 Found 284 hits with Last Name = 'kurihara' and Initial = 'h'
Found 284 hits with Last Name = 'kurihara' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50095997
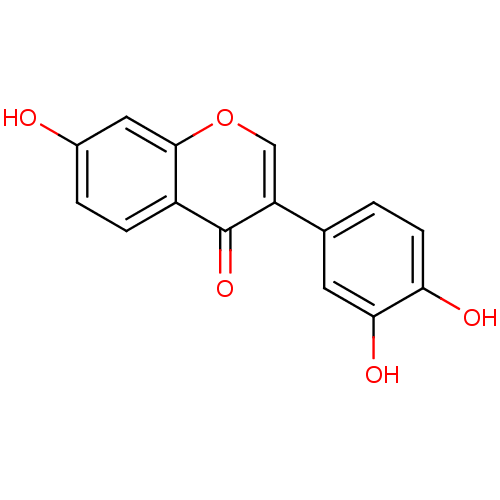
(3',4',7-trihydroxyisoflavone | CHEMBL13486)Show InChI InChI=1S/C15H10O5/c16-9-2-3-10-14(6-9)20-7-11(15(10)19)8-1-4-12(17)13(18)5-8/h1-7,16-18H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) |
J Med Chem 44: 540-7 (2001)
BindingDB Entry DOI: 10.7270/Q2W66MGR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50096004
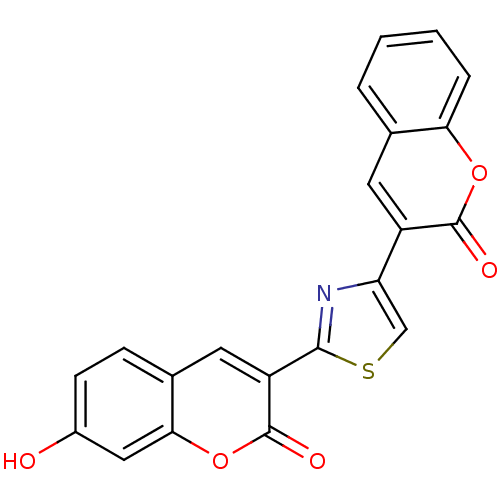
(7-Hydroxy-3-[4-(2-oxo-2H-chromen-3-yl)-thiazol-2-y...)Show SMILES Oc1ccc2cc(-c3nc(cs3)-c3cc4ccccc4oc3=O)c(=O)oc2c1 Show InChI InChI=1S/C21H11NO5S/c23-13-6-5-12-8-15(21(25)27-18(12)9-13)19-22-16(10-28-19)14-7-11-3-1-2-4-17(11)26-20(14)24/h1-10,23H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) |
J Med Chem 44: 540-7 (2001)
BindingDB Entry DOI: 10.7270/Q2W66MGR |
More data for this
Ligand-Target Pair | |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50096003
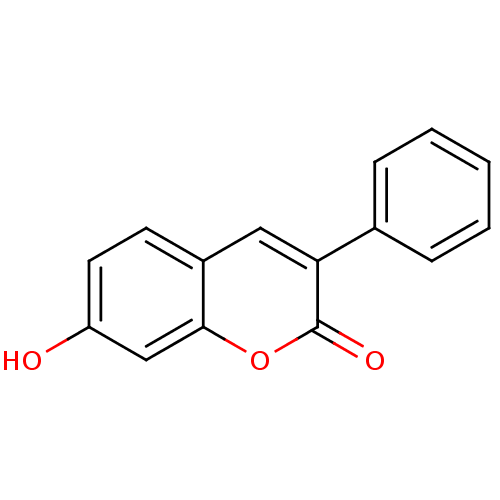
(7-Hydroxy-3-phenyl-chromen-2-one | 7-hydroxy-3-phe...)Show InChI InChI=1S/C15H10O3/c16-12-7-6-11-8-13(10-4-2-1-3-5-10)15(17)18-14(11)9-12/h1-9,16H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) |
J Med Chem 44: 540-7 (2001)
BindingDB Entry DOI: 10.7270/Q2W66MGR |
More data for this
Ligand-Target Pair | |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50096001
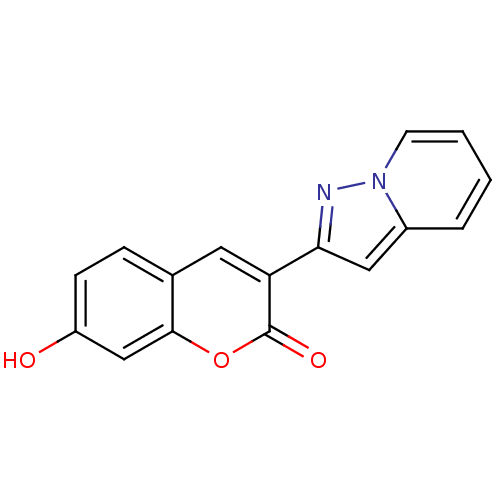
(7-Hydroxy-3-pyrazolo[1,5-a]pyridin-2-yl-chromen-2-...)Show InChI InChI=1S/C16H10N2O3/c19-12-5-4-10-7-13(16(20)21-15(10)9-12)14-8-11-3-1-2-6-18(11)17-14/h1-9,19H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) |
J Med Chem 44: 540-7 (2001)
BindingDB Entry DOI: 10.7270/Q2W66MGR |
More data for this
Ligand-Target Pair | |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50095993
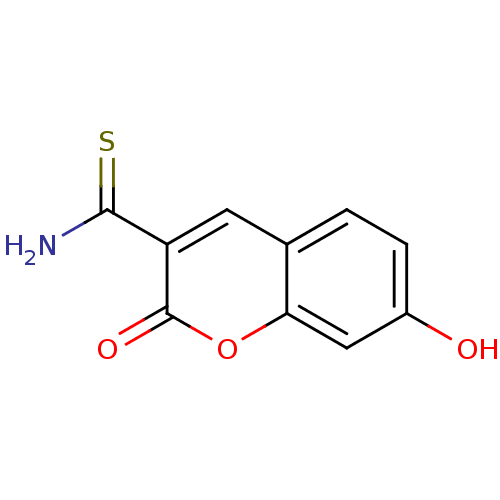
(7-Hydroxy-2-oxo-2H-chromene-3-carbothioic acid ami...)Show InChI InChI=1S/C10H7NO3S/c11-9(15)7-3-5-1-2-6(12)4-8(5)14-10(7)13/h1-4,12H,(H2,11,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) |
J Med Chem 44: 540-7 (2001)
BindingDB Entry DOI: 10.7270/Q2W66MGR |
More data for this
Ligand-Target Pair | |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50096002
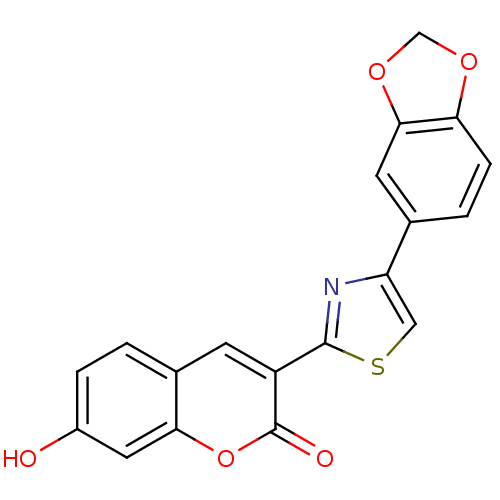
(3-(4-Benzo[1,3]dioxol-5-yl-thiazol-2-yl)-7-hydroxy...)Show SMILES Oc1ccc2cc(-c3nc(cs3)-c3ccc4OCOc4c3)c(=O)oc2c1 Show InChI InChI=1S/C19H11NO5S/c21-12-3-1-11-5-13(19(22)25-16(11)7-12)18-20-14(8-26-18)10-2-4-15-17(6-10)24-9-23-15/h1-8,21H,9H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) |
J Med Chem 44: 540-7 (2001)
BindingDB Entry DOI: 10.7270/Q2W66MGR |
More data for this
Ligand-Target Pair | |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50096007
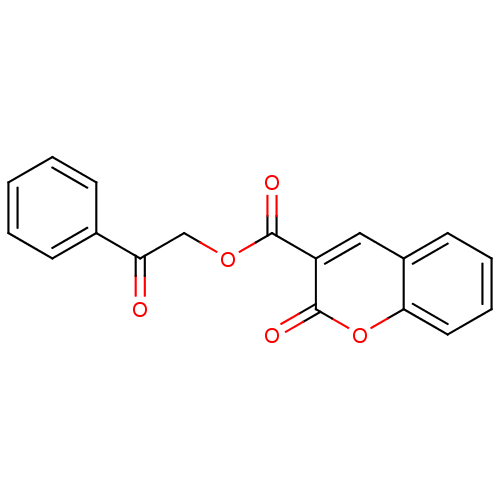
(2-Oxo-2H-chromene-3-carboxylic acid 2-oxo-2-phenyl...)Show InChI InChI=1S/C18H12O5/c19-15(12-6-2-1-3-7-12)11-22-17(20)14-10-13-8-4-5-9-16(13)23-18(14)21/h1-10H,11H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) |
J Med Chem 44: 540-7 (2001)
BindingDB Entry DOI: 10.7270/Q2W66MGR |
More data for this
Ligand-Target Pair | |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50096006
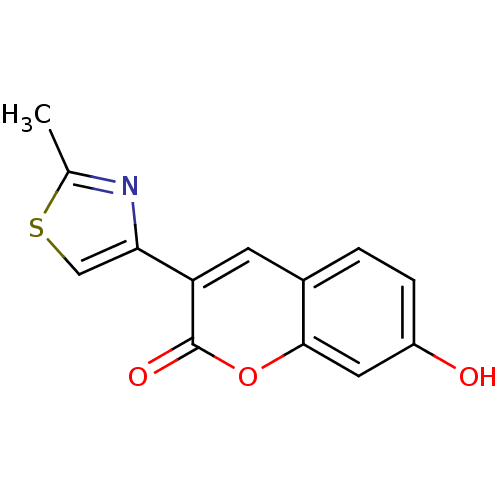
(7-Hydroxy-3-(2-methyl-thiazol-4-yl)-chromen-2-one ...)Show InChI InChI=1S/C13H9NO3S/c1-7-14-11(6-18-7)10-4-8-2-3-9(15)5-12(8)17-13(10)16/h2-6,15H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) |
J Med Chem 44: 540-7 (2001)
BindingDB Entry DOI: 10.7270/Q2W66MGR |
More data for this
Ligand-Target Pair | |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50096000
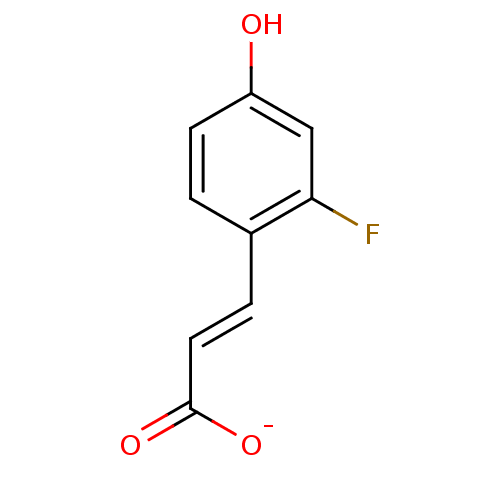
(3-(2-Fluoro-4-hydroxy-phenyl)-acrylic acid anion)Show InChI InChI=1S/C9H7FO3/c10-8-5-7(11)3-1-6(8)2-4-9(12)13/h1-5,11H,(H,12,13)/p-1/b4-2+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| PubMed
| 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) |
J Med Chem 44: 540-7 (2001)
BindingDB Entry DOI: 10.7270/Q2W66MGR |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50366376

(CHEMBL1159523)Show SMILES CC(=O)OCC(CO[C@H]1O[C@H](CS(O)(=O)=O)[C@@H](O)[C@H](O)[C@H]1O)OC(C)=O |r| Show InChI InChI=1S/C13H22O12S/c1-6(14)22-3-8(24-7(2)15)4-23-13-12(18)11(17)10(16)9(25-13)5-26(19,20)21/h8-13,16-18H,3-5H2,1-2H3,(H,19,20,21)/t8?,9-,10-,11+,12-,13+/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibitory constant against yeast alpha-glucosidase |
Bioorg Med Chem Lett 5: 1241-1244 (1995)
Article DOI: 10.1016/0960-894X(95)00196-Z
BindingDB Entry DOI: 10.7270/Q2MP53SJ |
More data for this
Ligand-Target Pair | |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50095994
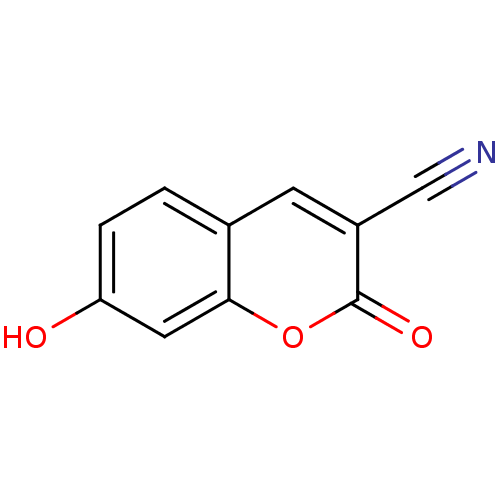
(3-Cyano-7-hydroxycoumarin (2) | 7-Hydroxy-2-oxo-2H...)Show InChI InChI=1S/C10H5NO3/c11-5-7-3-6-1-2-8(12)4-9(6)14-10(7)13/h1-4,12H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) |
J Med Chem 44: 540-7 (2001)
BindingDB Entry DOI: 10.7270/Q2W66MGR |
More data for this
Ligand-Target Pair | |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50096005

(7-Hydroxy-3-(4-methyl-thiazol-2-yl)-chromen-2-one ...)Show InChI InChI=1S/C13H9NO3S/c1-7-6-18-12(14-7)10-4-8-2-3-9(15)5-11(8)17-13(10)16/h2-6,15H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) |
J Med Chem 44: 540-7 (2001)
BindingDB Entry DOI: 10.7270/Q2W66MGR |
More data for this
Ligand-Target Pair | |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50096008
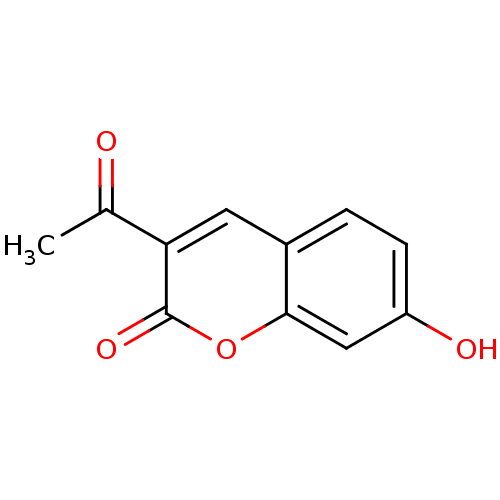
(3-Acetyl-7-hydroxy-chromen-2-one | 3-acetyl-7-hydr...)Show InChI InChI=1S/C11H8O4/c1-6(12)9-4-7-2-3-8(13)5-10(7)15-11(9)14/h2-5,13H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) |
J Med Chem 44: 540-7 (2001)
BindingDB Entry DOI: 10.7270/Q2W66MGR |
More data for this
Ligand-Target Pair | |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50095996
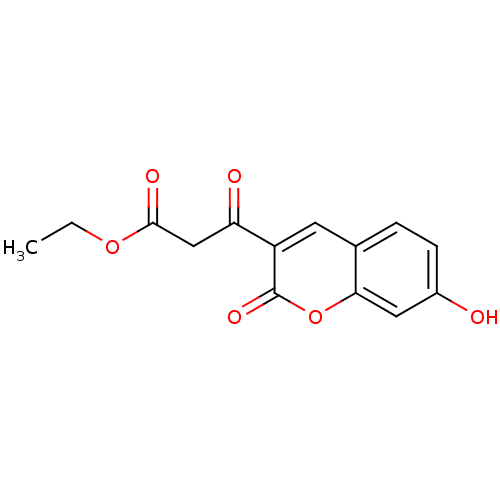
(3-(7-Hydroxy-2-oxo-2H-chromen-3-yl)-3-oxo-propioni...)Show InChI InChI=1S/C14H12O6/c1-2-19-13(17)7-11(16)10-5-8-3-4-9(15)6-12(8)20-14(10)18/h3-6,15H,2,7H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) |
J Med Chem 44: 540-7 (2001)
BindingDB Entry DOI: 10.7270/Q2W66MGR |
More data for this
Ligand-Target Pair | |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50096009
(3-(4-Dimethylamino-benzylidene)-chroman-2,4-dione ...)Show InChI InChI=1S/C18H15NO3/c1-19(2)13-9-7-12(8-10-13)11-15-17(20)14-5-3-4-6-16(14)22-18(15)21/h3-11H,1-2H3/b15-11- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) |
J Med Chem 44: 540-7 (2001)
BindingDB Entry DOI: 10.7270/Q2W66MGR |
More data for this
Ligand-Target Pair | |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50095995
(7-HYDROXY-2-OXO-CHROMENE-3-CARBOXYLIC ACID ETHYL E...)Show InChI InChI=1S/C12H10O5/c1-2-16-11(14)9-5-7-3-4-8(13)6-10(7)17-12(9)15/h3-6,13H,2H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) |
J Med Chem 44: 540-7 (2001)
BindingDB Entry DOI: 10.7270/Q2W66MGR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM50186373
(CHEMBL424696 | N-(5-(2-(cyclohexyloxy)pyrimidin-4-...)Show InChI InChI=1S/C18H19N5OS/c1-2-6-13(7-3-1)24-17-20-11-9-14(22-17)15-12-21-18(25-15)23-16-8-4-5-10-19-16/h4-5,8-13H,1-3,6-7H2,(H,19,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CDK9 |
Bioorg Med Chem Lett 16: 3751-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.048
BindingDB Entry DOI: 10.7270/Q2N29WK0 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM1434

(11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...)Show InChI InChI=1S/C15H14N4O/c1-9-6-8-17-14-12(9)18-15(20)11-3-2-7-16-13(11)19(14)10-4-5-10/h2-3,6-8,10H,4-5H2,1H3,(H,18,20) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
Bioorg Med Chem 12: 6171-82 (2004)
Article DOI: 10.1016/j.bmc.2004.08.050
BindingDB Entry DOI: 10.7270/Q23R0R24 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [588-1127,Y768C]/[588-1147,Y768C]
(Human immunodeficiency virus type 1) | BDBM2483

((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...)Show SMILES FC(F)(F)[C@]1(OC(=O)Nc2ccc(Cl)cc12)C#CC1CC1 |r| Show InChI InChI=1S/C14H9ClF3NO2/c15-9-3-4-11-10(7-9)13(14(16,17)18,21-12(20)19-11)6-5-8-1-2-8/h3-4,7-8H,1-2H2,(H,19,20)/t13-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
Bioorg Med Chem 13: 949-61 (2005)
Article DOI: 10.1016/j.bmc.2004.11.045
BindingDB Entry DOI: 10.7270/Q2C53J1Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [588-1127,Y768C]/[588-1147,Y768C]
(Human immunodeficiency virus type 1) | BDBM2483

((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...)Show SMILES FC(F)(F)[C@]1(OC(=O)Nc2ccc(Cl)cc12)C#CC1CC1 |r| Show InChI InChI=1S/C14H9ClF3NO2/c15-9-3-4-11-10(7-9)13(14(16,17)18,21-12(20)19-11)6-5-8-1-2-8/h3-4,7-8H,1-2H2,(H,19,20)/t13-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
Bioorg Med Chem 12: 6171-82 (2004)
Article DOI: 10.1016/j.bmc.2004.08.050
BindingDB Entry DOI: 10.7270/Q23R0R24 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50186373

(CHEMBL424696 | N-(5-(2-(cyclohexyloxy)pyrimidin-4-...)Show InChI InChI=1S/C18H19N5OS/c1-2-6-13(7-3-1)24-17-20-11-9-14(22-17)15-12-21-18(25-15)23-16-8-4-5-10-19-16/h4-5,8-13H,1-3,6-7H2,(H,19,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CDK4 |
Bioorg Med Chem Lett 16: 3751-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.048
BindingDB Entry DOI: 10.7270/Q2N29WK0 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM5052
(5-bromo-N-[(2Z)-4-chloro-3-methyl-5-(propan-2-yl)-...)Show SMILES CC(C)c1s\c(=N/S(=O)(=O)c2cc(Br)ccc2O)n(C)c1Cl Show InChI InChI=1S/C13H14BrClN2O3S2/c1-7(2)11-12(15)17(3)13(21-11)16-22(19,20)10-6-8(14)4-5-9(10)18/h4-7,18H,1-3H3/b16-13- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
Bioorg Med Chem 13: 949-61 (2005)
Article DOI: 10.1016/j.bmc.2004.11.045
BindingDB Entry DOI: 10.7270/Q2C53J1Z |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50297965

((R)-3-(1-(2-chloro-4-(hydroxymethyl)phenyl)ethoxy)...)Show SMILES C[C@@H](Oc1cc(sc1C(N)=O)-c1cnc2ccccn12)c1ccc(CO)cc1Cl |r| Show InChI InChI=1S/C21H18ClN3O3S/c1-12(14-6-5-13(11-26)8-15(14)22)28-17-9-18(29-20(17)21(23)27)16-10-24-19-4-2-3-7-25(16)19/h2-10,12,26H,11H2,1H3,(H2,23,27)/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 |
Bioorg Med Chem Lett 19: 4673-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.084
BindingDB Entry DOI: 10.7270/Q22R3RQG |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM2483

((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...)Show SMILES FC(F)(F)[C@]1(OC(=O)Nc2ccc(Cl)cc12)C#CC1CC1 |r| Show InChI InChI=1S/C14H9ClF3NO2/c15-9-3-4-11-10(7-9)13(14(16,17)18,21-12(20)19-11)6-5-8-1-2-8/h3-4,7-8H,1-2H2,(H,19,20)/t13-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
Bioorg Med Chem 13: 949-61 (2005)
Article DOI: 10.1016/j.bmc.2004.11.045
BindingDB Entry DOI: 10.7270/Q2C53J1Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM2483

((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...)Show SMILES FC(F)(F)[C@]1(OC(=O)Nc2ccc(Cl)cc12)C#CC1CC1 |r| Show InChI InChI=1S/C14H9ClF3NO2/c15-9-3-4-11-10(7-9)13(14(16,17)18,21-12(20)19-11)6-5-8-1-2-8/h3-4,7-8H,1-2H2,(H,19,20)/t13-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
Bioorg Med Chem 12: 6171-82 (2004)
Article DOI: 10.1016/j.bmc.2004.08.050
BindingDB Entry DOI: 10.7270/Q23R0R24 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50297962
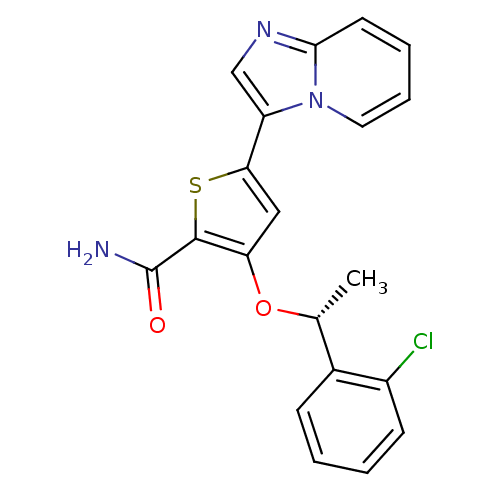
((R)-3-(1-(2-chlorophenyl)ethoxy)-5-(imidazo[1,2-a]...)Show SMILES C[C@@H](Oc1cc(sc1C(N)=O)-c1cnc2ccccn12)c1ccccc1Cl |r| Show InChI InChI=1S/C20H16ClN3O2S/c1-12(13-6-2-3-7-14(13)21)26-16-10-17(27-19(16)20(22)25)15-11-23-18-8-4-5-9-24(15)18/h2-12H,1H3,(H2,22,25)/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 |
Bioorg Med Chem Lett 19: 4673-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.084
BindingDB Entry DOI: 10.7270/Q22R3RQG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50297966
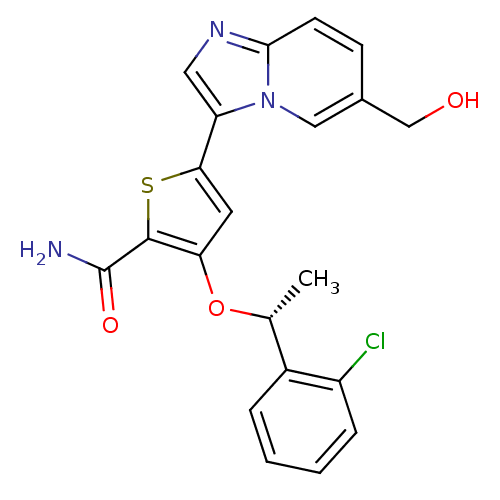
((R)-3-(1-(2-chlorophenyl)ethoxy)-5-(6-(hydroxymeth...)Show SMILES C[C@@H](Oc1cc(sc1C(N)=O)-c1cnc2ccc(CO)cn12)c1ccccc1Cl |r| Show InChI InChI=1S/C21H18ClN3O3S/c1-12(14-4-2-3-5-15(14)22)28-17-8-18(29-20(17)21(23)27)16-9-24-19-7-6-13(11-26)10-25(16)19/h2-10,12,26H,11H2,1H3,(H2,23,27)/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 |
Bioorg Med Chem Lett 19: 4673-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.084
BindingDB Entry DOI: 10.7270/Q22R3RQG |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 6
(Homo sapiens (Human)) | BDBM50186375

(CHEMBL210540 | N-(5-(2-(cyclohexyloxy)-6-methylpyr...)Show SMILES CN1CCN(Cc2cnc(Nc3ncc(s3)-c3cc(C)nc(OC4CCCCC4)n3)cn2)CC1 Show InChI InChI=1S/C24H32N8OS/c1-17-12-20(29-23(28-17)33-19-6-4-3-5-7-19)21-14-27-24(34-21)30-22-15-25-18(13-26-22)16-32-10-8-31(2)9-11-32/h12-15,19H,3-11,16H2,1-2H3,(H,26,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CDK6 |
Bioorg Med Chem Lett 16: 3751-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.048
BindingDB Entry DOI: 10.7270/Q2N29WK0 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM5054

(5-Chloro-N-(4-chloro-5-isopropyl-3-methyl-1,3-thia...)Show SMILES CC(C)c1s\c(=N/S(=O)(=O)c2cc(Cl)ccc2C#N)n(C)c1Cl Show InChI InChI=1S/C14H13Cl2N3O2S2/c1-8(2)12-13(16)19(3)14(22-12)18-23(20,21)11-6-10(15)5-4-9(11)7-17/h4-6,8H,1-3H3/b18-14- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
Bioorg Med Chem 13: 949-61 (2005)
Article DOI: 10.1016/j.bmc.2004.11.045
BindingDB Entry DOI: 10.7270/Q2C53J1Z |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50186375

(CHEMBL210540 | N-(5-(2-(cyclohexyloxy)-6-methylpyr...)Show SMILES CN1CCN(Cc2cnc(Nc3ncc(s3)-c3cc(C)nc(OC4CCCCC4)n3)cn2)CC1 Show InChI InChI=1S/C24H32N8OS/c1-17-12-20(29-23(28-17)33-19-6-4-3-5-7-19)21-14-27-24(34-21)30-22-15-25-18(13-26-22)16-32-10-8-31(2)9-11-32/h12-15,19H,3-11,16H2,1-2H3,(H,26,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CDK4 |
Bioorg Med Chem Lett 16: 3751-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.048
BindingDB Entry DOI: 10.7270/Q2N29WK0 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM5051
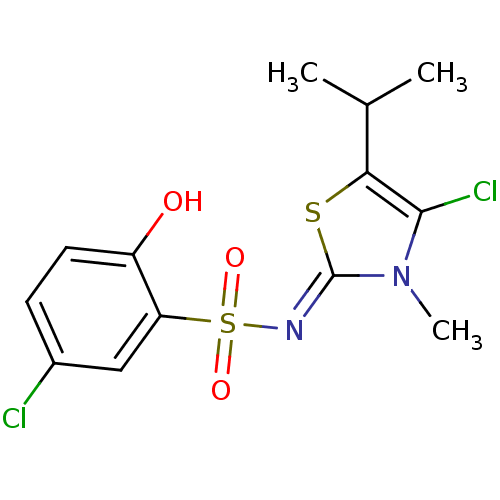
(5-Chloro-N-(4-chloro-5-isopropyl-3-methyl-13-thiaz...)Show SMILES CC(C)c1s\c(=N/S(=O)(=O)c2cc(Cl)ccc2O)n(C)c1Cl Show InChI InChI=1S/C13H14Cl2N2O3S2/c1-7(2)11-12(15)17(3)13(21-11)16-22(19,20)10-6-8(14)4-5-9(10)18/h4-7,18H,1-3H3/b16-13- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
Bioorg Med Chem 13: 949-61 (2005)
Article DOI: 10.1016/j.bmc.2004.11.045
BindingDB Entry DOI: 10.7270/Q2C53J1Z |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM5055

(N-(4-Chloro-5-isopropyl-3-methyl-1,3-thiazol-2(3H)...)Show SMILES CC(C)c1s\c(=N/S(=O)(=O)c2cc(ccc2C#N)C#N)n(C)c1Cl Show InChI InChI=1S/C15H13ClN4O2S2/c1-9(2)13-14(16)20(3)15(23-13)19-24(21,22)12-6-10(7-17)4-5-11(12)8-18/h4-6,9H,1-3H3/b19-15- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
Bioorg Med Chem 13: 949-61 (2005)
Article DOI: 10.1016/j.bmc.2004.11.045
BindingDB Entry DOI: 10.7270/Q2C53J1Z |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50297985
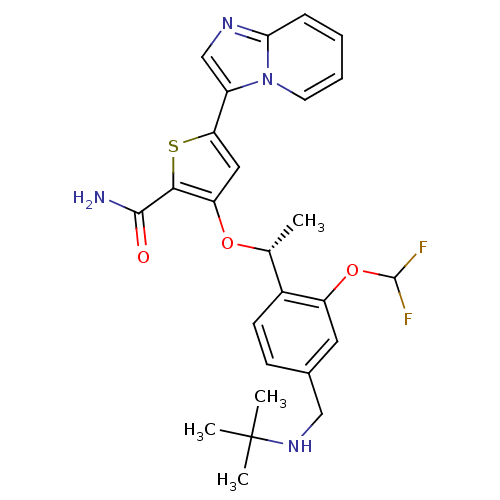
((R)-3-(1-(4-((tert-butylamino)methyl)-2-(difluorom...)Show SMILES C[C@@H](Oc1cc(sc1C(N)=O)-c1cnc2ccccn12)c1ccc(CNC(C)(C)C)cc1OC(F)F |r| Show InChI InChI=1S/C26H28F2N4O3S/c1-15(17-9-8-16(13-31-26(2,3)4)11-19(17)35-25(27)28)34-20-12-21(36-23(20)24(29)33)18-14-30-22-7-5-6-10-32(18)22/h5-12,14-15,25,31H,13H2,1-4H3,(H2,29,33)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 |
Bioorg Med Chem Lett 19: 4673-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.084
BindingDB Entry DOI: 10.7270/Q22R3RQG |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [588-1127,Y768C]/[588-1147,Y768C]
(Human immunodeficiency virus type 1) | BDBM5041

(N-(5-tert-Butyl-3,4-dimethyl-1,3-thiazol-2(3H)-yli...)Show SMILES Cc1c(s\c(=N/S(=O)(=O)c2cc(Cl)ccc2O)n1C)C(C)(C)C Show InChI InChI=1S/C15H19ClN2O3S2/c1-9-13(15(2,3)4)22-14(18(9)5)17-23(20,21)12-8-10(16)6-7-11(12)19/h6-8,19H,1-5H3/b17-14- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
Bioorg Med Chem 13: 949-61 (2005)
Article DOI: 10.1016/j.bmc.2004.11.045
BindingDB Entry DOI: 10.7270/Q2C53J1Z |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM5048
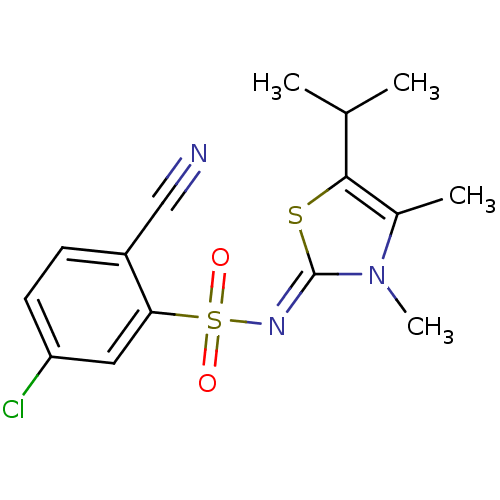
(5-Chloro-2-cyano-N-(5-isopropyl-3,4-dimethyl-1,3-t...)Show SMILES CC(C)c1s\c(=N/S(=O)(=O)c2cc(Cl)ccc2C#N)n(C)c1C Show InChI InChI=1S/C15H16ClN3O2S2/c1-9(2)14-10(3)19(4)15(22-14)18-23(20,21)13-7-12(16)6-5-11(13)8-17/h5-7,9H,1-4H3/b18-15- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
Bioorg Med Chem 13: 949-61 (2005)
Article DOI: 10.1016/j.bmc.2004.11.045
BindingDB Entry DOI: 10.7270/Q2C53J1Z |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM5053
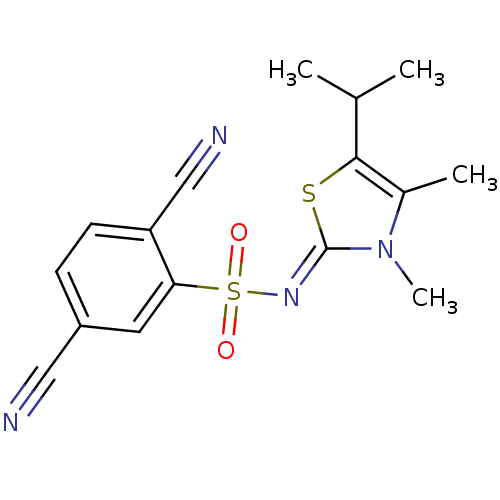
(2,5-dicyano-N-[(2Z)-3,4-dimethyl-5-(propan-2-yl)-2...)Show SMILES CC(C)c1s\c(=N/S(=O)(=O)c2cc(ccc2C#N)C#N)n(C)c1C Show InChI InChI=1S/C16H16N4O2S2/c1-10(2)15-11(3)20(4)16(23-15)19-24(21,22)14-7-12(8-17)5-6-13(14)9-18/h5-7,10H,1-4H3/b19-16- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
Bioorg Med Chem 13: 949-61 (2005)
Article DOI: 10.1016/j.bmc.2004.11.045
BindingDB Entry DOI: 10.7270/Q2C53J1Z |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50297971

((R)-3-(1-(2-chloro-4-((cyclopropylamino)methyl)phe...)Show SMILES C[C@@H](Oc1cc(sc1C(N)=O)-c1cnc2ccccn12)c1ccc(CNC2CC2)cc1Cl |r| Show InChI InChI=1S/C24H23ClN4O2S/c1-14(17-8-5-15(10-18(17)25)12-27-16-6-7-16)31-20-11-21(32-23(20)24(26)30)19-13-28-22-4-2-3-9-29(19)22/h2-5,8-11,13-14,16,27H,6-7,12H2,1H3,(H2,26,30)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 |
Bioorg Med Chem Lett 19: 4673-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.084
BindingDB Entry DOI: 10.7270/Q22R3RQG |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 7
(Rattus norvegicus (Rat)) | BDBM50305863
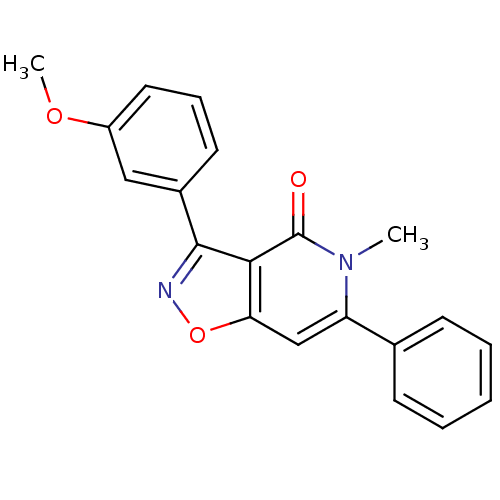
(3-(3-methoxyphenyl)-5-methyl-6-phenylisoxazolo[4,5...)Show SMILES COc1cccc(c1)-c1noc2cc(-c3ccccc3)n(C)c(=O)c12 Show InChI InChI=1S/C20H16N2O3/c1-22-16(13-7-4-3-5-8-13)12-17-18(20(22)23)19(21-25-17)14-9-6-10-15(11-14)24-2/h3-12H,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at rat mGluR7 expressed in CHO cells assessed as inhibition of LAP-induced intracellular calcium mobilization by FLIPR |
Bioorg Med Chem Lett 20: 726-9 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.070
BindingDB Entry DOI: 10.7270/Q2F18ZSX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50297981
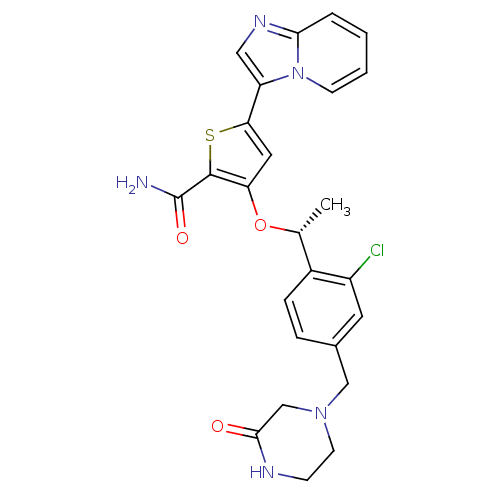
((R)-3-(1-(2-chloro-4-((3-oxopiperazin-1-yl)methyl)...)Show SMILES C[C@@H](Oc1cc(sc1C(N)=O)-c1cnc2ccccn12)c1ccc(CN2CCNC(=O)C2)cc1Cl |r| Show InChI InChI=1S/C25H24ClN5O3S/c1-15(17-6-5-16(10-18(17)26)13-30-9-7-28-23(32)14-30)34-20-11-21(35-24(20)25(27)33)19-12-29-22-4-2-3-8-31(19)22/h2-6,8,10-12,15H,7,9,13-14H2,1H3,(H2,27,33)(H,28,32)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 |
Bioorg Med Chem Lett 19: 4673-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.084
BindingDB Entry DOI: 10.7270/Q22R3RQG |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [588-1127,Y768C]/[588-1147,Y768C]
(Human immunodeficiency virus type 1) | BDBM5052

(5-bromo-N-[(2Z)-4-chloro-3-methyl-5-(propan-2-yl)-...)Show SMILES CC(C)c1s\c(=N/S(=O)(=O)c2cc(Br)ccc2O)n(C)c1Cl Show InChI InChI=1S/C13H14BrClN2O3S2/c1-7(2)11-12(15)17(3)13(21-11)16-22(19,20)10-6-8(14)4-5-9(10)18/h4-7,18H,1-3H3/b16-13- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
Bioorg Med Chem 13: 949-61 (2005)
Article DOI: 10.1016/j.bmc.2004.11.045
BindingDB Entry DOI: 10.7270/Q2C53J1Z |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50186374

(CHEMBL212049 | N-(5-(2-(cyclohexyloxy)-5-methylpyr...)Show InChI InChI=1S/C19H21N5OS/c1-13-11-21-18(25-14-7-3-2-4-8-14)24-17(13)15-12-22-19(26-15)23-16-9-5-6-10-20-16/h5-6,9-12,14H,2-4,7-8H2,1H3,(H,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CDK4 |
Bioorg Med Chem Lett 16: 3751-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.048
BindingDB Entry DOI: 10.7270/Q2N29WK0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50297977

((R)-3-(1-(2-chloro-4-((4-fluoropiperidin-1-yl)meth...)Show SMILES C[C@@H](Oc1cc(sc1C(N)=O)-c1cnc2ccccn12)c1ccc(CN2CCC(F)CC2)cc1Cl |r| Show InChI InChI=1S/C26H26ClFN4O2S/c1-16(19-6-5-17(12-20(19)27)15-31-10-7-18(28)8-11-31)34-22-13-23(35-25(22)26(29)33)21-14-30-24-4-2-3-9-32(21)24/h2-6,9,12-14,16,18H,7-8,10-11,15H2,1H3,(H2,29,33)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 |
Bioorg Med Chem Lett 19: 4673-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.084
BindingDB Entry DOI: 10.7270/Q22R3RQG |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50186373

(CHEMBL424696 | N-(5-(2-(cyclohexyloxy)pyrimidin-4-...)Show InChI InChI=1S/C18H19N5OS/c1-2-6-13(7-3-1)24-17-20-11-9-14(22-17)15-12-21-18(25-15)23-16-8-4-5-10-19-16/h4-5,8-13H,1-3,6-7H2,(H,19,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
Bioorg Med Chem Lett 16: 3751-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.048
BindingDB Entry DOI: 10.7270/Q2N29WK0 |
More data for this
Ligand-Target Pair | |
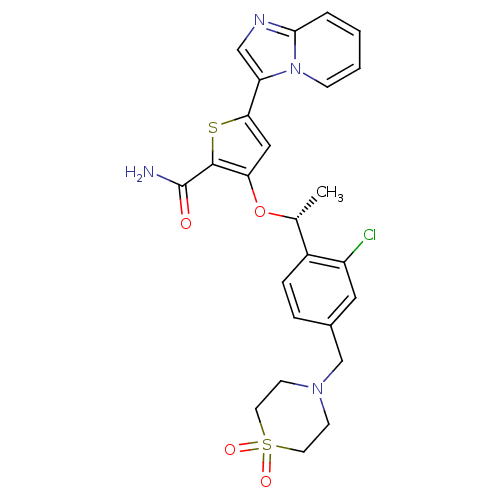
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50297982
(3-{(R)-1-[2-Chloro-4-(1,1-dioxo-1lambda*6*-thiomor...)Show SMILES C[C@@H](Oc1cc(sc1C(N)=O)-c1cnc2ccccn12)c1ccc(CN2CCS(=O)(=O)CC2)cc1Cl |r| Show InChI InChI=1S/C25H25ClN4O4S2/c1-16(18-6-5-17(12-19(18)26)15-29-8-10-36(32,33)11-9-29)34-21-13-22(35-24(21)25(27)31)20-14-28-23-4-2-3-7-30(20)23/h2-7,12-14,16H,8-11,15H2,1H3,(H2,27,31)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 |
Bioorg Med Chem Lett 19: 4673-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.084
BindingDB Entry DOI: 10.7270/Q22R3RQG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50297967

((R)-3-(1-(2-chloro-4-((methylamino)methyl)phenyl)e...)Show SMILES CNCc1ccc([C@@H](C)Oc2cc(sc2C(N)=O)-c2cnc3ccccn23)c(Cl)c1 |r| Show InChI InChI=1S/C22H21ClN4O2S/c1-13(15-7-6-14(11-25-2)9-16(15)23)29-18-10-19(30-21(18)22(24)28)17-12-26-20-5-3-4-8-27(17)20/h3-10,12-13,25H,11H2,1-2H3,(H2,24,28)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 |
Bioorg Med Chem Lett 19: 4673-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.084
BindingDB Entry DOI: 10.7270/Q22R3RQG |
More data for this
Ligand-Target Pair | |
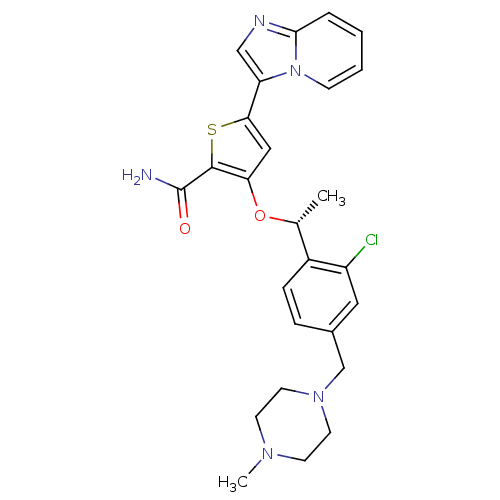
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50297978
((R)-3-(1-(2-chloro-4-((4-methylpiperazin-1-yl)meth...)Show SMILES C[C@@H](Oc1cc(sc1C(N)=O)-c1cnc2ccccn12)c1ccc(CN2CCN(C)CC2)cc1Cl |r| Show InChI InChI=1S/C26H28ClN5O2S/c1-17(19-7-6-18(13-20(19)27)16-31-11-9-30(2)10-12-31)34-22-14-23(35-25(22)26(28)33)21-15-29-24-5-3-4-8-32(21)24/h3-8,13-15,17H,9-12,16H2,1-2H3,(H2,28,33)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 |
Bioorg Med Chem Lett 19: 4673-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.084
BindingDB Entry DOI: 10.7270/Q22R3RQG |
More data for this
Ligand-Target Pair | |
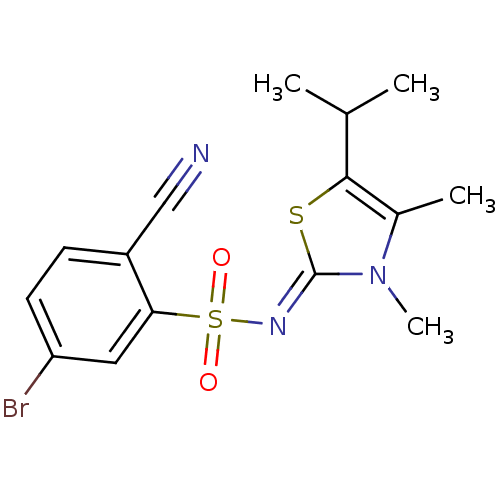
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM5050
(5-Bromo-2-cyano-N-(5-isopropyl-3,4-dimethyl-1,3-th...)Show SMILES CC(C)c1s\c(=N/S(=O)(=O)c2cc(Br)ccc2C#N)n(C)c1C Show InChI InChI=1S/C15H16BrN3O2S2/c1-9(2)14-10(3)19(4)15(22-14)18-23(20,21)13-7-12(16)6-5-11(13)8-17/h5-7,9H,1-4H3/b18-15- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
Bioorg Med Chem 13: 949-61 (2005)
Article DOI: 10.1016/j.bmc.2004.11.045
BindingDB Entry DOI: 10.7270/Q2C53J1Z |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50297975

((R)-3-(1-(2-chloro-4-((3-hydroxypropylamino)methyl...)Show SMILES C[C@@H](Oc1cc(sc1C(N)=O)-c1cnc2ccccn12)c1ccc(CNCCCO)cc1Cl |r| Show InChI InChI=1S/C24H25ClN4O3S/c1-15(17-7-6-16(11-18(17)25)13-27-8-4-10-30)32-20-12-21(33-23(20)24(26)31)19-14-28-22-5-2-3-9-29(19)22/h2-3,5-7,9,11-12,14-15,27,30H,4,8,10,13H2,1H3,(H2,26,31)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 |
Bioorg Med Chem Lett 19: 4673-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.084
BindingDB Entry DOI: 10.7270/Q22R3RQG |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 7
(Homo sapiens (Human)) | BDBM50186373

(CHEMBL424696 | N-(5-(2-(cyclohexyloxy)pyrimidin-4-...)Show InChI InChI=1S/C18H19N5OS/c1-2-6-13(7-3-1)24-17-20-11-9-14(22-17)15-12-21-18(25-15)23-16-8-4-5-10-19-16/h4-5,8-13H,1-3,6-7H2,(H,19,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CDK7 |
Bioorg Med Chem Lett 16: 3751-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.048
BindingDB Entry DOI: 10.7270/Q2N29WK0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50297980
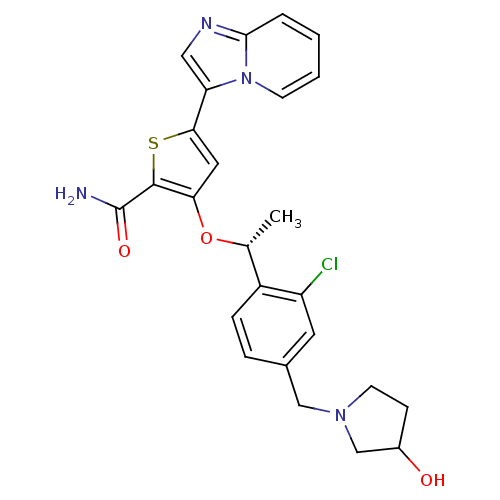
(3-((1R)-1-(2-chloro-4-((3-hydroxypyrrolidin-1-yl)m...)Show SMILES C[C@@H](Oc1cc(sc1C(N)=O)-c1cnc2ccccn12)c1ccc(CN2CCC(O)C2)cc1Cl |r| Show InChI InChI=1S/C25H25ClN4O3S/c1-15(18-6-5-16(10-19(18)26)13-29-9-7-17(31)14-29)33-21-11-22(34-24(21)25(27)32)20-12-28-23-4-2-3-8-30(20)23/h2-6,8,10-12,15,17,31H,7,9,13-14H2,1H3,(H2,27,32)/t15-,17?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 |
Bioorg Med Chem Lett 19: 4673-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.084
BindingDB Entry DOI: 10.7270/Q22R3RQG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data