

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50315548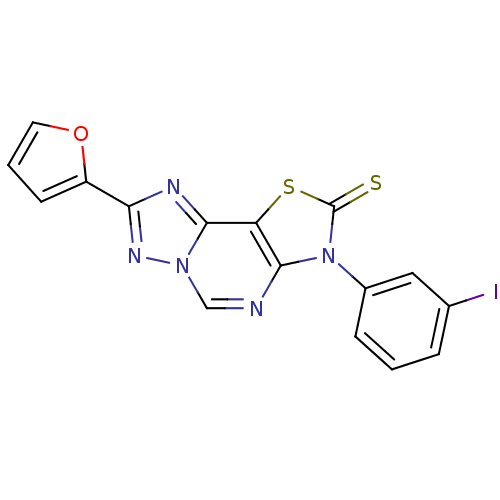 (8-(2-Thioxo-7(3-m-iodophenyl)-2-(2-furyl)thiazolo[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells after 60 mins by rapid filtration assay | Bioorg Med Chem 18: 2491-500 (2010) Article DOI: 10.1016/j.bmc.2010.02.048 BindingDB Entry DOI: 10.7270/Q27W6D57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcium/calmodulin-dependent protein kinase type IV [15-340] (Homo sapiens (Human)) | BDBM223209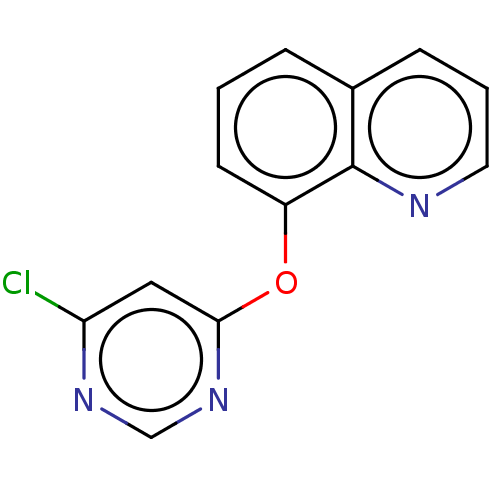 (8-((6-Chloropyrimidin-4-yl)oxy)quinoline (Compound...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 0.0109 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
B.R. Ambedkar Bihar University | Assay Description The docking and scoring of ligands with CAMKIV protein was accomplished using ParDOCK module of Sanjeevini drug design suite, which is based on physi... | Chem Biol Drug Des 89: 741-754 (2017) Article DOI: 10.1111/cbdd.12898 BindingDB Entry DOI: 10.7270/Q28C9V30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50315541 (8-(2-Thioxo-7(3-allyl)-2-(2-furyl)thiazole[4,3-e]1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells after 60 mins by rapid filtration assay | Bioorg Med Chem 18: 2491-500 (2010) Article DOI: 10.1016/j.bmc.2010.02.048 BindingDB Entry DOI: 10.7270/Q27W6D57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50315544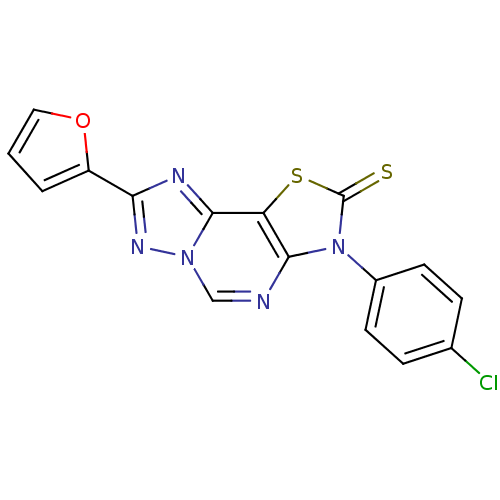 (8-(2-Thioxo-7(3-p-chlorophenyl.)-2-(2-furyl)thiazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in HEK293 cells after 60 mins by rapid filtration assay | Bioorg Med Chem 18: 2491-500 (2010) Article DOI: 10.1016/j.bmc.2010.02.048 BindingDB Entry DOI: 10.7270/Q27W6D57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50315545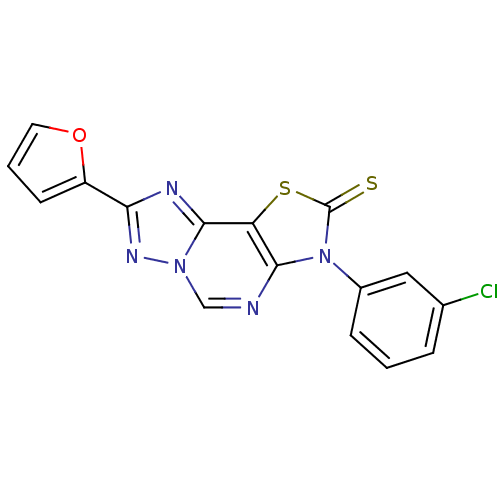 (8-(2-Thioxo-7(3-m-chlorophenyl)-2-(2-furyl)thiazol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells after 60 mins by rapid filtration assay | Bioorg Med Chem 18: 2491-500 (2010) Article DOI: 10.1016/j.bmc.2010.02.048 BindingDB Entry DOI: 10.7270/Q27W6D57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50315538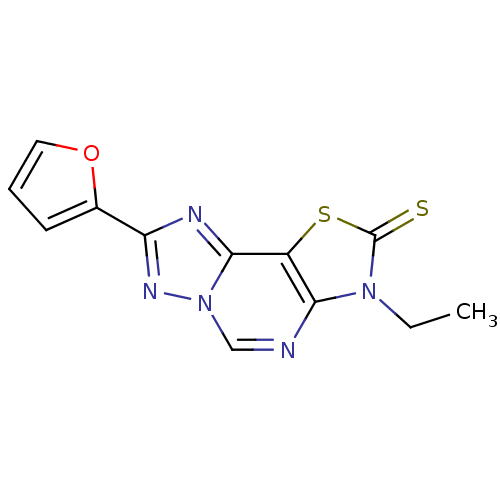 (8-(2-Thioxo-7(3-ethyl)-2-(2-furyl)thiazolo[4,3-e]1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in HEK293 cells after 60 mins by rapid filtration assay | Bioorg Med Chem 18: 2491-500 (2010) Article DOI: 10.1016/j.bmc.2010.02.048 BindingDB Entry DOI: 10.7270/Q27W6D57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50315540 (8-(2-Thioxo-7(3-butyl)-2-(2-furyl),thiazolo,[4,3-e...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells after 60 mins by rapid filtration assay | Bioorg Med Chem 18: 2491-500 (2010) Article DOI: 10.1016/j.bmc.2010.02.048 BindingDB Entry DOI: 10.7270/Q27W6D57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/2C (Homo sapiens (Human)) | BDBM50024605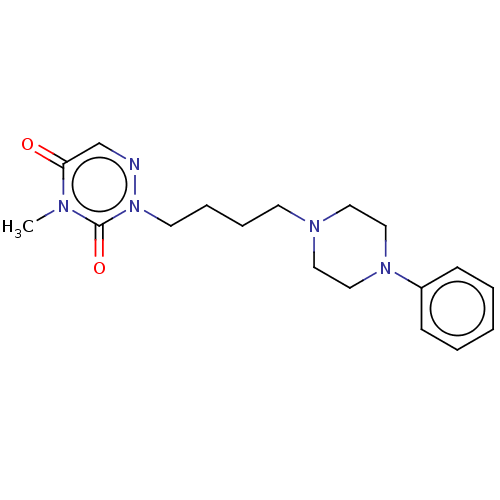 (CHEMBL3330603 | US9290463, E) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.150 | -52.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
The Trustees of Columbia University in the City of New York US Patent | Assay Description Preparation of Membrane Fractions from CHO-h5-HT1A Cells. Membranes from CHO cells stably expressing the human 5-HT1A receptor at a density of 8 pmol... | US Patent US9290463 (2016) BindingDB Entry DOI: 10.7270/Q2MS3RK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM86757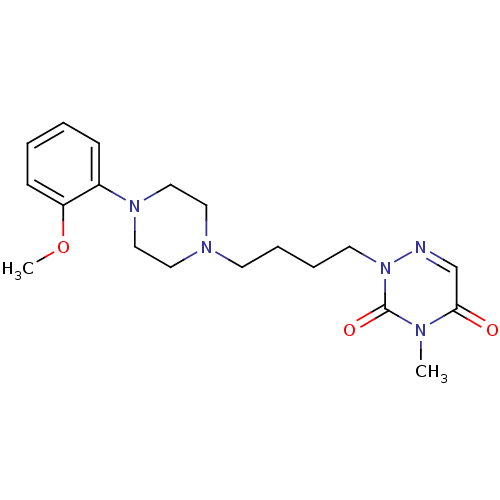 (CAS_0 | NSC_11603174 | [11C]MMP) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1AR (unknown origin) by competition binding assay | Bioorg Med Chem Lett 24: 4759-62 (2014) Article DOI: 10.1016/j.bmcl.2014.07.048 BindingDB Entry DOI: 10.7270/Q2NZ896M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50315544 (8-(2-Thioxo-7(3-p-chlorophenyl.)-2-(2-furyl)thiazo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.152 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells after 60 mins by rapid filtration assay | Bioorg Med Chem 18: 2491-500 (2010) Article DOI: 10.1016/j.bmc.2010.02.048 BindingDB Entry DOI: 10.7270/Q27W6D57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50315545 (8-(2-Thioxo-7(3-m-chlorophenyl)-2-(2-furyl)thiazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in HEK293 cells after 60 mins by rapid filtration assay | Bioorg Med Chem 18: 2491-500 (2010) Article DOI: 10.1016/j.bmc.2010.02.048 BindingDB Entry DOI: 10.7270/Q27W6D57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50024574 (CHEMBL3330616) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1AR (unknown origin) by competition binding assay | Bioorg Med Chem Lett 24: 4759-62 (2014) Article DOI: 10.1016/j.bmcl.2014.07.048 BindingDB Entry DOI: 10.7270/Q2NZ896M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50315539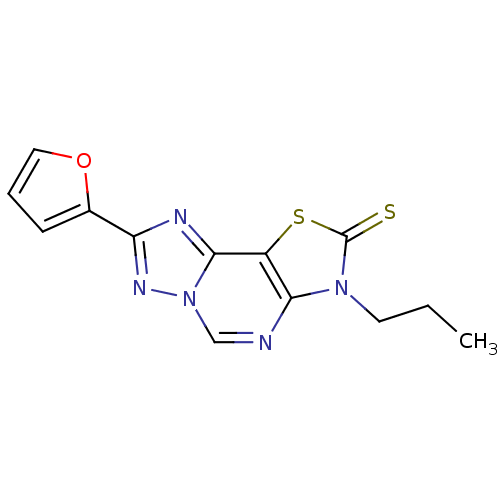 (8-(2-Thioxo-7(3-propyl)-2-(2-furyl)thiazolo[4,3-e]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells after 60 mins by rapid filtration assay | Bioorg Med Chem 18: 2491-500 (2010) Article DOI: 10.1016/j.bmc.2010.02.048 BindingDB Entry DOI: 10.7270/Q27W6D57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50315546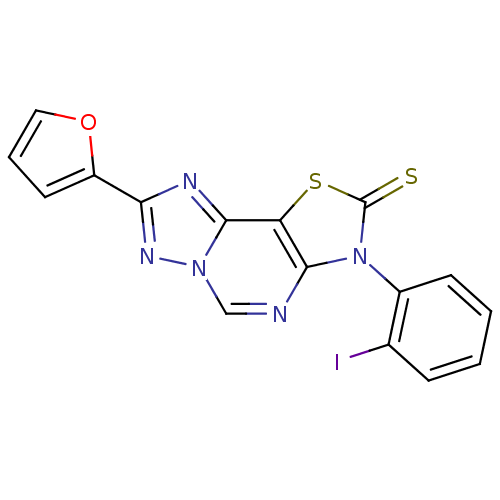 (8-(2-Thioxo-7(3-o-iodophenyl)-2-(2-furyl)thiazolo[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in HEK293 cells after 60 mins by rapid filtration assay | Bioorg Med Chem 18: 2491-500 (2010) Article DOI: 10.1016/j.bmc.2010.02.048 BindingDB Entry DOI: 10.7270/Q27W6D57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50024567 (CHEMBL3330623) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1AR (unknown origin) by competition binding assay | Bioorg Med Chem Lett 24: 4759-62 (2014) Article DOI: 10.1016/j.bmcl.2014.07.048 BindingDB Entry DOI: 10.7270/Q2NZ896M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50024643 (CHEMBL3329234) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1AR (unknown origin) by competition binding assay | Bioorg Med Chem Lett 24: 4759-62 (2014) Article DOI: 10.1016/j.bmcl.2014.07.048 BindingDB Entry DOI: 10.7270/Q2NZ896M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50315547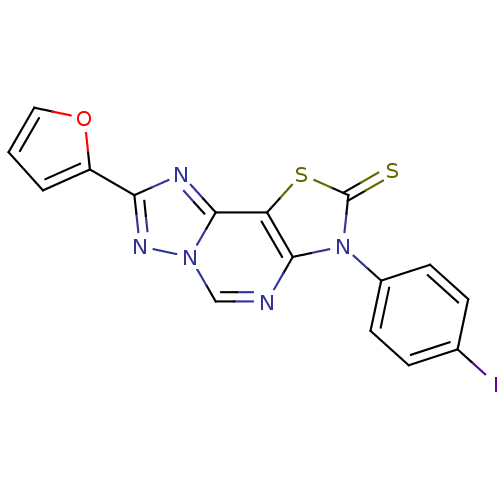 (8-(2-Thioxo-7(3-p-iodophenyl)-2-(2-furyl)thiazolo[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells after 60 mins by rapid filtration assay | Bioorg Med Chem 18: 2491-500 (2010) Article DOI: 10.1016/j.bmc.2010.02.048 BindingDB Entry DOI: 10.7270/Q27W6D57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50024647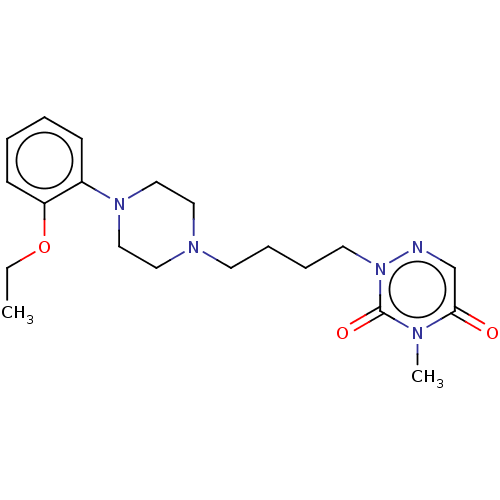 (CHEMBL3330599) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1AR (unknown origin) by competition binding assay | Bioorg Med Chem Lett 24: 4759-62 (2014) Article DOI: 10.1016/j.bmcl.2014.07.048 BindingDB Entry DOI: 10.7270/Q2NZ896M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50315546 (8-(2-Thioxo-7(3-o-iodophenyl)-2-(2-furyl)thiazolo[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells after 60 mins by rapid filtration assay | Bioorg Med Chem 18: 2491-500 (2010) Article DOI: 10.1016/j.bmc.2010.02.048 BindingDB Entry DOI: 10.7270/Q27W6D57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50419052 (SB-399885) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.776 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Medical Center Curated by ChEMBL | Assay Description Displacement of [125I]SB-258585 from human recombinant 5HT6 receptor using methiothepin after 45 mins by liquid scintillation spectrometry | Bioorg Med Chem 19: 5255-9 (2011) Article DOI: 10.1016/j.bmc.2011.06.090 BindingDB Entry DOI: 10.7270/Q2TM7CDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50024646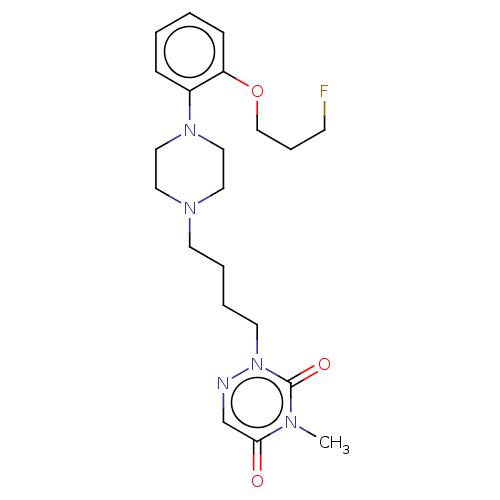 (CHEMBL3330600) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1AR (unknown origin) by competition binding assay | Bioorg Med Chem Lett 24: 4759-62 (2014) Article DOI: 10.1016/j.bmcl.2014.07.048 BindingDB Entry DOI: 10.7270/Q2NZ896M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50024595 (CHEMBL3330605) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.835 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1AR (unknown origin) by competition binding assay | Bioorg Med Chem Lett 24: 4759-62 (2014) Article DOI: 10.1016/j.bmcl.2014.07.048 BindingDB Entry DOI: 10.7270/Q2NZ896M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50182020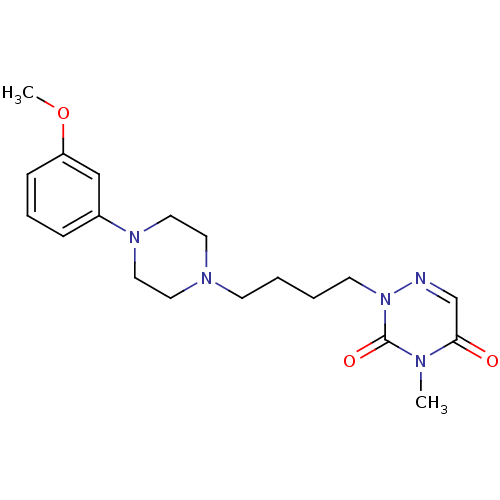 (2-(4-(4-(3-methoxyphenyl)piperazin-1-yl)butyl)-4-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description Agonist activity assessed by stimulation of [35S]GTP-gamma-S binding to human 5HT1A receptor expressed in CHO cells | Bioorg Med Chem Lett 16: 2101-4 (2006) Article DOI: 10.1016/j.bmcl.2006.01.052 BindingDB Entry DOI: 10.7270/Q2ZC82FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50392592 (CHEMBL2153381) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Inhibition of F10a assessed as S-2765 substrate hydrolysis by microplate reader analysis | Eur J Med Chem 58: 136-52 (2012) Article DOI: 10.1016/j.ejmech.2012.10.005 BindingDB Entry DOI: 10.7270/Q2571D4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50315542 (8-(2-Thioxo-7(3-phenyl)-2-(2-furyl)thiazolo[4,3-e]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells after 60 mins by rapid filtration assay | Bioorg Med Chem 18: 2491-500 (2010) Article DOI: 10.1016/j.bmc.2010.02.048 BindingDB Entry DOI: 10.7270/Q27W6D57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50024580 (CHEMBL3330612) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1AR (unknown origin) by competition binding assay | Bioorg Med Chem Lett 24: 4759-62 (2014) Article DOI: 10.1016/j.bmcl.2014.07.048 BindingDB Entry DOI: 10.7270/Q2NZ896M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50024577 (CHEMBL3330614) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1AR (unknown origin) by competition binding assay | Bioorg Med Chem Lett 24: 4759-62 (2014) Article DOI: 10.1016/j.bmcl.2014.07.048 BindingDB Entry DOI: 10.7270/Q2NZ896M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50048466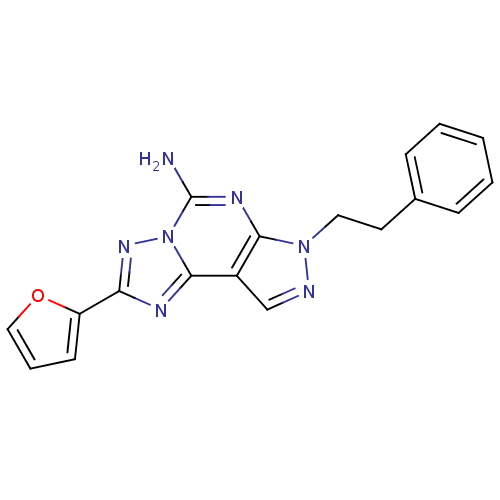 (2-(furan-2-yl)-7-phenethyl-7H-pyrazolo[4,3-e][1,2,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells after 60 mins by rapid filtration assay | Bioorg Med Chem 18: 2491-500 (2010) Article DOI: 10.1016/j.bmc.2010.02.048 BindingDB Entry DOI: 10.7270/Q27W6D57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50180054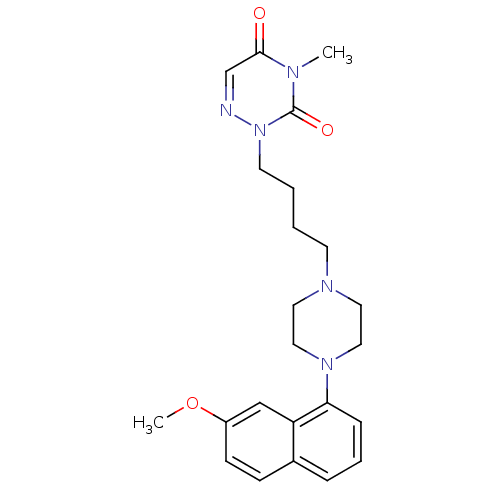 (CHEMBL199824 | [O-methyl-11C]2-{4-[4-(7-methoxynap...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 49: 125-34 (2006) Article DOI: 10.1021/jm050725j BindingDB Entry DOI: 10.7270/Q2R49QBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50024573 (CHEMBL3330617 | US9290463, A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1AR (unknown origin) by competition binding assay | Bioorg Med Chem Lett 24: 4759-62 (2014) Article DOI: 10.1016/j.bmcl.2014.07.048 BindingDB Entry DOI: 10.7270/Q2NZ896M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50392589 (CHEMBL2153377) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Inhibition of F10a assessed as S-2765 substrate hydrolysis by microplate reader analysis | Eur J Med Chem 58: 136-52 (2012) Article DOI: 10.1016/j.ejmech.2012.10.005 BindingDB Entry DOI: 10.7270/Q2571D4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50320446 (CHEMBL1085510 | [N-methyl]5-methyl-3-[4-(3-phenyla...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Displacement of [3H]rauwolscine from adrenergic alpha2A receptor | Bioorg Med Chem Lett 20: 3654-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.099 BindingDB Entry DOI: 10.7270/Q2474B1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50024584 (CHEMBL3330609) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1AR (unknown origin) by competition binding assay | Bioorg Med Chem Lett 24: 4759-62 (2014) Article DOI: 10.1016/j.bmcl.2014.07.048 BindingDB Entry DOI: 10.7270/Q2NZ896M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50024582 (CHEMBL3330611) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1AR (unknown origin) by competition binding assay | Bioorg Med Chem Lett 24: 4759-62 (2014) Article DOI: 10.1016/j.bmcl.2014.07.048 BindingDB Entry DOI: 10.7270/Q2NZ896M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50315543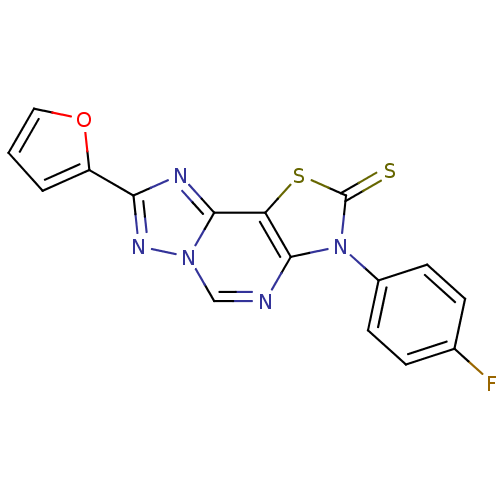 (8-(2-Thioxo-7(3-p fluorophenyl)-2-(2-furyl)thiazol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells after 60 mins by rapid filtration assay | Bioorg Med Chem 18: 2491-500 (2010) Article DOI: 10.1016/j.bmc.2010.02.048 BindingDB Entry DOI: 10.7270/Q27W6D57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50024566 (CHEMBL3330624) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1AR (unknown origin) by competition binding assay | Bioorg Med Chem Lett 24: 4759-62 (2014) Article DOI: 10.1016/j.bmcl.2014.07.048 BindingDB Entry DOI: 10.7270/Q2NZ896M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50024605 (CHEMBL3330603 | US9290463, E) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1AR (unknown origin) by competition binding assay | Bioorg Med Chem Lett 24: 4759-62 (2014) Article DOI: 10.1016/j.bmcl.2014.07.048 BindingDB Entry DOI: 10.7270/Q2NZ896M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50315548 (8-(2-Thioxo-7(3-m-iodophenyl)-2-(2-furyl)thiazolo[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in HEK293 cells after 60 mins by rapid filtration assay | Bioorg Med Chem 18: 2491-500 (2010) Article DOI: 10.1016/j.bmc.2010.02.048 BindingDB Entry DOI: 10.7270/Q27W6D57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50024583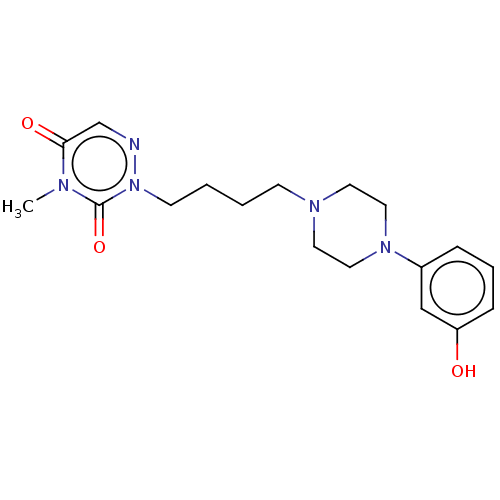 (CHEMBL3330610) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1AR (unknown origin) by competition binding assay | Bioorg Med Chem Lett 24: 4759-62 (2014) Article DOI: 10.1016/j.bmcl.2014.07.048 BindingDB Entry DOI: 10.7270/Q2NZ896M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50315543 (8-(2-Thioxo-7(3-p fluorophenyl)-2-(2-furyl)thiazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in HEK293 cells after 60 mins by rapid filtration assay | Bioorg Med Chem 18: 2491-500 (2010) Article DOI: 10.1016/j.bmc.2010.02.048 BindingDB Entry DOI: 10.7270/Q27W6D57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50315538 (8-(2-Thioxo-7(3-ethyl)-2-(2-furyl)thiazolo[4,3-e]1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells after 60 mins by rapid filtration assay | Bioorg Med Chem 18: 2491-500 (2010) Article DOI: 10.1016/j.bmc.2010.02.048 BindingDB Entry DOI: 10.7270/Q27W6D57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/2C (Homo sapiens (Human)) | BDBM50024573 (CHEMBL3330617 | US9290463, A) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 4 | -44.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
The Trustees of Columbia University in the City of New York US Patent | Assay Description Preparation of Membrane Fractions from CHO-h5-HT1A Cells. Membranes from CHO cells stably expressing the human 5-HT1A receptor at a density of 8 pmol... | US Patent US9290463 (2016) BindingDB Entry DOI: 10.7270/Q2MS3RK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50320446 (CHEMBL1085510 | [N-methyl]5-methyl-3-[4-(3-phenyla...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Displacement of [3H]rauwolscine from adrenergic alpha2C receptor | Bioorg Med Chem Lett 20: 3654-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.099 BindingDB Entry DOI: 10.7270/Q2474B1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50024587 (CHEMBL3330606) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1AR (unknown origin) by competition binding assay | Bioorg Med Chem Lett 24: 4759-62 (2014) Article DOI: 10.1016/j.bmcl.2014.07.048 BindingDB Entry DOI: 10.7270/Q2NZ896M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581953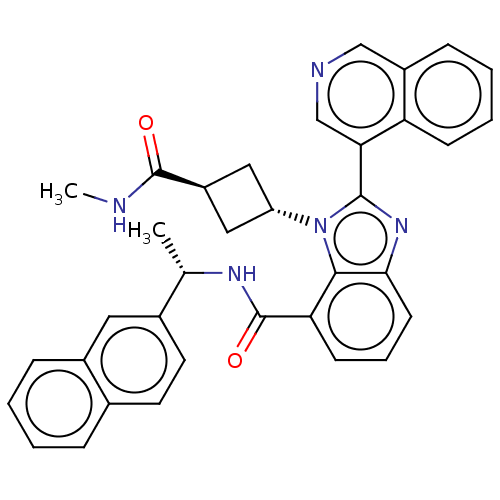 (WO2023004291, Compound CDD-1819) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | MCE PC cid PC sid UniChem | WIPO WO2023004291 | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BV7MGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/2C (Homo sapiens (Human)) | BDBM210829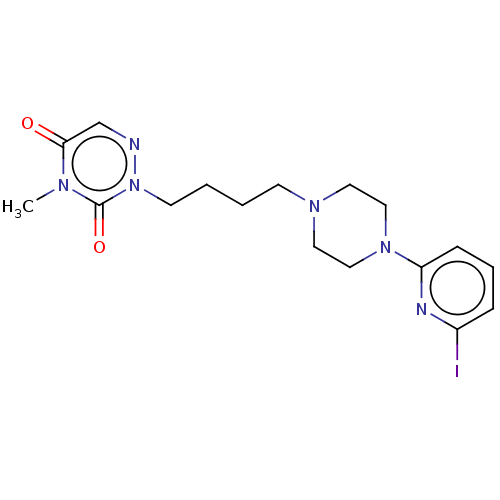 (US9290463, B) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.5 | -43.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
The Trustees of Columbia University in the City of New York US Patent | Assay Description Preparation of Membrane Fractions from CHO-h5-HT1A Cells. Membranes from CHO cells stably expressing the human 5-HT1A receptor at a density of 8 pmol... | US Patent US9290463 (2016) BindingDB Entry DOI: 10.7270/Q2MS3RK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581952 (WO2023004291, Compound CDD-1830) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2023004291 | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BV7MGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/2C (Homo sapiens (Human)) | BDBM210830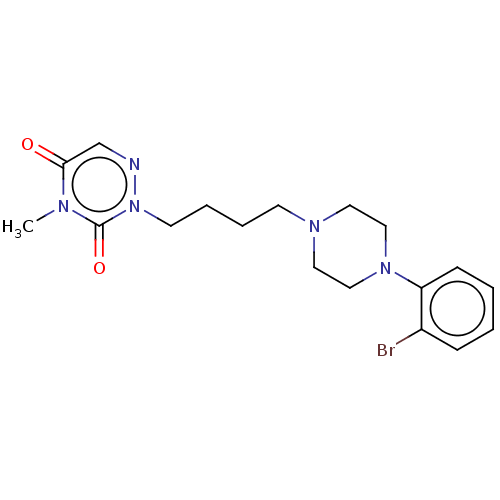 (US9290463, D) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6 | -43.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
The Trustees of Columbia University in the City of New York US Patent | Assay Description Preparation of Membrane Fractions from CHO-h5-HT1A Cells. Membranes from CHO cells stably expressing the human 5-HT1A receptor at a density of 8 pmol... | US Patent US9290463 (2016) BindingDB Entry DOI: 10.7270/Q2MS3RK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50315547 (8-(2-Thioxo-7(3-p-iodophenyl)-2-(2-furyl)thiazolo[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in HEK293 cells after 60 mins by rapid filtration assay | Bioorg Med Chem 18: 2491-500 (2010) Article DOI: 10.1016/j.bmc.2010.02.048 BindingDB Entry DOI: 10.7270/Q27W6D57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 22 member 1 (BOVINE) | BDBM86757 (CAS_0 | NSC_11603174 | [11C]MMP) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 6.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by PDSP Ki Database | Eur J Nucl Med Mol Imaging 34: 1050-60 (2007) Article DOI: 10.1007/s00259-006-0324-y BindingDB Entry DOI: 10.7270/Q2D7990W | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 766 total ) | Next | Last >> |