72,;3.83,-8.11,;2.33,-8.43,;1.3,-7.28,;-.21,-7.6,;-1.24,-6.45,;-2.74,-6.77,;-3.22,-8.23,;-3.77,-5.62,;-4.54,-6.96,;-5.05,-5.05,;1.99,3.68,;3.48,4.08,;.9,4.77,;1.3,6.26,;.21,7.35,;.61,8.83,;2.1,9.23,;2.5,10.72,;3.19,8.14,;2.79,6.66,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50606712
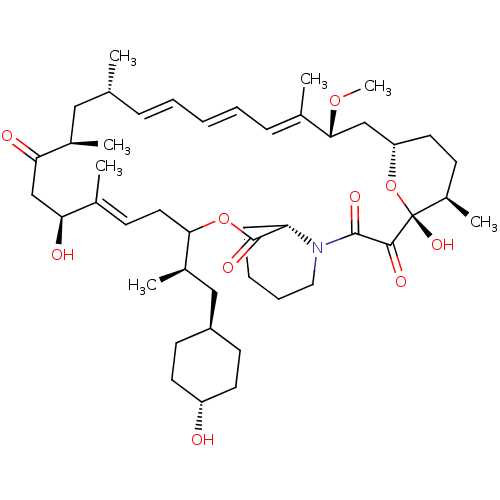
(CHEMBL5219200)Show SMILES [H][C@@]12CC[C@@H](C)[C@@](O)(O1)C(=O)C(=O)N1CCCC[C@H]1C(=O)OC(C\C=C(C)\[C@@H](O)CC(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C2)OC)[C@H](C)C[C@H]1CC[C@H](O)CC1 |r,wU:4.4,18.20,48.51,51.53,6.6,1.0,44.48,wD:54.57,27.29,32.34,35.37,c:26,43,t:39,41,(-3.43,-3.47,;-4.92,-3.86,;-6.38,-4.38,;-7.46,-3.39,;-7.18,-1.89,;-8.35,-.89,;-5.81,-1.37,;-6.9,-.28,;-4.64,-2.36,;-5.55,.05,;-6.64,1.03,;-4.09,.55,;-3.01,-.42,;-3.83,1.96,;-5.01,2.96,;-4.72,4.47,;-3.27,4.97,;-2.19,3.99,;-2.47,2.48,;-.98,2.88,;-.58,4.37,;.11,1.79,;1.59,2.19,;5.2,.36,;4.72,-1.11,;5.75,-2.25,;7.25,-1.94,;5.27,-3.72,;3.76,-4.04,;6.3,-4.86,;5.82,-6.33,;4.31,-6.65,;6.85,-7.47,;8.35,-7.16,;6.37,-8.94,;4.86,-9.26,;4.38,-10.72,;3.83,-8.11,;2.33,-8.43,;1.3,-7.28,;-.21,-7.6,;-1.24,-6.45,;-2.74,-6.77,;-3.22,-8.23,;-3.77,-5.62,;-5.05,-5.05,;-4.54,-6.96,;-6.08,-6.96,;1.99,3.68,;3.48,4.08,;.9,4.77,;1.3,6.26,;.21,7.35,;.61,8.83,;2.1,9.23,;2.5,10.72,;3.19,8.14,;2.79,6.66,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005114

(CHEMBL3093804)Show SMILES [Br-].[Br-].C(CCCCCC[n+]1cccc2ccccc12)CCCCC[n+]1ccccc1 Show InChI InChI=1S/C26H36N2.2BrH/c1(3-5-7-12-20-27-21-13-9-14-22-27)2-4-6-8-15-23-28-24-16-18-25-17-10-11-19-26(25)28;;/h9-11,13-14,16-19,21-22,24H,1-8,12,15,20,23H2;2*1H/q+2;;/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Non competitive inhibition of human recombinant AChE using ATChCl as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10624

(1,5-bis(4-allyldimethylammoniumphenyl)-pentan-3-on...)Show SMILES C[N+](C)(CC=C)c1ccc(CCC(=O)CCc2ccc(cc2)[N+](C)(C)CC=C)cc1 Show InChI InChI=1S/C27H38N2O/c1-7-21-28(3,4)25-15-9-23(10-16-25)13-19-27(30)20-14-24-11-17-26(18-12-24)29(5,6)22-8-2/h7-12,15-18H,1-2,13-14,19-22H2,3-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex by Lineweaver-Burk plot |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10624

(1,5-bis(4-allyldimethylammoniumphenyl)-pentan-3-on...)Show SMILES C[N+](C)(CC=C)c1ccc(CCC(=O)CCc2ccc(cc2)[N+](C)(C)CC=C)cc1 Show InChI InChI=1S/C27H38N2O/c1-7-21-28(3,4)25-15-9-23(10-16-25)13-19-27(30)20-14-24-11-17-26(18-12-24)29(5,6)22-8-2/h7-12,15-18H,1-2,13-14,19-22H2,3-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex |
Bioorg Med Chem Lett 21: 2505-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.047
BindingDB Entry DOI: 10.7270/Q2JW8F6N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10624

(1,5-bis(4-allyldimethylammoniumphenyl)-pentan-3-on...)Show SMILES C[N+](C)(CC=C)c1ccc(CCC(=O)CCc2ccc(cc2)[N+](C)(C)CC=C)cc1 Show InChI InChI=1S/C27H38N2O/c1-7-21-28(3,4)25-15-9-23(10-16-25)13-19-27(30)20-14-24-11-17-26(18-12-24)29(5,6)22-8-2/h7-12,15-18H,1-2,13-14,19-22H2,3-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Dissociation constant for enzyme-inhibitor complex of human recombinant AChE by Lineweaver-Burk plot analysis |
Eur J Med Chem 46: 811-8 (2011)
Article DOI: 10.1016/j.ejmech.2010.12.011
BindingDB Entry DOI: 10.7270/Q2PR7W8D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50389380

(CHEMBL2064468)Show SMILES CN(CCCN1CCN(CCNc2c3CCCCc3nc3ccccc23)CC1)C1CCCCC1 Show InChI InChI=1S/C29H45N5/c1-32(24-10-3-2-4-11-24)17-9-18-33-20-22-34(23-21-33)19-16-30-29-25-12-5-7-14-27(25)31-28-15-8-6-13-26(28)29/h5,7,12,14,24H,2-4,6,8-11,13,15-23H2,1H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex using acetylthiocholine substrate preincubated fo... |
Eur J Med Chem 55: 23-31 (2012)
Article DOI: 10.1016/j.ejmech.2012.06.051
BindingDB Entry DOI: 10.7270/Q2HH6M4Z |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM582478

(5-(2-aminobenzoxazol-5-yl)-7-isobutyl-6, 7-dihydro...) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50389381
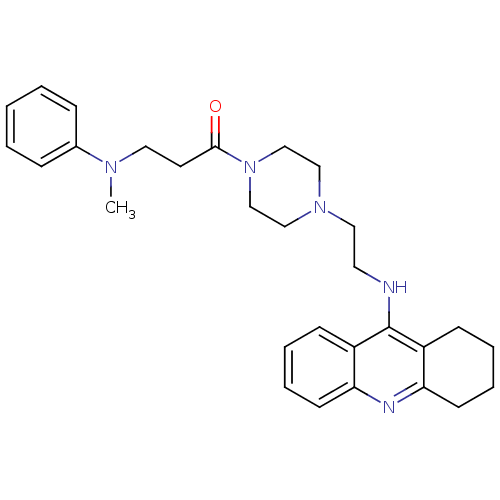
(CHEMBL2064466)Show SMILES CN(CCC(=O)N1CCN(CCNc2c3CCCCc3nc3ccccc23)CC1)c1ccccc1 Show InChI InChI=1S/C29H37N5O/c1-32(23-9-3-2-4-10-23)17-15-28(35)34-21-19-33(20-22-34)18-16-30-29-24-11-5-7-13-26(24)31-27-14-8-6-12-25(27)29/h2-5,7,9-11,13H,6,8,12,14-22H2,1H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex using acetylthiocholine substrate preincubated fo... |
Eur J Med Chem 55: 23-31 (2012)
Article DOI: 10.1016/j.ejmech.2012.06.051
BindingDB Entry DOI: 10.7270/Q2HH6M4Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50389384

(CHEMBL2064404)Show SMILES O=C(CCN1CCCCC1)N1CCN(CCNc2c3CCCCc3nc3ccccc23)CC1 Show InChI InChI=1S/C27H39N5O/c33-26(12-16-30-14-6-1-7-15-30)32-20-18-31(19-21-32)17-13-28-27-22-8-2-4-10-24(22)29-25-11-5-3-9-23(25)27/h2,4,8,10H,1,3,5-7,9,11-21H2,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex using acetylthiocholine substrate preincubated fo... |
Eur J Med Chem 55: 23-31 (2012)
Article DOI: 10.1016/j.ejmech.2012.06.051
BindingDB Entry DOI: 10.7270/Q2HH6M4Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50119798
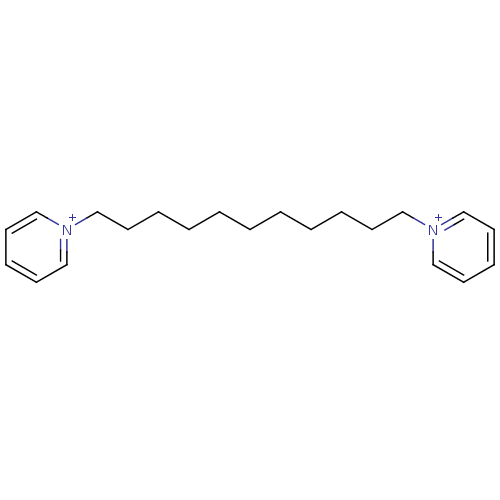
(1,11-bis(pyridinium)-undecane dibromide | 1,11-di(...)Show InChI InChI=1S/C21H32N2/c1(2-4-6-10-16-22-18-12-8-13-19-22)3-5-7-11-17-23-20-14-9-15-21-23/h8-9,12-15,18-21H,1-7,10-11,16-17H2/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Military Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE after 5 mins by noncompetitive Lineweaver-burk plot analysis for enzyme-inhibitor complex |
Bioorg Med Chem Lett 20: 1763-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.034
BindingDB Entry DOI: 10.7270/Q2BK1CGS |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005114

(CHEMBL3093804)Show SMILES [Br-].[Br-].C(CCCCCC[n+]1cccc2ccccc12)CCCCC[n+]1ccccc1 Show InChI InChI=1S/C26H36N2.2BrH/c1(3-5-7-12-20-27-21-13-9-14-22-27)2-4-6-8-15-23-28-24-16-18-25-17-10-11-19-26(25)28;;/h9-11,13-14,16-19,21-22,24H,1-8,12,15,20,23H2;2*1H/q+2;;/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056134

(CHEMBL3322160)Show SMILES Oc1ccc2c(CC(=O)NCCCCNc3c4CCCCc4nc4ccccc34)cc(=O)oc2c1 Show InChI InChI=1S/C28H29N3O4/c32-19-11-12-20-18(16-27(34)35-25(20)17-19)15-26(33)29-13-5-6-14-30-28-21-7-1-3-9-23(21)31-24-10-4-2-8-22(24)28/h1,3,7,9,11-12,16-17,32H,2,4-6,8,10,13-15H2,(H,29,33)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex by Lineweaver-Burk plot |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50389379
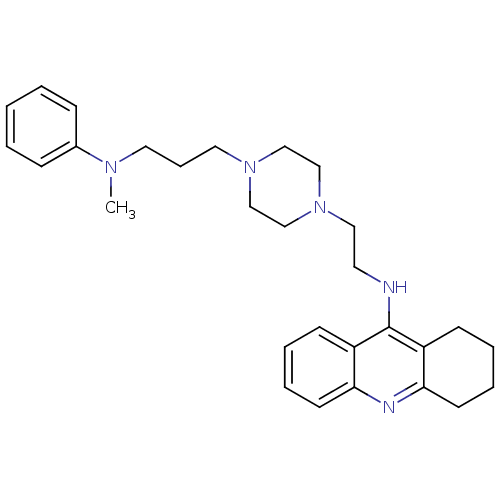
(CHEMBL2064470)Show SMILES CN(CCCN1CCN(CCNc2c3CCCCc3nc3ccccc23)CC1)c1ccccc1 Show InChI InChI=1S/C29H39N5/c1-32(24-10-3-2-4-11-24)17-9-18-33-20-22-34(23-21-33)19-16-30-29-25-12-5-7-14-27(25)31-28-15-8-6-13-26(28)29/h2-5,7,10-12,14H,6,8-9,13,15-23H2,1H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex using acetylthiocholine substrate prein... |
Eur J Med Chem 55: 23-31 (2012)
Article DOI: 10.1016/j.ejmech.2012.06.051
BindingDB Entry DOI: 10.7270/Q2HH6M4Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50119798

(1,11-bis(pyridinium)-undecane dibromide | 1,11-di(...)Show InChI InChI=1S/C21H32N2/c1(2-4-6-10-16-22-18-12-8-13-19-22)3-5-7-11-17-23-20-14-9-15-21-23/h8-9,12-15,18-21H,1-7,10-11,16-17H2/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Military Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE after 5 mins by noncompetitive Lineweaver-burk plot analysis |
Bioorg Med Chem Lett 20: 1763-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.034
BindingDB Entry DOI: 10.7270/Q2BK1CGS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606734

(CHEMBL5221072) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50119797

(1,1'-(decane-1,10-diyl)diquinolinium iodide | 1,10...)Show SMILES C(CCCCC[n+]1cccc2ccccc12)CCCC[n+]1cccc2ccccc12 Show InChI InChI=1S/C28H34N2/c1(3-5-11-21-29-23-13-17-25-15-7-9-19-27(25)29)2-4-6-12-22-30-24-14-18-26-16-8-10-20-28(26)30/h7-10,13-20,23-24H,1-6,11-12,21-22H2/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex |
Bioorg Med Chem Lett 21: 2505-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.047
BindingDB Entry DOI: 10.7270/Q2JW8F6N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50389380

(CHEMBL2064468)Show SMILES CN(CCCN1CCN(CCNc2c3CCCCc3nc3ccccc23)CC1)C1CCCCC1 Show InChI InChI=1S/C29H45N5/c1-32(24-10-3-2-4-11-24)17-9-18-33-20-22-34(23-21-33)19-16-30-29-25-12-5-7-14-27(25)31-28-15-8-6-13-26(28)29/h5,7,12,14,24H,2-4,6,8-11,13,15-23H2,1H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 47.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex using acetylthiocholine substrate prein... |
Eur J Med Chem 55: 23-31 (2012)
Article DOI: 10.1016/j.ejmech.2012.06.051
BindingDB Entry DOI: 10.7270/Q2HH6M4Z |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM10624

(1,5-bis(4-allyldimethylammoniumphenyl)-pentan-3-on...)Show SMILES C[N+](C)(CC=C)c1ccc(CCC(=O)CCc2ccc(cc2)[N+](C)(C)CC=C)cc1 Show InChI InChI=1S/C27H38N2O/c1-7-21-28(3,4)25-15-9-23(10-16-25)13-19-27(30)20-14-24-11-17-26(18-12-24)29(5,6)22-8-2/h7-12,15-18H,1-2,13-14,19-22H2,3-6H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex by Lineweaver-Burk plot |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10624

(1,5-bis(4-allyldimethylammoniumphenyl)-pentan-3-on...)Show SMILES C[N+](C)(CC=C)c1ccc(CCC(=O)CCc2ccc(cc2)[N+](C)(C)CC=C)cc1 Show InChI InChI=1S/C27H38N2O/c1-7-21-28(3,4)25-15-9-23(10-16-25)13-19-27(30)20-14-24-11-17-26(18-12-24)29(5,6)22-8-2/h7-12,15-18H,1-2,13-14,19-22H2,3-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex |
Bioorg Med Chem Lett 21: 2505-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.047
BindingDB Entry DOI: 10.7270/Q2JW8F6N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10624

(1,5-bis(4-allyldimethylammoniumphenyl)-pentan-3-on...)Show SMILES C[N+](C)(CC=C)c1ccc(CCC(=O)CCc2ccc(cc2)[N+](C)(C)CC=C)cc1 Show InChI InChI=1S/C27H38N2O/c1-7-21-28(3,4)25-15-9-23(10-16-25)13-19-27(30)20-14-24-11-17-26(18-12-24)29(5,6)22-8-2/h7-12,15-18H,1-2,13-14,19-22H2,3-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Non competitive inhibition of human recombinant AChE using ATChCl as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10624

(1,5-bis(4-allyldimethylammoniumphenyl)-pentan-3-on...)Show SMILES C[N+](C)(CC=C)c1ccc(CCC(=O)CCc2ccc(cc2)[N+](C)(C)CC=C)cc1 Show InChI InChI=1S/C27H38N2O/c1-7-21-28(3,4)25-15-9-23(10-16-25)13-19-27(30)20-14-24-11-17-26(18-12-24)29(5,6)22-8-2/h7-12,15-18H,1-2,13-14,19-22H2,3-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Dissociation constant for enzyme-inhibitor-substrate complex of human recombinant AChE by Lineweaver-Burk plot analysis |
Eur J Med Chem 46: 811-8 (2011)
Article DOI: 10.1016/j.ejmech.2010.12.011
BindingDB Entry DOI: 10.7270/Q2PR7W8D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50133473
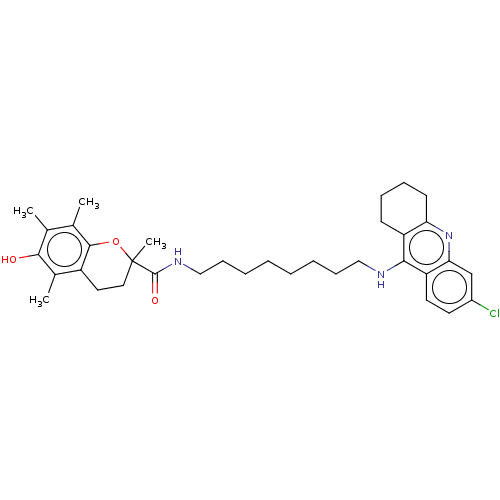
(CHEMBL3632994)Show SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(=O)NCCCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C35H46ClN3O3/c1-22-23(2)33-26(24(3)32(22)40)17-18-35(4,42-33)34(41)38-20-12-8-6-5-7-11-19-37-31-27-13-9-10-14-29(27)39-30-21-25(36)15-16-28(30)31/h15-16,21,40H,5-14,17-20H2,1-4H3,(H,37,39)(H,38,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant AChE using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data