 Found 160 hits with Last Name = 'kuwahara' and Initial = 's'
Found 160 hits with Last Name = 'kuwahara' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50083552

(1,9-di{4-[5-amino(imino)methylbenzo[b]furan-2-ylca...)Show SMILES NC(=N)c1ccc2oc(cc2c1)C(=O)N1CCN(CC1)C(=O)CCCCCCCC(=O)N1CCN(CC1)C(=O)c1cc2cc(ccc2o1)C(N)=N Show InChI InChI=1S/C37H44N8O6/c38-34(39)24-8-10-28-26(20-24)22-30(50-28)36(48)44-16-12-42(13-17-44)32(46)6-4-2-1-3-5-7-33(47)43-14-18-45(19-15-43)37(49)31-23-27-21-25(35(40)41)9-11-29(27)51-31/h8-11,20-23H,1-7,12-19H2,(H3,38,39)(H3,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of tryptase activity |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50083561

(1-{4-[5-amino(imino)methylbenzo[b]thiophen-2-ylcar...)Show SMILES NC(=N)c1ccc2sc(cc2c1)C(=O)N1CCN(CC1)C(=O)COc1ccc(OCC(=O)N2CCN(CC2)C(=O)c2cc3cc(ccc3s2)C(N)=N)cc1 Show InChI InChI=1S/C38H38N8O6S2/c39-35(40)23-1-7-29-25(17-23)19-31(53-29)37(49)45-13-9-43(10-14-45)33(47)21-51-27-3-5-28(6-4-27)52-22-34(48)44-11-15-46(16-12-44)38(50)32-20-26-18-24(36(41)42)2-8-30(26)54-32/h1-8,17-20H,9-16,21-22H2,(H3,39,40)(H3,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of tryptase activity |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50083556
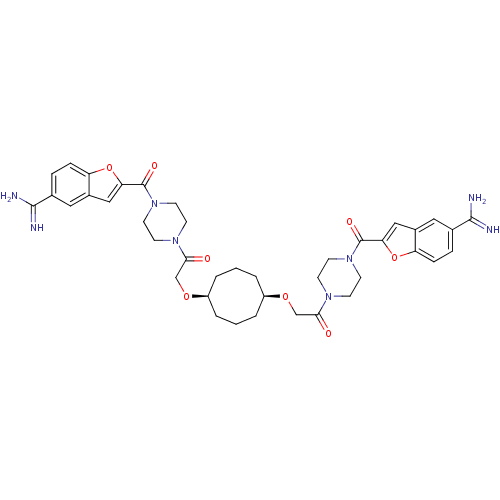
(1-{4-[5-amino(imino)methylbenzo[b]furan-2-ylcarbon...)Show SMILES NC(=N)c1ccc2oc(cc2c1)C(=O)N1CCN(CC1)C(=O)CO[C@H]1CCC[C@H](CCC1)OCC(=O)N1CCN(CC1)C(=O)c1cc2cc(ccc2o1)C(N)=N Show InChI InChI=1S/C40H48N8O8/c41-37(42)25-7-9-31-27(19-25)21-33(55-31)39(51)47-15-11-45(12-16-47)35(49)23-53-29-3-1-4-30(6-2-5-29)54-24-36(50)46-13-17-48(18-14-46)40(52)34-22-28-20-26(38(43)44)8-10-32(28)56-34/h7-10,19-22,29-30H,1-6,11-18,23-24H2,(H3,41,42)(H3,43,44)/t29-,30+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of tryptase activity |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50083541
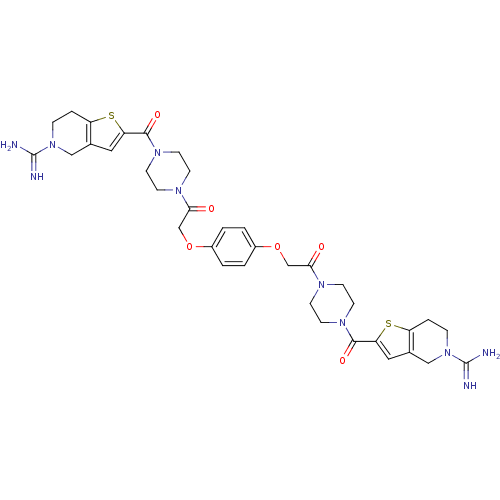
(1-{4-[5-amino(imino)methyl-4,5,6,7-tetrahydrothien...)Show SMILES NC(=N)N1CCc2sc(cc2C1)C(=O)N1CCN(CC1)C(=O)COc1ccc(OCC(=O)N2CCN(CC2)C(=O)c2cc3CN(CCc3s2)C(N)=N)cc1 Show InChI InChI=1S/C36H44N10O6S2/c37-35(38)45-7-5-27-23(19-45)17-29(53-27)33(49)43-13-9-41(10-14-43)31(47)21-51-25-1-2-26(4-3-25)52-22-32(48)42-11-15-44(16-12-42)34(50)30-18-24-20-46(36(39)40)8-6-28(24)54-30/h1-4,17-18H,5-16,19-22H2,(H3,37,38)(H3,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of tryptase activity |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50083548

(1-{4-[5-amino(imino)methylbenzo[b]furan-2-ylcarbon...)Show SMILES NC(=N)c1ccc2oc(cc2c1)C(=O)N1CCN(CC1)C(=O)COc1ccc(OCC(=O)N2CCN(CC2)C(=O)c2cc3cc(ccc3o2)C(N)=N)cc1 Show InChI InChI=1S/C38H38N8O8/c39-35(40)23-1-7-29-25(17-23)19-31(53-29)37(49)45-13-9-43(10-14-45)33(47)21-51-27-3-5-28(6-4-27)52-22-34(48)44-11-15-46(16-12-44)38(50)32-20-26-18-24(36(41)42)2-8-30(26)54-32/h1-8,17-20H,9-16,21-22H2,(H3,39,40)(H3,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of tryptase activity |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50217306

(CHEMBL112049)Show SMILES NC(=N)c1ccc2oc(cc2c1)C(=O)N1CCN(CC1)C(=O)CCCCCCC(=O)N1CCN(CC1)C(=O)c1cc2cc(ccc2o1)C(N)=N Show InChI InChI=1S/C36H42N8O6/c37-33(38)23-7-9-27-25(19-23)21-29(49-27)35(47)43-15-11-41(12-16-43)31(45)5-3-1-2-4-6-32(46)42-13-17-44(18-14-42)36(48)30-22-26-20-24(34(39)40)8-10-28(26)50-30/h7-10,19-22H,1-6,11-18H2,(H3,37,38)(H3,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of tryptase activity |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50083549
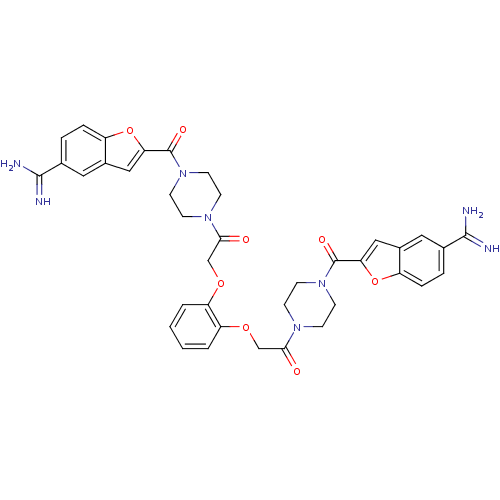
(1-{4-[5-amino(imino)methylbenzo[b]furan-2-ylcarbon...)Show SMILES NC(=N)c1ccc2oc(cc2c1)C(=O)N1CCN(CC1)C(=O)COc1ccccc1OCC(=O)N1CCN(CC1)C(=O)c1cc2cc(ccc2o1)C(N)=N Show InChI InChI=1S/C38H38N8O8/c39-35(40)23-5-7-27-25(17-23)19-31(53-27)37(49)45-13-9-43(10-14-45)33(47)21-51-29-3-1-2-4-30(29)52-22-34(48)44-11-15-46(16-12-44)38(50)32-20-26-18-24(36(41)42)6-8-28(26)54-32/h1-8,17-20H,9-16,21-22H2,(H3,39,40)(H3,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of tryptase activity |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50098853
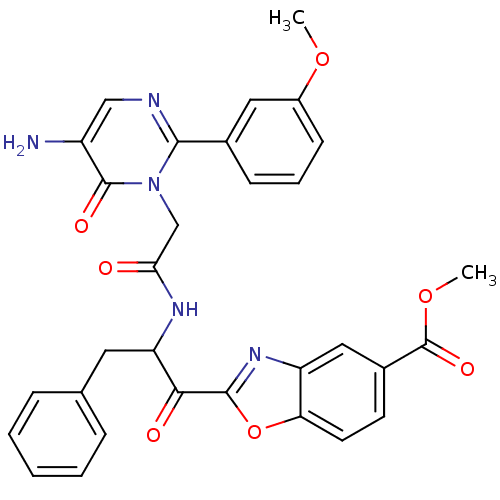
(2-(2-{2-[5-Amino-2-(3-methoxy-phenyl)-6-oxo-6H-pyr...)Show SMILES COC(=O)c1ccc2oc(nc2c1)C(=O)C(Cc1ccccc1)NC(=O)Cn1c(ncc(N)c1=O)-c1cccc(OC)c1 Show InChI InChI=1S/C31H27N5O7/c1-41-21-10-6-9-19(14-21)28-33-16-22(32)30(39)36(28)17-26(37)34-24(13-18-7-4-3-5-8-18)27(38)29-35-23-15-20(31(40)42-2)11-12-25(23)43-29/h3-12,14-16,24H,13,17,32H2,1-2H3,(H,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against canine skin chymase |
J Med Chem 44: 1286-96 (2001)
BindingDB Entry DOI: 10.7270/Q26H4J4K |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50098874

(4-{2-[5-Amino-2-(3-chloro-phenyl)-6-oxo-6H-pyrimid...)Show SMILES Nc1cnc(-c2cccc(Cl)c2)n(CC(=O)NC(Cc2ccccc2)C(=O)C(F)(F)C(=O)NCc2ccccc2)c1=O Show InChI InChI=1S/C30H26ClF2N5O4/c31-22-13-7-12-21(15-22)27-35-17-23(34)28(41)38(27)18-25(39)37-24(14-19-8-3-1-4-9-19)26(40)30(32,33)29(42)36-16-20-10-5-2-6-11-20/h1-13,15,17,24H,14,16,18,34H2,(H,36,42)(H,37,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity was determined against human heart chymase |
J Med Chem 44: 1297-304 (2001)
BindingDB Entry DOI: 10.7270/Q22R3SCD |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50098868
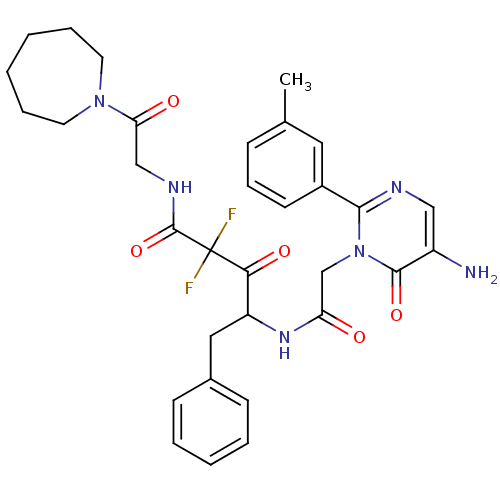
(4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl)-a...)Show SMILES Cc1cccc(c1)-c1ncc(N)c(=O)n1CC(=O)NC(Cc1ccccc1)C(=O)C(F)(F)C(=O)NCC(=O)N1CCCCCC1 Show InChI InChI=1S/C32H36F2N6O5/c1-21-10-9-13-23(16-21)29-36-18-24(35)30(44)40(29)20-26(41)38-25(17-22-11-5-4-6-12-22)28(43)32(33,34)31(45)37-19-27(42)39-14-7-2-3-8-15-39/h4-6,9-13,16,18,25H,2-3,7-8,14-15,17,19-20,35H2,1H3,(H,37,45)(H,38,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity was determined against human heart chymase |
J Med Chem 44: 1297-304 (2001)
BindingDB Entry DOI: 10.7270/Q22R3SCD |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50098847
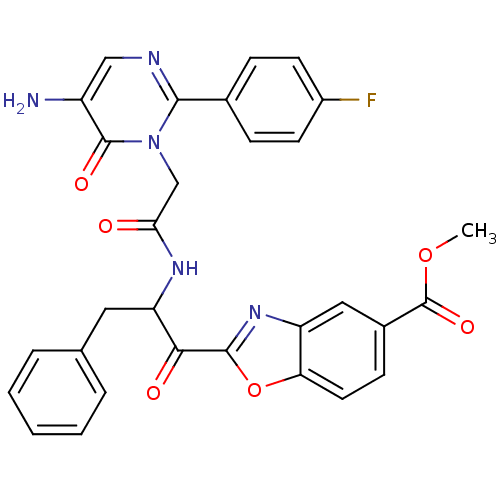
(2-(2-{2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyri...)Show SMILES COC(=O)c1ccc2oc(nc2c1)C(=O)C(Cc1ccccc1)NC(=O)Cn1c(ncc(N)c1=O)-c1ccc(F)cc1 Show InChI InChI=1S/C30H24FN5O6/c1-41-30(40)19-9-12-24-22(14-19)35-28(42-24)26(38)23(13-17-5-3-2-4-6-17)34-25(37)16-36-27(33-15-21(32)29(36)39)18-7-10-20(31)11-8-18/h2-12,14-15,23H,13,16,32H2,1H3,(H,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against canine skin chymase |
J Med Chem 44: 1286-96 (2001)
BindingDB Entry DOI: 10.7270/Q26H4J4K |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50098853

(2-(2-{2-[5-Amino-2-(3-methoxy-phenyl)-6-oxo-6H-pyr...)Show SMILES COC(=O)c1ccc2oc(nc2c1)C(=O)C(Cc1ccccc1)NC(=O)Cn1c(ncc(N)c1=O)-c1cccc(OC)c1 Show InChI InChI=1S/C31H27N5O7/c1-41-21-10-6-9-19(14-21)28-33-16-22(32)30(39)36(28)17-26(37)34-24(13-18-7-4-3-5-8-18)27(38)29-35-23-15-20(31(40)42-2)11-12-25(23)43-29/h3-12,14-16,24H,13,17,32H2,1-2H3,(H,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4.85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against chymase from human heart. |
J Med Chem 44: 1286-96 (2001)
BindingDB Entry DOI: 10.7270/Q26H4J4K |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50098854

(2-{2-[2-(5-Amino-6-oxo-2-phenyl-6H-pyrimidin-1-yl)...)Show SMILES COC(=O)c1ccc2oc(nc2c1)C(=O)C(Cc1ccccc1)NC(=O)Cn1c(ncc(N)c1=O)-c1ccccc1 Show InChI InChI=1S/C30H25N5O6/c1-40-30(39)20-12-13-24-22(15-20)34-28(41-24)26(37)23(14-18-8-4-2-5-9-18)33-25(36)17-35-27(19-10-6-3-7-11-19)32-16-21(31)29(35)38/h2-13,15-16,23H,14,17,31H2,1H3,(H,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5.57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against chymase from human heart. |
J Med Chem 44: 1286-96 (2001)
BindingDB Entry DOI: 10.7270/Q26H4J4K |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50068894
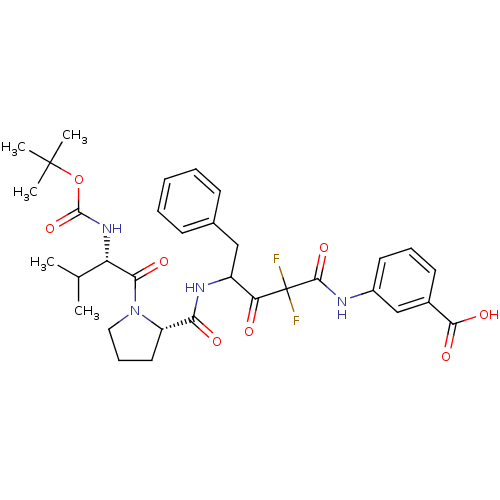
(3-(4-{[(S)-1-((S)-2-tert-Butoxycarbonylamino-3-met...)Show SMILES CC(C)[C@H](NC(=O)OC(C)(C)C)C(=O)N1CCC[C@H]1C(=O)NC(Cc1ccccc1)C(=O)C(F)(F)C(=O)Nc1cccc(c1)C(O)=O Show InChI InChI=1S/C33H40F2N4O8/c1-19(2)25(38-31(46)47-32(3,4)5)28(42)39-16-10-15-24(39)27(41)37-23(17-20-11-7-6-8-12-20)26(40)33(34,35)30(45)36-22-14-9-13-21(18-22)29(43)44/h6-9,11-14,18-19,23-25H,10,15-17H2,1-5H3,(H,36,45)(H,37,41)(H,38,46)(H,43,44)/t23?,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity was determined against human heart chymase |
J Med Chem 44: 1297-304 (2001)
BindingDB Entry DOI: 10.7270/Q22R3SCD |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50098841
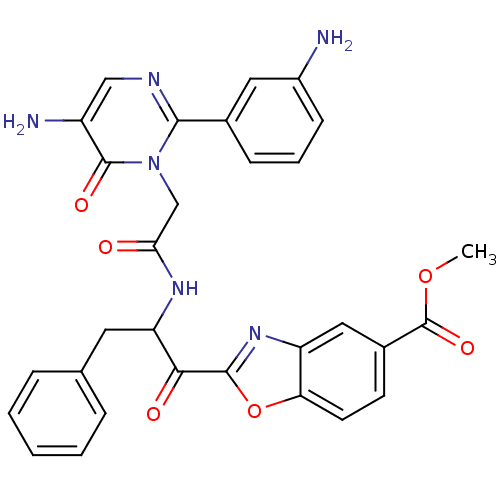
(2-(2-{2-[5-Amino-2-(3-amino-phenyl)-6-oxo-6H-pyrim...)Show SMILES COC(=O)c1ccc2oc(nc2c1)C(=O)C(Cc1ccccc1)NC(=O)Cn1c(ncc(N)c1=O)-c1cccc(N)c1 Show InChI InChI=1S/C30H26N6O6/c1-41-30(40)19-10-11-24-22(14-19)35-28(42-24)26(38)23(12-17-6-3-2-4-7-17)34-25(37)16-36-27(33-15-21(32)29(36)39)18-8-5-9-20(31)13-18/h2-11,13-15,23H,12,16,31-32H2,1H3,(H,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against chymase from human heart. |
J Med Chem 44: 1286-96 (2001)
BindingDB Entry DOI: 10.7270/Q26H4J4K |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50098892
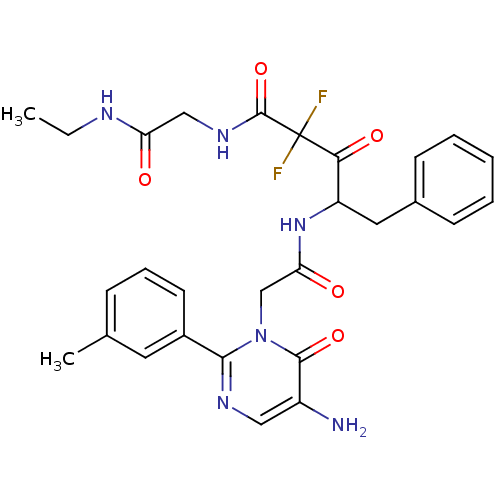
(4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl)-a...)Show SMILES CCNC(=O)CNC(=O)C(F)(F)C(=O)C(Cc1ccccc1)NC(=O)Cn1c(ncc(N)c1=O)-c1cccc(C)c1 Show InChI InChI=1S/C28H30F2N6O5/c1-3-32-22(37)15-34-27(41)28(29,30)24(39)21(13-18-9-5-4-6-10-18)35-23(38)16-36-25(33-14-20(31)26(36)40)19-11-7-8-17(2)12-19/h4-12,14,21H,3,13,15-16,31H2,1-2H3,(H,32,37)(H,34,41)(H,35,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity was determined against human heart chymase |
J Med Chem 44: 1297-304 (2001)
BindingDB Entry DOI: 10.7270/Q22R3SCD |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50098879

(4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl)-a...)Show SMILES CCN(CC)C(=O)CNC(=O)C(F)(F)C(=O)C(Cc1ccccc1)NC(=O)Cn1c(ncc(N)c1=O)-c1cccc(C)c1 Show InChI InChI=1S/C30H34F2N6O5/c1-4-37(5-2)25(40)17-35-29(43)30(31,32)26(41)23(15-20-11-7-6-8-12-20)36-24(39)18-38-27(34-16-22(33)28(38)42)21-13-9-10-19(3)14-21/h6-14,16,23H,4-5,15,17-18,33H2,1-3H3,(H,35,43)(H,36,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity was determined against human heart chymase |
J Med Chem 44: 1297-304 (2001)
BindingDB Entry DOI: 10.7270/Q22R3SCD |
More data for this
Ligand-Target Pair | |
Chymotrypsin-C
(Homo sapiens (Human)) | BDBM87059
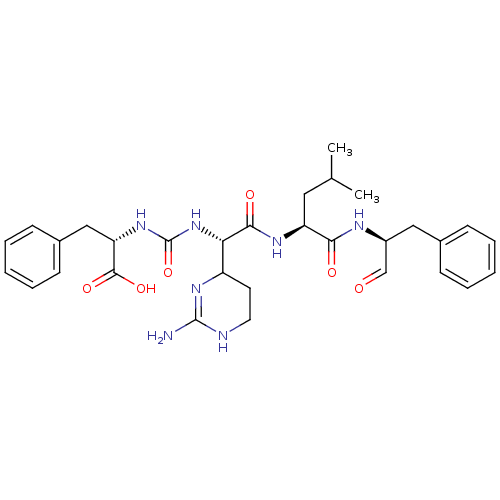
(CHEMBL247767 | Chymostatin)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)N[C@@H](Cc1ccccc1)C(O)=O)C1CCNC(N)=N1)C(=O)N[C@@H](Cc1ccccc1)C=O |c:30| Show InChI InChI=1S/C31H41N7O6/c1-19(2)15-24(27(40)34-22(18-39)16-20-9-5-3-6-10-20)35-28(41)26(23-13-14-33-30(32)36-23)38-31(44)37-25(29(42)43)17-21-11-7-4-8-12-21/h3-12,18-19,22-26H,13-17H2,1-2H3,(H,34,40)(H,35,41)(H,42,43)(H3,32,33,36)(H2,37,38,44)/t22-,23?,24-,25-,26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
inhibitory activity against alpha chymotrypsin from bovine pancreas. |
J Med Chem 44: 1286-96 (2001)
BindingDB Entry DOI: 10.7270/Q26H4J4K |
More data for this
Ligand-Target Pair | |
Chymotrypsin-C
(Homo sapiens (Human)) | BDBM87059

(CHEMBL247767 | Chymostatin)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)N[C@@H](Cc1ccccc1)C(O)=O)C1CCNC(N)=N1)C(=O)N[C@@H](Cc1ccccc1)C=O |c:30| Show InChI InChI=1S/C31H41N7O6/c1-19(2)15-24(27(40)34-22(18-39)16-20-9-5-3-6-10-20)35-28(41)26(23-13-14-33-30(32)36-23)38-31(44)37-25(29(42)43)17-21-11-7-4-8-12-21/h3-12,18-19,22-26H,13-17H2,1-2H3,(H,34,40)(H,35,41)(H,42,43)(H3,32,33,36)(H2,37,38,44)/t22-,23?,24-,25-,26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity was determined against bovine pancreas chymotrypsin |
J Med Chem 44: 1297-304 (2001)
BindingDB Entry DOI: 10.7270/Q22R3SCD |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50098880
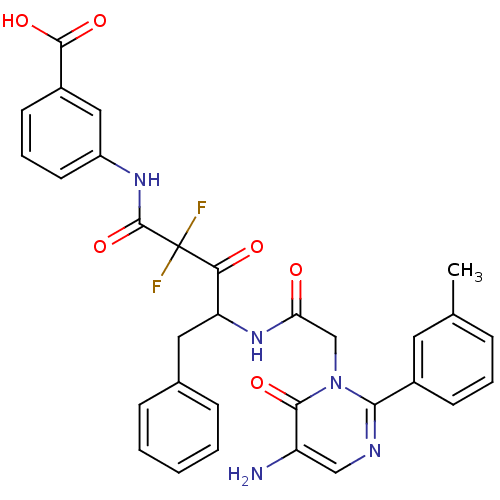
(3-{4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl...)Show SMILES Cc1cccc(c1)-c1ncc(N)c(=O)n1CC(=O)NC(Cc1ccccc1)C(=O)C(F)(F)C(=O)Nc1cccc(c1)C(O)=O Show InChI InChI=1S/C31H27F2N5O6/c1-18-7-5-10-20(13-18)27-35-16-23(34)28(41)38(27)17-25(39)37-24(14-19-8-3-2-4-9-19)26(40)31(32,33)30(44)36-22-12-6-11-21(15-22)29(42)43/h2-13,15-16,24H,14,17,34H2,1H3,(H,36,44)(H,37,39)(H,42,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity was determined against human heart chymase |
J Med Chem 44: 1297-304 (2001)
BindingDB Entry DOI: 10.7270/Q22R3SCD |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50098878

(4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl)-a...)Show SMILES Cc1cccc(c1)-c1ncc(N)c(=O)n1CC(=O)NC(Cc1ccccc1)C(=O)C(F)(F)C(=O)NCC(=O)N1CCCCC1 Show InChI InChI=1S/C31H34F2N6O5/c1-20-9-8-12-22(15-20)28-35-17-23(34)29(43)39(28)19-25(40)37-24(16-21-10-4-2-5-11-21)27(42)31(32,33)30(44)36-18-26(41)38-13-6-3-7-14-38/h2,4-5,8-12,15,17,24H,3,6-7,13-14,16,18-19,34H2,1H3,(H,36,44)(H,37,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 10.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity was determined against human heart chymase |
J Med Chem 44: 1297-304 (2001)
BindingDB Entry DOI: 10.7270/Q22R3SCD |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50098870
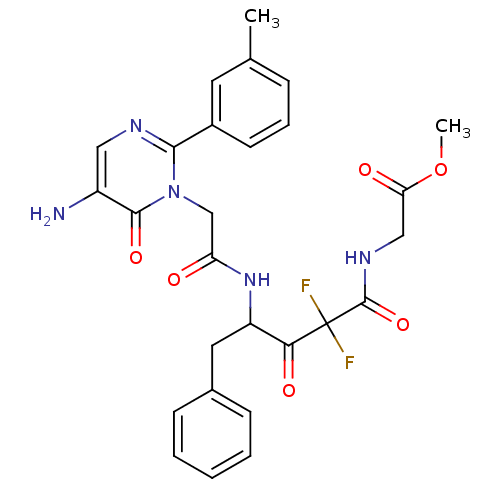
(CHEMBL26182 | {4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-py...)Show SMILES COC(=O)CNC(=O)C(F)(F)C(=O)C(Cc1ccccc1)NC(=O)Cn1c(ncc(N)c1=O)-c1cccc(C)c1 Show InChI InChI=1S/C27H27F2N5O6/c1-16-7-6-10-18(11-16)24-31-13-19(30)25(38)34(24)15-21(35)33-20(12-17-8-4-3-5-9-17)23(37)27(28,29)26(39)32-14-22(36)40-2/h3-11,13,20H,12,14-15,30H2,1-2H3,(H,32,39)(H,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 10.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity was determined against human heart chymase |
J Med Chem 44: 1297-304 (2001)
BindingDB Entry DOI: 10.7270/Q22R3SCD |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50098875

(4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl)-a...)Show SMILES Cc1cccc(c1)-c1ncc(N)c(=O)n1CC(=O)NC(Cc1ccccc1)C(=O)C(F)(F)C(=O)NCC(N)=O Show InChI InChI=1S/C26H26F2N6O5/c1-15-6-5-9-17(10-15)23-31-12-18(29)24(38)34(23)14-21(36)33-19(11-16-7-3-2-4-8-16)22(37)26(27,28)25(39)32-13-20(30)35/h2-10,12,19H,11,13-14,29H2,1H3,(H2,30,35)(H,32,39)(H,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity was determined against human heart chymase |
J Med Chem 44: 1297-304 (2001)
BindingDB Entry DOI: 10.7270/Q22R3SCD |
More data for this
Ligand-Target Pair | |
Chymotrypsin-C
(Homo sapiens (Human)) | BDBM50098882
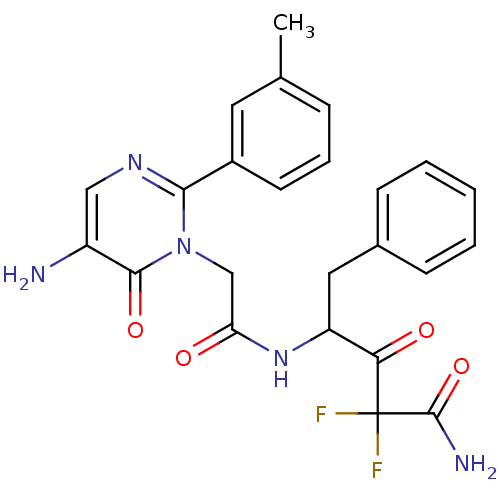
(4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl)-a...)Show SMILES Cc1cccc(c1)-c1ncc(N)c(=O)n1CC(=O)NC(Cc1ccccc1)C(=O)C(F)(F)C(N)=O Show InChI InChI=1S/C24H23F2N5O4/c1-14-6-5-9-16(10-14)21-29-12-17(27)22(34)31(21)13-19(32)30-18(11-15-7-3-2-4-8-15)20(33)24(25,26)23(28)35/h2-10,12,18H,11,13,27H2,1H3,(H2,28,35)(H,30,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity was determined against bovine pancreas chymotrypsin |
J Med Chem 44: 1297-304 (2001)
BindingDB Entry DOI: 10.7270/Q22R3SCD |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM87059

(CHEMBL247767 | Chymostatin)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)N[C@@H](Cc1ccccc1)C(O)=O)C1CCNC(N)=N1)C(=O)N[C@@H](Cc1ccccc1)C=O |c:30| Show InChI InChI=1S/C31H41N7O6/c1-19(2)15-24(27(40)34-22(18-39)16-20-9-5-3-6-10-20)35-28(41)26(23-13-14-33-30(32)36-23)38-31(44)37-25(29(42)43)17-21-11-7-4-8-12-21/h3-12,18-19,22-26H,13-17H2,1-2H3,(H,34,40)(H,35,41)(H,42,43)(H3,32,33,36)(H2,37,38,44)/t22-,23?,24-,25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 13.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity was determined against human heart chymase |
J Med Chem 44: 1297-304 (2001)
BindingDB Entry DOI: 10.7270/Q22R3SCD |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM87059

(CHEMBL247767 | Chymostatin)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)N[C@@H](Cc1ccccc1)C(O)=O)C1CCNC(N)=N1)C(=O)N[C@@H](Cc1ccccc1)C=O |c:30| Show InChI InChI=1S/C31H41N7O6/c1-19(2)15-24(27(40)34-22(18-39)16-20-9-5-3-6-10-20)35-28(41)26(23-13-14-33-30(32)36-23)38-31(44)37-25(29(42)43)17-21-11-7-4-8-12-21/h3-12,18-19,22-26H,13-17H2,1-2H3,(H,34,40)(H,35,41)(H,42,43)(H3,32,33,36)(H2,37,38,44)/t22-,23?,24-,25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 13.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
inhibitory activity was evaluated against chymase from human heart. |
J Med Chem 44: 1286-96 (2001)
BindingDB Entry DOI: 10.7270/Q26H4J4K |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50098882

(4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl)-a...)Show SMILES Cc1cccc(c1)-c1ncc(N)c(=O)n1CC(=O)NC(Cc1ccccc1)C(=O)C(F)(F)C(N)=O Show InChI InChI=1S/C24H23F2N5O4/c1-14-6-5-9-16(10-14)21-29-12-17(27)22(34)31(21)13-19(32)30-18(11-15-7-3-2-4-8-15)20(33)24(25,26)23(28)35/h2-10,12,18H,11,13,27H2,1H3,(H2,28,35)(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 13.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity was determined against human heart chymase |
J Med Chem 44: 1297-304 (2001)
BindingDB Entry DOI: 10.7270/Q22R3SCD |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50098877

(4-{2-[5-Amino-2-(3-methoxy-phenyl)-6-oxo-6H-pyrimi...)Show SMILES COc1cccc(c1)-c1ncc(N)c(=O)n1CC(=O)NC(Cc1ccccc1)C(=O)C(F)(F)C(=O)NCc1ccccc1 Show InChI InChI=1S/C31H29F2N5O5/c1-43-23-14-8-13-22(16-23)28-35-18-24(34)29(41)38(28)19-26(39)37-25(15-20-9-4-2-5-10-20)27(40)31(32,33)30(42)36-17-21-11-6-3-7-12-21/h2-14,16,18,25H,15,17,19,34H2,1H3,(H,36,42)(H,37,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 13.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity was determined against human heart chymase |
J Med Chem 44: 1297-304 (2001)
BindingDB Entry DOI: 10.7270/Q22R3SCD |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50098849
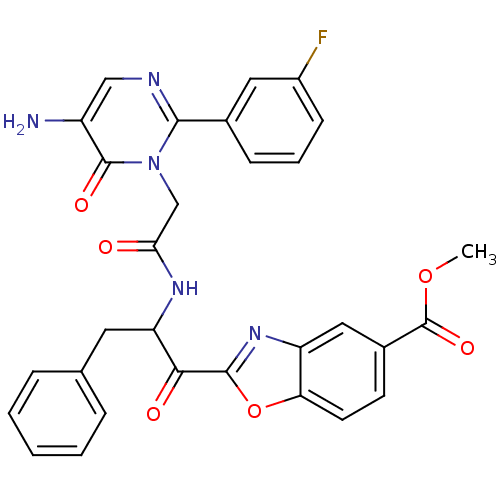
(2-(2-{2-[5-Amino-2-(3-fluoro-phenyl)-6-oxo-6H-pyri...)Show SMILES COC(=O)c1ccc2oc(nc2c1)C(=O)C(Cc1ccccc1)NC(=O)Cn1c(ncc(N)c1=O)-c1cccc(F)c1 Show InChI InChI=1S/C30H24FN5O6/c1-41-30(40)19-10-11-24-22(14-19)35-28(42-24)26(38)23(12-17-6-3-2-4-7-17)34-25(37)16-36-27(33-15-21(32)29(36)39)18-8-5-9-20(31)13-18/h2-11,13-15,23H,12,16,32H2,1H3,(H,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 14.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against chymase from human heart. |
J Med Chem 44: 1286-96 (2001)
BindingDB Entry DOI: 10.7270/Q26H4J4K |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50098876
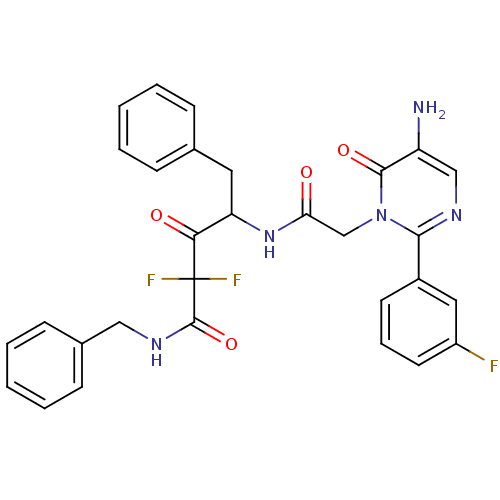
(4-{2-[5-Amino-2-(3-fluoro-phenyl)-6-oxo-6H-pyrimid...)Show SMILES Nc1cnc(-c2cccc(F)c2)n(CC(=O)NC(Cc2ccccc2)C(=O)C(F)(F)C(=O)NCc2ccccc2)c1=O Show InChI InChI=1S/C30H26F3N5O4/c31-22-13-7-12-21(15-22)27-35-17-23(34)28(41)38(27)18-25(39)37-24(14-19-8-3-1-4-9-19)26(40)30(32,33)29(42)36-16-20-10-5-2-6-11-20/h1-13,15,17,24H,14,16,18,34H2,(H,36,42)(H,37,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 15.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity was determined against human heart chymase |
J Med Chem 44: 1297-304 (2001)
BindingDB Entry DOI: 10.7270/Q22R3SCD |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50098866
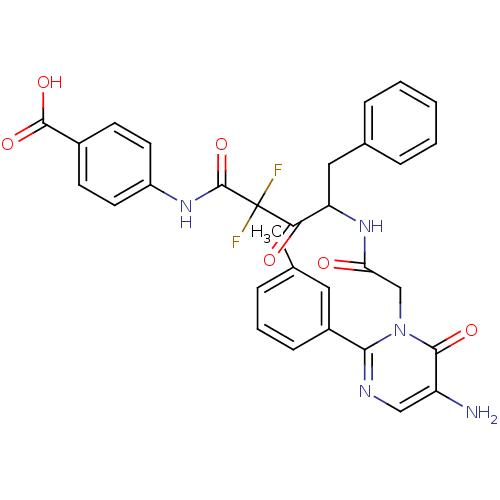
(4-({4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-y...)Show SMILES Cc1cccc(c1)-c1ncc(N)c(=O)n1CC(=O)NC(Cc1ccccc1)C(=O)C(F)(F)C(=O)Nc1ccc(cc1)C(O)=O Show InChI InChI=1S/C31H27F2N5O6/c1-18-6-5-9-21(14-18)27-35-16-23(34)28(41)38(27)17-25(39)37-24(15-19-7-3-2-4-8-19)26(40)31(32,33)30(44)36-22-12-10-20(11-13-22)29(42)43/h2-14,16,24H,15,17,34H2,1H3,(H,36,44)(H,37,39)(H,42,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 16.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity was determined against human heart chymase |
J Med Chem 44: 1297-304 (2001)
BindingDB Entry DOI: 10.7270/Q22R3SCD |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50098872

(CHEMBL442146 | {4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-p...)Show SMILES Cc1cccc(c1)-c1ncc(N)c(=O)n1CC(=O)NC(Cc1ccccc1)C(=O)C(F)(F)C(=O)NCC(O)=O Show InChI InChI=1S/C26H25F2N5O6/c1-15-6-5-9-17(10-15)23-30-12-18(29)24(38)33(23)14-20(34)32-19(11-16-7-3-2-4-8-16)22(37)26(27,28)25(39)31-13-21(35)36/h2-10,12,19H,11,13-14,29H2,1H3,(H,31,39)(H,32,34)(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 16.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity was determined against human heart chymase |
J Med Chem 44: 1297-304 (2001)
BindingDB Entry DOI: 10.7270/Q22R3SCD |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50098891

(4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl)-a...)Show SMILES Cc1cccc(c1)-c1ncc(N)c(=O)n1CC(=O)NC(Cc1ccccc1)C(=O)C(F)(F)C(=O)NCc1ccccc1 Show InChI InChI=1S/C31H29F2N5O4/c1-20-9-8-14-23(15-20)28-35-18-24(34)29(41)38(28)19-26(39)37-25(16-21-10-4-2-5-11-21)27(40)31(32,33)30(42)36-17-22-12-6-3-7-13-22/h2-15,18,25H,16-17,19,34H2,1H3,(H,36,42)(H,37,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 16.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity was determined against human heart chymase |
J Med Chem 44: 1297-304 (2001)
BindingDB Entry DOI: 10.7270/Q22R3SCD |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50098889
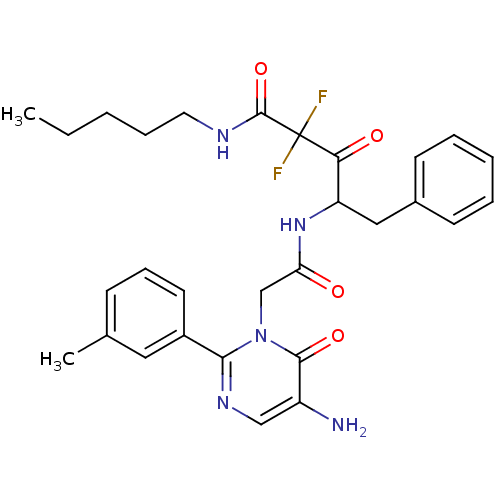
(4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl)-a...)Show SMILES CCCCCNC(=O)C(F)(F)C(=O)C(Cc1ccccc1)NC(=O)Cn1c(ncc(N)c1=O)-c1cccc(C)c1 Show InChI InChI=1S/C29H33F2N5O4/c1-3-4-8-14-33-28(40)29(30,31)25(38)23(16-20-11-6-5-7-12-20)35-24(37)18-36-26(34-17-22(32)27(36)39)21-13-9-10-19(2)15-21/h5-7,9-13,15,17,23H,3-4,8,14,16,18,32H2,1-2H3,(H,33,40)(H,35,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 18.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity was determined against human heart chymase |
J Med Chem 44: 1297-304 (2001)
BindingDB Entry DOI: 10.7270/Q22R3SCD |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50098847

(2-(2-{2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyri...)Show SMILES COC(=O)c1ccc2oc(nc2c1)C(=O)C(Cc1ccccc1)NC(=O)Cn1c(ncc(N)c1=O)-c1ccc(F)cc1 Show InChI InChI=1S/C30H24FN5O6/c1-41-30(40)19-9-12-24-22(14-19)35-28(42-24)26(38)23(13-17-5-3-2-4-6-17)34-25(37)16-36-27(33-15-21(32)29(36)39)18-7-10-20(31)11-8-18/h2-12,14-15,23H,13,16,32H2,1H3,(H,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 22.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against chymase from human heart. |
J Med Chem 44: 1286-96 (2001)
BindingDB Entry DOI: 10.7270/Q26H4J4K |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50098867

(3-{4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl...)Show SMILES Cc1cccc(c1)-c1ncc(N)c(=O)n1CC(=O)NC(Cc1ccccc1)C(=O)C(F)(F)C(=O)NCCC(O)=O Show InChI InChI=1S/C27H27F2N5O6/c1-16-6-5-9-18(12-16)24-32-14-19(30)25(39)34(24)15-21(35)33-20(13-17-7-3-2-4-8-17)23(38)27(28,29)26(40)31-11-10-22(36)37/h2-9,12,14,20H,10-11,13,15,30H2,1H3,(H,31,40)(H,33,35)(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity was determined against human heart chymase |
J Med Chem 44: 1297-304 (2001)
BindingDB Entry DOI: 10.7270/Q22R3SCD |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50098842
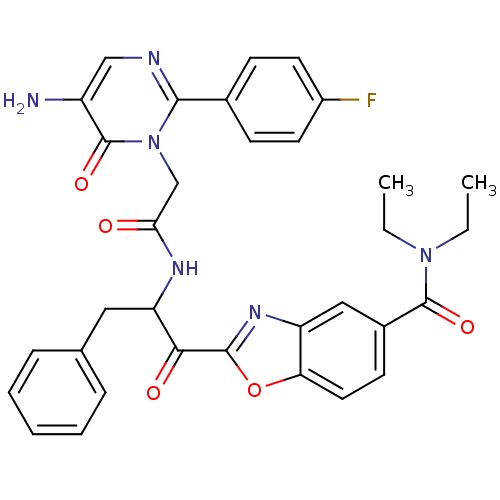
(2-(2-{2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyri...)Show SMILES CCN(CC)C(=O)c1ccc2oc(nc2c1)C(=O)C(Cc1ccccc1)NC(=O)Cn1c(ncc(N)c1=O)-c1ccc(F)cc1 Show InChI InChI=1S/C33H31FN6O5/c1-3-39(4-2)32(43)22-12-15-27-25(17-22)38-31(45-27)29(42)26(16-20-8-6-5-7-9-20)37-28(41)19-40-30(36-18-24(35)33(40)44)21-10-13-23(34)14-11-21/h5-15,17-18,26H,3-4,16,19,35H2,1-2H3,(H,37,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 23.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against alpha chymotrypsin from bovine pancreas. |
J Med Chem 44: 1286-96 (2001)
BindingDB Entry DOI: 10.7270/Q26H4J4K |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50098844

(2-(2-{2-[5-Amino-2-(3-chloro-phenyl)-6-oxo-6H-pyri...)Show SMILES COC(=O)c1ccc2oc(nc2c1)C(=O)C(Cc1ccccc1)NC(=O)Cn1c(ncc(N)c1=O)-c1cccc(Cl)c1 Show InChI InChI=1S/C30H24ClN5O6/c1-41-30(40)19-10-11-24-22(14-19)35-28(42-24)26(38)23(12-17-6-3-2-4-7-17)34-25(37)16-36-27(33-15-21(32)29(36)39)18-8-5-9-20(31)13-18/h2-11,13-15,23H,12,16,32H2,1H3,(H,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 26.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against chymase from human heart. |
J Med Chem 44: 1286-96 (2001)
BindingDB Entry DOI: 10.7270/Q26H4J4K |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50098874

(4-{2-[5-Amino-2-(3-chloro-phenyl)-6-oxo-6H-pyrimid...)Show SMILES Nc1cnc(-c2cccc(Cl)c2)n(CC(=O)NC(Cc2ccccc2)C(=O)C(F)(F)C(=O)NCc2ccccc2)c1=O Show InChI InChI=1S/C30H26ClF2N5O4/c31-22-13-7-12-21(15-22)27-35-17-23(34)28(41)38(27)18-25(39)37-24(14-19-8-3-1-4-9-19)26(40)30(32,33)29(42)36-16-20-10-5-2-6-11-20/h1-13,15,17,24H,14,16,18,34H2,(H,36,42)(H,37,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against canine skin chymase |
J Med Chem 44: 1297-304 (2001)
BindingDB Entry DOI: 10.7270/Q22R3SCD |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50098881
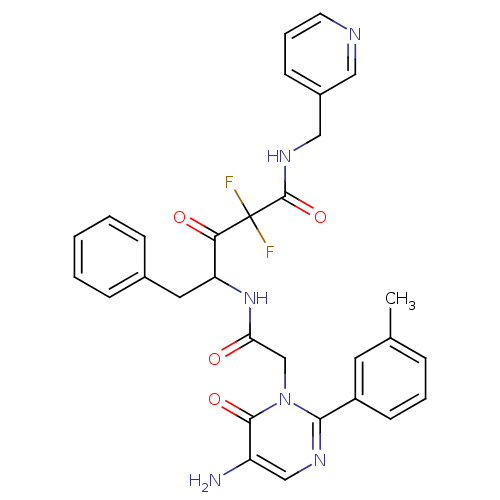
(4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl)-a...)Show SMILES Cc1cccc(c1)-c1ncc(N)c(=O)n1CC(=O)NC(Cc1ccccc1)C(=O)C(F)(F)C(=O)NCc1cccnc1 Show InChI InChI=1S/C30H28F2N6O4/c1-19-7-5-11-22(13-19)27-35-17-23(33)28(41)38(27)18-25(39)37-24(14-20-8-3-2-4-9-20)26(40)30(31,32)29(42)36-16-21-10-6-12-34-15-21/h2-13,15,17,24H,14,16,18,33H2,1H3,(H,36,42)(H,37,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 28.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity was determined against human heart chymase |
J Med Chem 44: 1297-304 (2001)
BindingDB Entry DOI: 10.7270/Q22R3SCD |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50098861
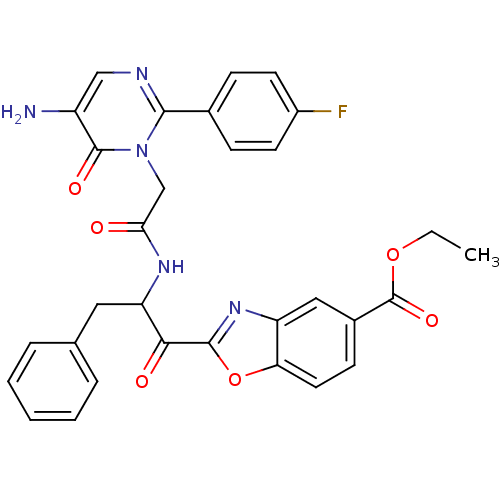
(2-(2-{2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyri...)Show SMILES CCOC(=O)c1ccc2oc(nc2c1)C(=O)C(Cc1ccccc1)NC(=O)Cn1c(ncc(N)c1=O)-c1ccc(F)cc1 Show InChI InChI=1S/C31H26FN5O6/c1-2-42-31(41)20-10-13-25-23(15-20)36-29(43-25)27(39)24(14-18-6-4-3-5-7-18)35-26(38)17-37-28(34-16-22(33)30(37)40)19-8-11-21(32)12-9-19/h3-13,15-16,24H,2,14,17,33H2,1H3,(H,35,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against chymase from human heart. |
J Med Chem 44: 1286-96 (2001)
BindingDB Entry DOI: 10.7270/Q26H4J4K |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50098886

(4-[2-(5-Amino-6-oxo-2-phenyl-6H-pyrimidin-1-yl)-ac...)Show SMILES Nc1cnc(-c2ccccc2)n(CC(=O)NC(Cc2ccccc2)C(=O)C(F)(F)C(=O)NCc2ccccc2)c1=O Show InChI InChI=1S/C30H27F2N5O4/c31-30(32,29(41)35-17-21-12-6-2-7-13-21)26(39)24(16-20-10-4-1-5-11-20)36-25(38)19-37-27(22-14-8-3-9-15-22)34-18-23(33)28(37)40/h1-15,18,24H,16-17,19,33H2,(H,35,41)(H,36,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 32.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity was determined against human heart chymase |
J Med Chem 44: 1297-304 (2001)
BindingDB Entry DOI: 10.7270/Q22R3SCD |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50098862

(2-(2-{2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyri...)Show SMILES CCNC(=O)c1ccc2oc(nc2c1)C(=O)C(Cc1ccccc1)NC(=O)Cn1c(ncc(N)c1=O)-c1ccc(F)cc1 Show InChI InChI=1S/C31H27FN6O5/c1-2-34-29(41)20-10-13-25-23(15-20)37-30(43-25)27(40)24(14-18-6-4-3-5-7-18)36-26(39)17-38-28(35-16-22(33)31(38)42)19-8-11-21(32)12-9-19/h3-13,15-16,24H,2,14,17,33H2,1H3,(H,34,41)(H,36,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 32.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against alpha chymotrypsin from bovine pancreas. |
J Med Chem 44: 1286-96 (2001)
BindingDB Entry DOI: 10.7270/Q26H4J4K |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50098883
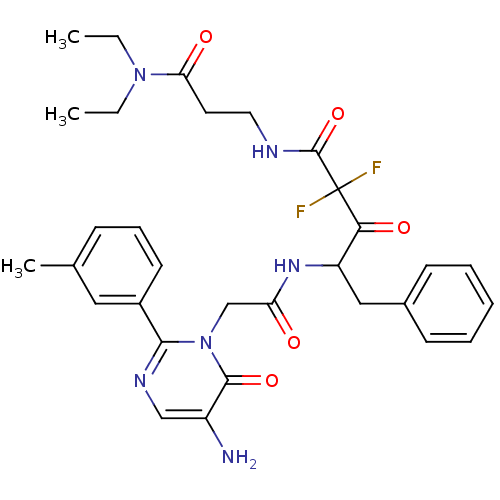
(4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl)-a...)Show SMILES CCN(CC)C(=O)CCNC(=O)C(F)(F)C(=O)C(Cc1ccccc1)NC(=O)Cn1c(ncc(N)c1=O)-c1cccc(C)c1 Show InChI InChI=1S/C31H36F2N6O5/c1-4-38(5-2)26(41)14-15-35-30(44)31(32,33)27(42)24(17-21-11-7-6-8-12-21)37-25(40)19-39-28(36-18-23(34)29(39)43)22-13-9-10-20(3)16-22/h6-13,16,18,24H,4-5,14-15,17,19,34H2,1-3H3,(H,35,44)(H,37,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 33.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity was determined against human heart chymase |
J Med Chem 44: 1297-304 (2001)
BindingDB Entry DOI: 10.7270/Q22R3SCD |
More data for this
Ligand-Target Pair | |
Mast cell protease 9
(Mus musculus) | BDBM50098847

(2-(2-{2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyri...)Show SMILES COC(=O)c1ccc2oc(nc2c1)C(=O)C(Cc1ccccc1)NC(=O)Cn1c(ncc(N)c1=O)-c1ccc(F)cc1 Show InChI InChI=1S/C30H24FN5O6/c1-41-30(40)19-9-12-24-22(14-19)35-28(42-24)26(38)23(13-17-5-3-2-4-6-17)34-25(37)16-36-27(33-15-21(32)29(36)39)18-7-10-20(31)11-8-18/h2-12,14-15,23H,13,16,32H2,1H3,(H,34,37) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 40.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against mouse peritoneal chymase |
J Med Chem 44: 1286-96 (2001)
BindingDB Entry DOI: 10.7270/Q26H4J4K |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50098864
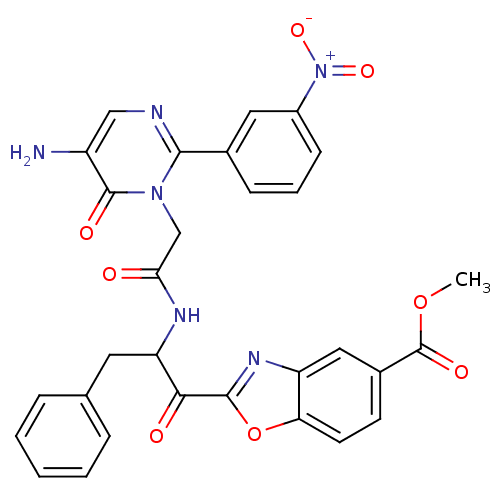
(2-(2-{2-[5-Amino-2-(3-nitro-phenyl)-6-oxo-6H-pyrim...)Show SMILES COC(=O)c1ccc2oc(nc2c1)C(=O)C(Cc1ccccc1)NC(=O)Cn1c(ncc(N)c1=O)-c1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C30H24N6O8/c1-43-30(40)19-10-11-24-22(14-19)34-28(44-24)26(38)23(12-17-6-3-2-4-7-17)33-25(37)16-35-27(32-15-21(31)29(35)39)18-8-5-9-20(13-18)36(41)42/h2-11,13-15,23H,12,16,31H2,1H3,(H,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 41.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against chymase from human heart. |
J Med Chem 44: 1286-96 (2001)
BindingDB Entry DOI: 10.7270/Q26H4J4K |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50098843
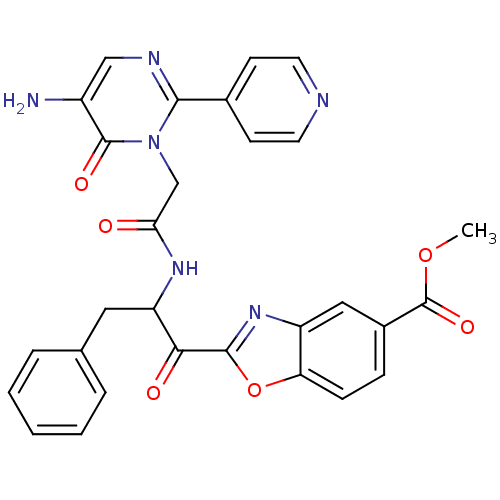
(2-{2-[2-(5-Amino-6-oxo-2-pyridin-4-yl-6H-pyrimidin...)Show SMILES COC(=O)c1ccc2oc(nc2c1)C(=O)C(Cc1ccccc1)NC(=O)Cn1c(ncc(N)c1=O)-c1ccncc1 Show InChI InChI=1S/C29H24N6O6/c1-40-29(39)19-7-8-23-21(14-19)34-27(41-23)25(37)22(13-17-5-3-2-4-6-17)33-24(36)16-35-26(18-9-11-31-12-10-18)32-15-20(30)28(35)38/h2-12,14-15,22H,13,16,30H2,1H3,(H,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 43.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against chymase from human heart. |
J Med Chem 44: 1286-96 (2001)
BindingDB Entry DOI: 10.7270/Q26H4J4K |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50098838

(2-{2-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl...)Show SMILES COC(=O)c1ccc2oc(nc2c1)C(=O)C(Cc1ccccc1)NC(=O)Cn1c(ncc(N)c1=O)-c1cccc(C)c1 Show InChI InChI=1S/C31H27N5O6/c1-18-7-6-10-20(13-18)28-33-16-22(32)30(39)36(28)17-26(37)34-24(14-19-8-4-3-5-9-19)27(38)29-35-23-15-21(31(40)41-2)11-12-25(23)42-29/h3-13,15-16,24H,14,17,32H2,1-2H3,(H,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 44.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against chymase from human heart. |
J Med Chem 44: 1286-96 (2001)
BindingDB Entry DOI: 10.7270/Q26H4J4K |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50098839
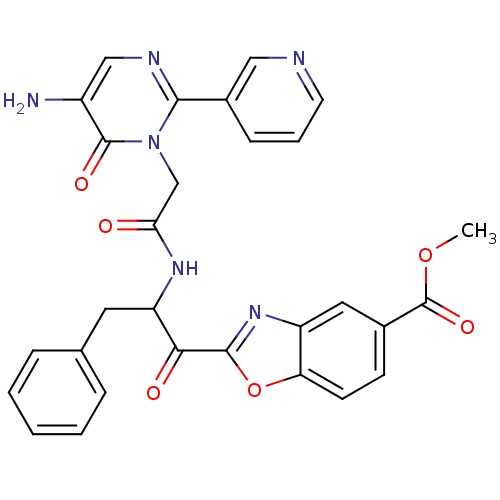
(2-{2-[2-(5-Amino-6-oxo-2-pyridin-3-yl-6H-pyrimidin...)Show SMILES COC(=O)c1ccc2oc(nc2c1)C(=O)C(Cc1ccccc1)NC(=O)Cn1c(ncc(N)c1=O)-c1cccnc1 Show InChI InChI=1S/C29H24N6O6/c1-40-29(39)18-9-10-23-21(13-18)34-27(41-23)25(37)22(12-17-6-3-2-4-7-17)33-24(36)16-35-26(19-8-5-11-31-14-19)32-15-20(30)28(35)38/h2-11,13-15,22H,12,16,30H2,1H3,(H,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 45.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against alpha chymotrypsin from bovine pancreas. |
J Med Chem 44: 1286-96 (2001)
BindingDB Entry DOI: 10.7270/Q26H4J4K |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50098854

(2-{2-[2-(5-Amino-6-oxo-2-phenyl-6H-pyrimidin-1-yl)...)Show SMILES COC(=O)c1ccc2oc(nc2c1)C(=O)C(Cc1ccccc1)NC(=O)Cn1c(ncc(N)c1=O)-c1ccccc1 Show InChI InChI=1S/C30H25N5O6/c1-40-30(39)20-12-13-24-22(15-20)34-28(41-24)26(37)23(14-18-8-4-2-5-9-18)33-25(36)17-35-27(19-10-6-3-7-11-19)32-16-21(31)29(35)38/h2-13,15-16,23H,14,17,31H2,1H3,(H,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 46.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against alpha chymotrypsin from bovine pancreas. |
J Med Chem 44: 1286-96 (2001)
BindingDB Entry DOI: 10.7270/Q26H4J4K |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data