

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50129260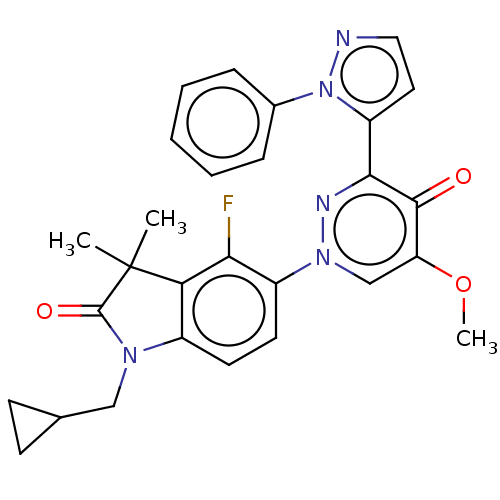 (CHEMBL3629735) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human PDE10A2 expressed in COS-7 cells using [3H]cGMP as substrate assessed as substrate hydrolysis after 60 mins by scintillation prox... | Bioorg Med Chem 23: 7138-49 (2015) Article DOI: 10.1016/j.bmc.2015.10.002 BindingDB Entry DOI: 10.7270/Q2NG4SGN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50129262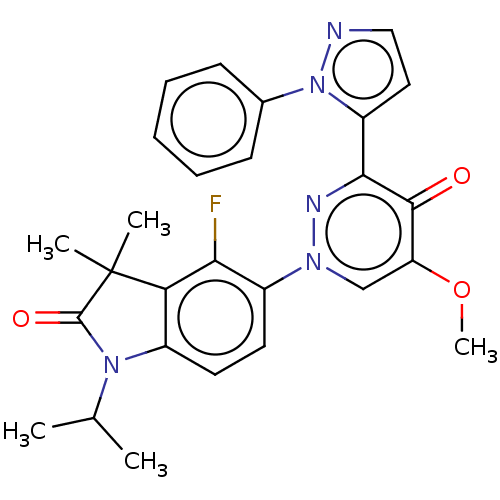 (CHEMBL3627741) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human PDE10A2 expressed in COS-7 cells using [3H]cGMP as substrate assessed as substrate hydrolysis after 60 mins by scintillation prox... | Bioorg Med Chem 23: 7138-49 (2015) Article DOI: 10.1016/j.bmc.2015.10.002 BindingDB Entry DOI: 10.7270/Q2NG4SGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50129261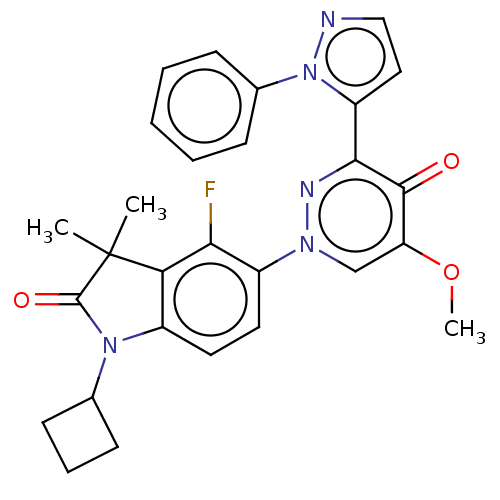 (CHEMBL3629734) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human PDE10A2 expressed in COS-7 cells using [3H]cGMP as substrate assessed as substrate hydrolysis after 60 mins by scintillation prox... | Bioorg Med Chem 23: 7138-49 (2015) Article DOI: 10.1016/j.bmc.2015.10.002 BindingDB Entry DOI: 10.7270/Q2NG4SGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50129263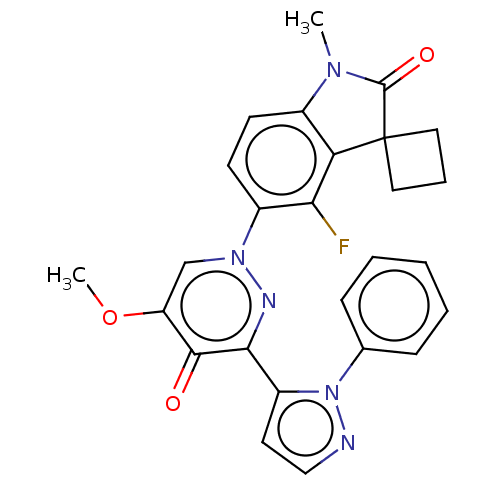 (CHEMBL3629733) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human PDE10A2 expressed in COS-7 cells using [3H]cGMP as substrate assessed as substrate hydrolysis after 60 mins by scintillation prox... | Bioorg Med Chem 23: 7138-49 (2015) Article DOI: 10.1016/j.bmc.2015.10.002 BindingDB Entry DOI: 10.7270/Q2NG4SGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451372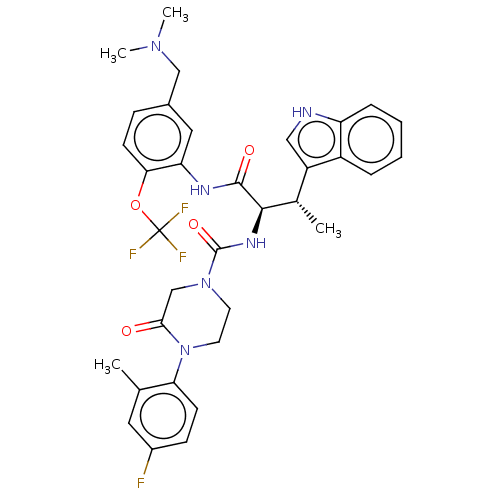 (CHEMBL4217405) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451373 (CHEMBL4206924) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451391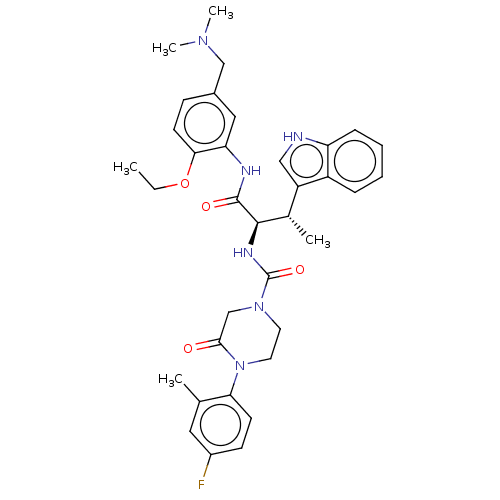 (CHEMBL4212088) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451392 (CHEMBL4214725) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451394 (CHEMBL4205606) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50129264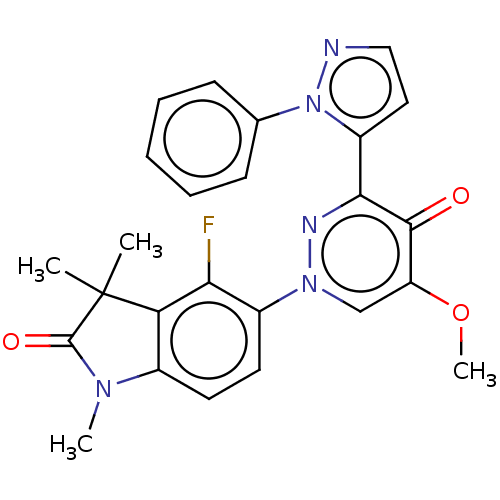 (CHEMBL3629732) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human PDE10A2 expressed in COS-7 cells using [3H]cGMP as substrate assessed as substrate hydrolysis after 60 mins by scintillation prox... | Bioorg Med Chem 23: 7138-49 (2015) Article DOI: 10.1016/j.bmc.2015.10.002 BindingDB Entry DOI: 10.7270/Q2NG4SGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50129265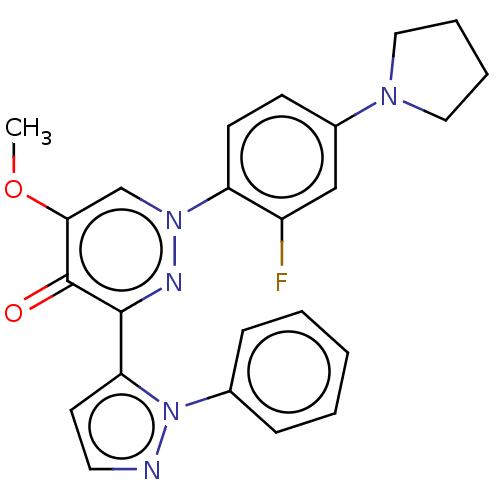 (CHEMBL3629542) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human PDE10A2 expressed in COS-7 cells using [3H]cGMP as substrate assessed as substrate hydrolysis after 60 mins by scintillation prox... | Bioorg Med Chem 23: 7138-49 (2015) Article DOI: 10.1016/j.bmc.2015.10.002 BindingDB Entry DOI: 10.7270/Q2NG4SGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451384 (CHEMBL4210064) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451385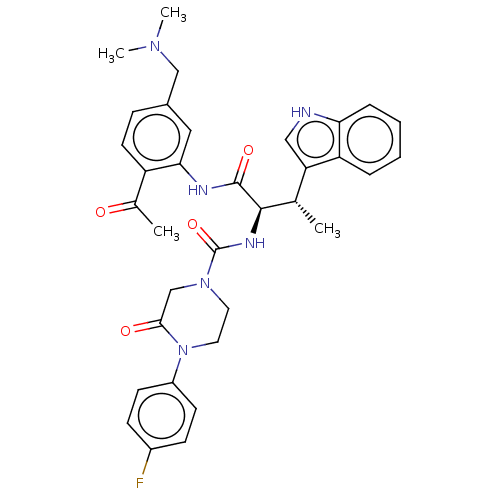 (CHEMBL4205969) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451382 (CHEMBL4205696) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50383112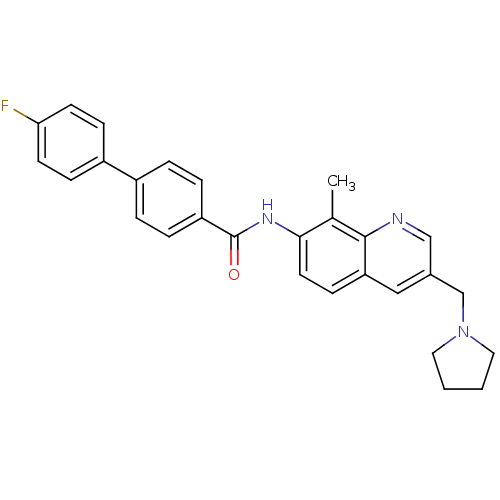 (CHEMBL2029372) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]MCH from human MCHR1 expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 55: 4336-51 (2012) Article DOI: 10.1021/jm300167z BindingDB Entry DOI: 10.7270/Q2JS9RHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451399 (CHEMBL4208528) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451395 (CHEMBL4211422) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451390 (CHEMBL4210627) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451388 (CHEMBL4215053) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50180268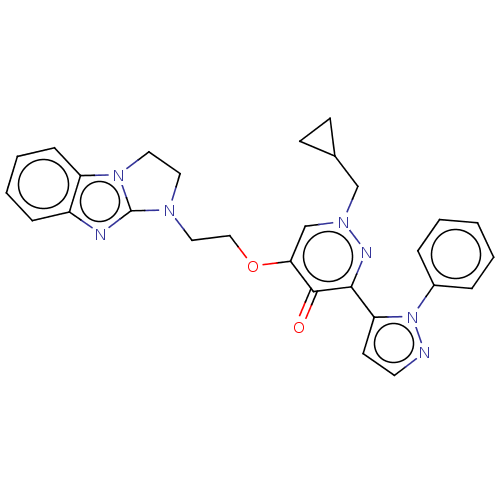 (CHEMBL3814662) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human full length PDE10A2 expressed in African green monkey COS7 cells using [3H]cGMP as substrate preincubated for 30 mins followed by... | Bioorg Med Chem 24: 3447-55 (2016) Article DOI: 10.1016/j.bmc.2016.05.049 BindingDB Entry DOI: 10.7270/Q2H9974G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50129266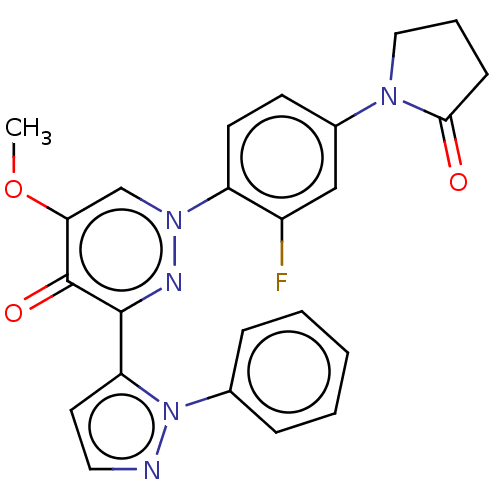 (CHEMBL3629541) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human PDE10A2 expressed in COS-7 cells using [3H]cGMP as substrate assessed as substrate hydrolysis after 60 mins by scintillation prox... | Bioorg Med Chem 23: 7138-49 (2015) Article DOI: 10.1016/j.bmc.2015.10.002 BindingDB Entry DOI: 10.7270/Q2NG4SGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50180271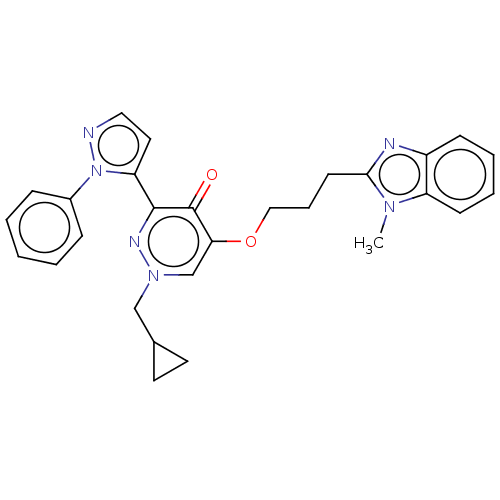 (CHEMBL3814601) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human full length PDE10A2 expressed in African green monkey COS7 cells using [3H]cGMP as substrate preincubated for 30 mins followed by... | Bioorg Med Chem 24: 3447-55 (2016) Article DOI: 10.1016/j.bmc.2016.05.049 BindingDB Entry DOI: 10.7270/Q2H9974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451396 (CHEMBL4216387) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50129267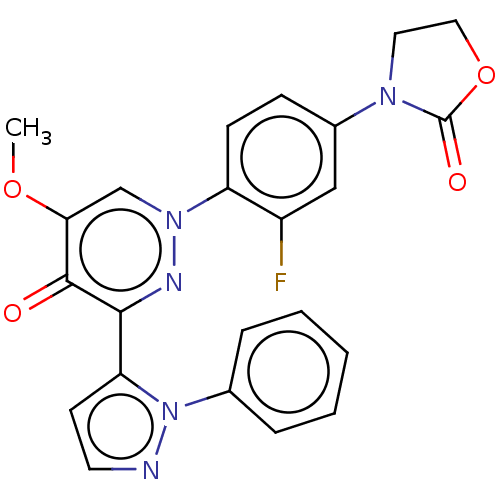 (CHEMBL3629540) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human PDE10A2 expressed in COS-7 cells using [3H]cGMP as substrate assessed as substrate hydrolysis after 60 mins by scintillation prox... | Bioorg Med Chem 23: 7138-49 (2015) Article DOI: 10.1016/j.bmc.2015.10.002 BindingDB Entry DOI: 10.7270/Q2NG4SGN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451386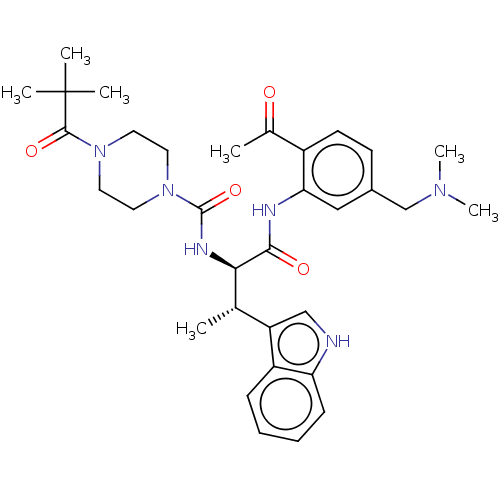 (CHEMBL4203793) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50383112 (CHEMBL2029372) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]MCH from rat MCHR1 expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 55: 4336-51 (2012) Article DOI: 10.1021/jm300167z BindingDB Entry DOI: 10.7270/Q2JS9RHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451397 (CHEMBL4208009) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50388452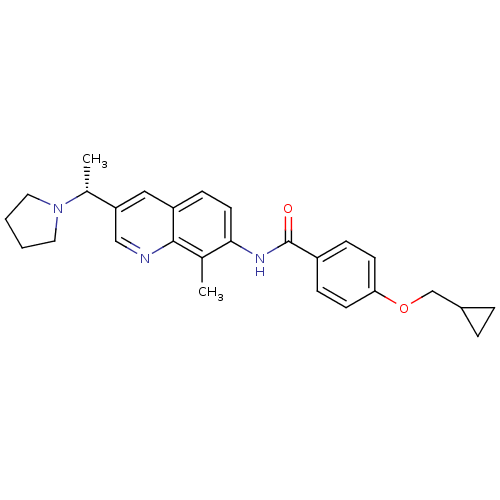 (CHEMBL2059420) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]MCH from human MCHR1 expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 55: 4336-51 (2012) Article DOI: 10.1021/jm300167z BindingDB Entry DOI: 10.7270/Q2JS9RHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50388442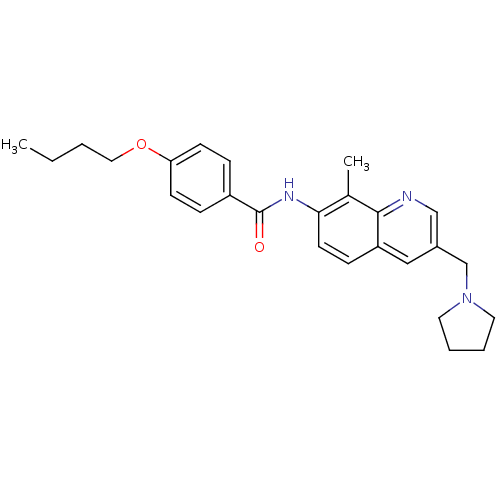 (CHEMBL2059408) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]MCH from rat MCHR1 expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 55: 4336-51 (2012) Article DOI: 10.1021/jm300167z BindingDB Entry DOI: 10.7270/Q2JS9RHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50388444 (CHEMBL2059410) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]MCH from human MCHR1 expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 55: 4336-51 (2012) Article DOI: 10.1021/jm300167z BindingDB Entry DOI: 10.7270/Q2JS9RHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50388460 (CHEMBL2059513) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]MCH from human MCHR1 expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 55: 4336-51 (2012) Article DOI: 10.1021/jm300167z BindingDB Entry DOI: 10.7270/Q2JS9RHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50388457 (CHEMBL2059426) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]MCH from human MCHR1 expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 55: 4336-51 (2012) Article DOI: 10.1021/jm300167z BindingDB Entry DOI: 10.7270/Q2JS9RHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50388443 (CHEMBL2059409) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]MCH from rat MCHR1 expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 55: 4336-51 (2012) Article DOI: 10.1021/jm300167z BindingDB Entry DOI: 10.7270/Q2JS9RHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50388450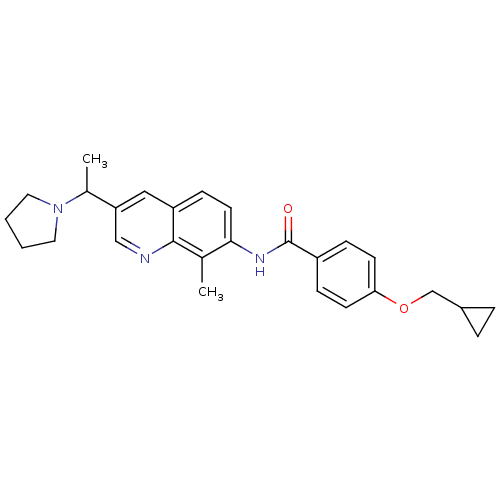 (CHEMBL2059418) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]MCH from human MCHR1 expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 55: 4336-51 (2012) Article DOI: 10.1021/jm300167z BindingDB Entry DOI: 10.7270/Q2JS9RHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451387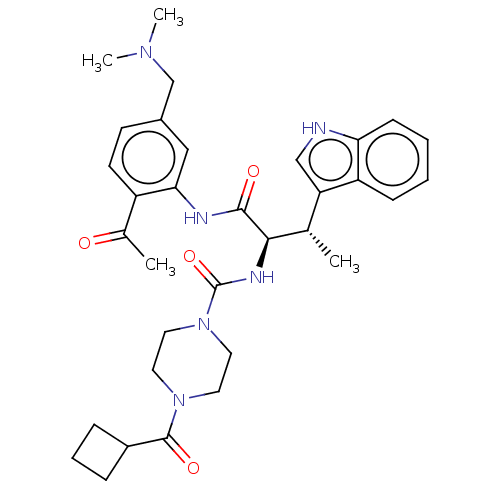 (CHEMBL4203387) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50388443 (CHEMBL2059409) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]MCH from human MCHR1 expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 55: 4336-51 (2012) Article DOI: 10.1021/jm300167z BindingDB Entry DOI: 10.7270/Q2JS9RHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50388458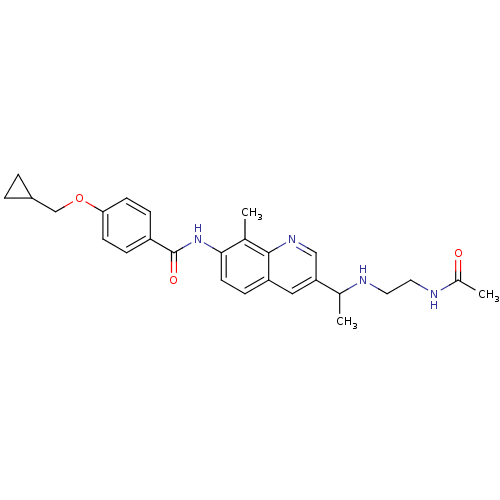 (CHEMBL2059511) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]MCH from human MCHR1 expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 55: 4336-51 (2012) Article DOI: 10.1021/jm300167z BindingDB Entry DOI: 10.7270/Q2JS9RHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50388452 (CHEMBL2059420) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]MCH from rat MCHR1 expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 55: 4336-51 (2012) Article DOI: 10.1021/jm300167z BindingDB Entry DOI: 10.7270/Q2JS9RHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50388454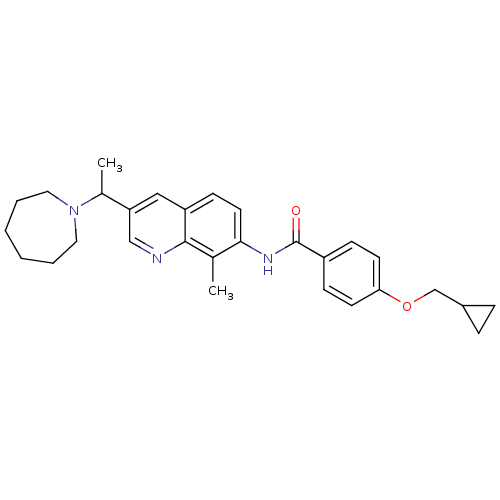 (CHEMBL2059422) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]MCH from human MCHR1 expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 55: 4336-51 (2012) Article DOI: 10.1021/jm300167z BindingDB Entry DOI: 10.7270/Q2JS9RHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50388442 (CHEMBL2059408) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]MCH from human MCHR1 expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 55: 4336-51 (2012) Article DOI: 10.1021/jm300167z BindingDB Entry DOI: 10.7270/Q2JS9RHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50388444 (CHEMBL2059410) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human MCHR1 expressed in CHO cells assessed as inhibition of MCH-stimulated arachidonic acid release after 45 mins by liquid s... | J Med Chem 55: 4336-51 (2012) Article DOI: 10.1021/jm300167z BindingDB Entry DOI: 10.7270/Q2JS9RHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50388444 (CHEMBL2059410) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]MCH from rat MCHR1 expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 55: 4336-51 (2012) Article DOI: 10.1021/jm300167z BindingDB Entry DOI: 10.7270/Q2JS9RHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451398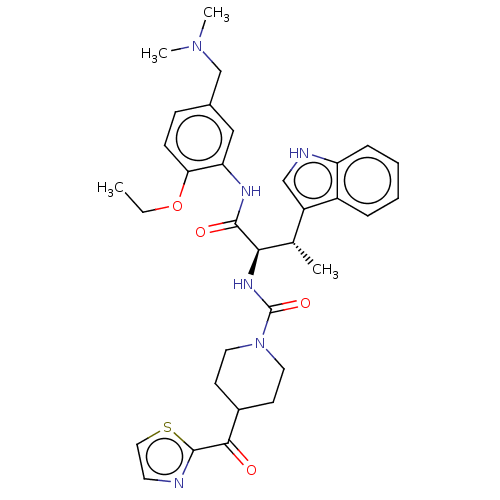 (CHEMBL4203652) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451389 (CHEMBL4215052) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451393 (CHEMBL4202861) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451370 (CHEMBL4204193) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM31587 (oxadiazole derivative, 20x) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Takeda Pharmaceutical Company Ltd. | Assay Description Human GSK-3beta was expressed as the N-terminal FLAG-tagged protein using a baculovirus expression system. The kinase assay was was conducted in a 96... | Bioorg Med Chem 17: 2017-29 (2009) Article DOI: 10.1016/j.bmc.2009.01.019 BindingDB Entry DOI: 10.7270/Q2DR2STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451369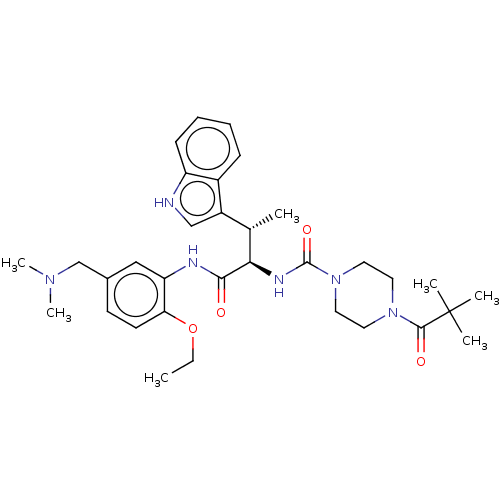 (CHEMBL4207735) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50388460 (CHEMBL2059513) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]MCH from rat MCHR1 expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 55: 4336-51 (2012) Article DOI: 10.1021/jm300167z BindingDB Entry DOI: 10.7270/Q2JS9RHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50388453 (CHEMBL2059421) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]MCH from human MCHR1 expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 55: 4336-51 (2012) Article DOI: 10.1021/jm300167z BindingDB Entry DOI: 10.7270/Q2JS9RHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 261 total ) | Next | Last >> |