

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ribosomal protein S6 kinase alpha-1 (Homo sapiens (Human)) | BDBM25004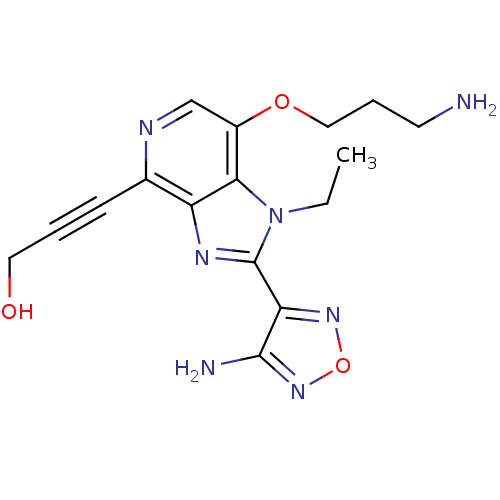 (3-[2-(4-amino-1,2,5-oxadiazol-3-yl)-7-(3-aminoprop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-5 (Homo sapiens (Human)) | BDBM24994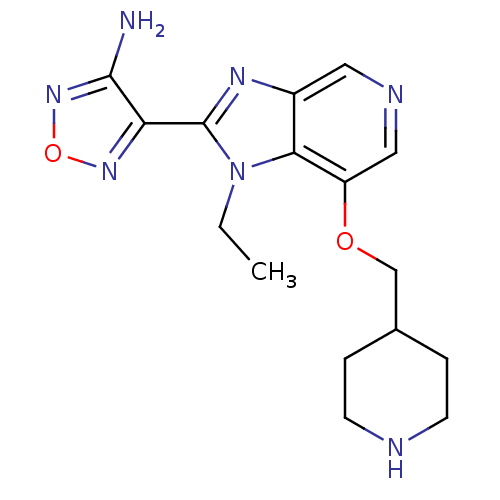 (4-[1-ethyl-7-(piperidin-4-ylmethoxy)-1H-imidazo[4,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM25004 (3-[2-(4-amino-1,2,5-oxadiazol-3-yl)-7-(3-aminoprop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 22 |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM25013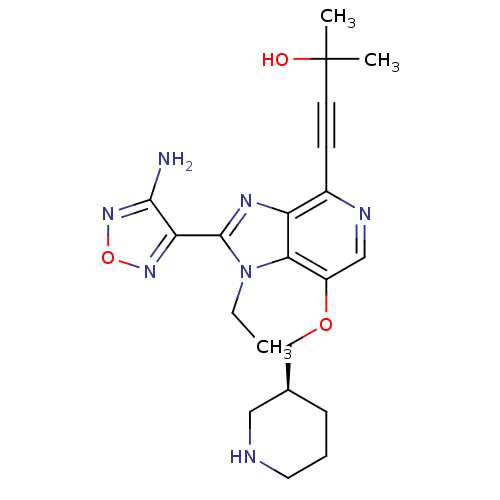 (4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-[(3S...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 22 |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM25009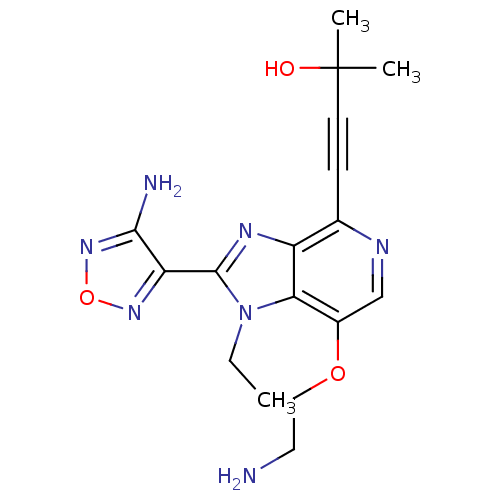 (4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-7-(2-aminoetho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 22 |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM25010 (4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-7-[(3S)-3-amin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM25014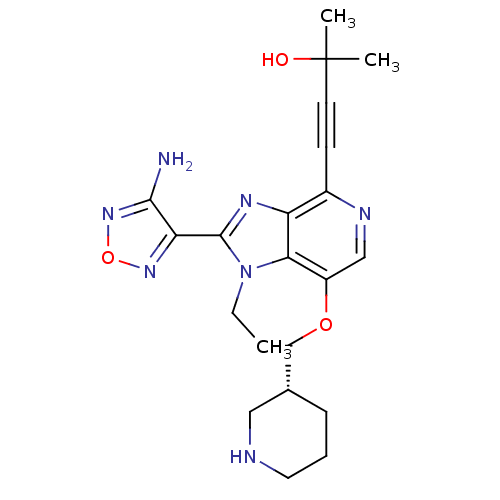 (4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-[(3R...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM24994 (4-[1-ethyl-7-(piperidin-4-ylmethoxy)-1H-imidazo[4,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-5 (Homo sapiens (Human)) | BDBM24991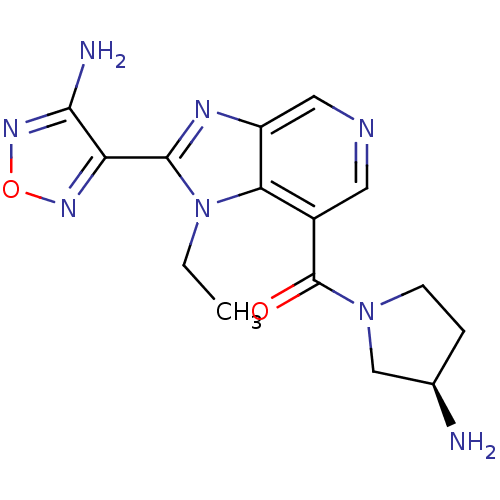 (4-(7-{[(3R)-3-aminopyrrolidin-1-yl]carbonyl}-1-eth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM25016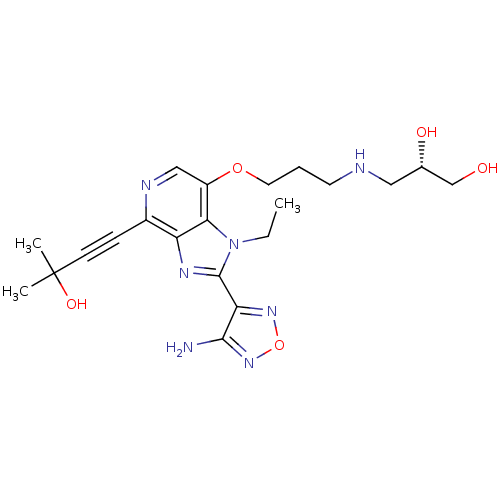 ((2S)-3-[(3-{[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-et...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM25010 (4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-7-[(3S)-3-amin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM25015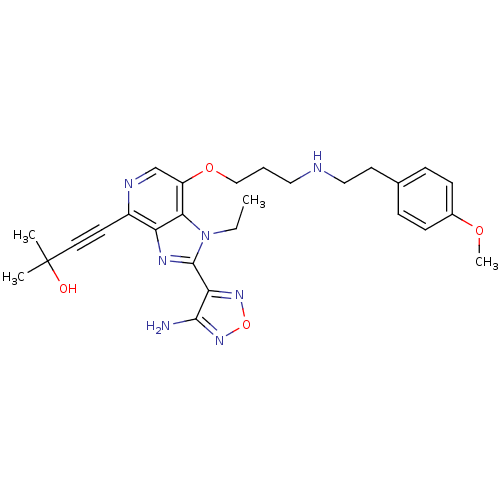 (4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-(3-{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM24991 (4-(7-{[(3R)-3-aminopyrrolidin-1-yl]carbonyl}-1-eth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM24990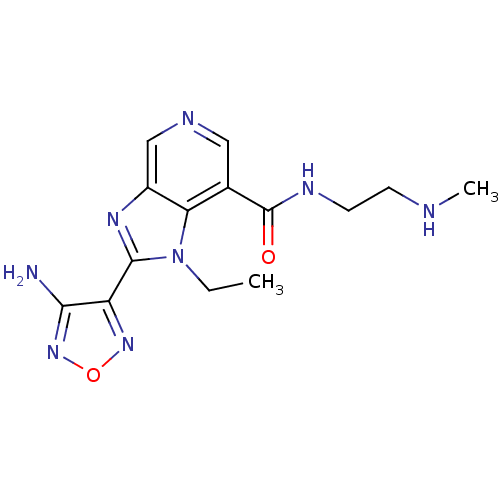 (2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-N-[2-(met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM25016 ((2S)-3-[(3-{[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-et...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM25003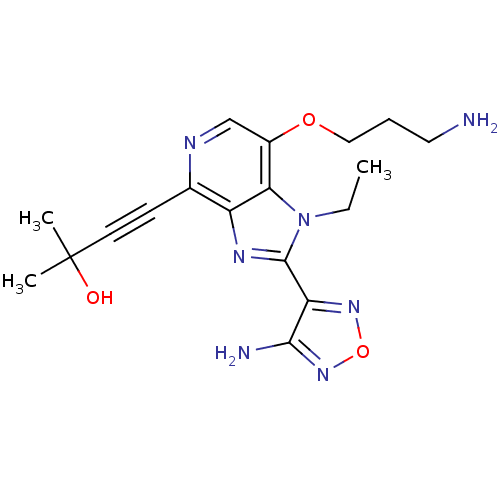 (4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-7-(3-aminoprop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.5 | 22 |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-1 (Homo sapiens (Human)) | BDBM25005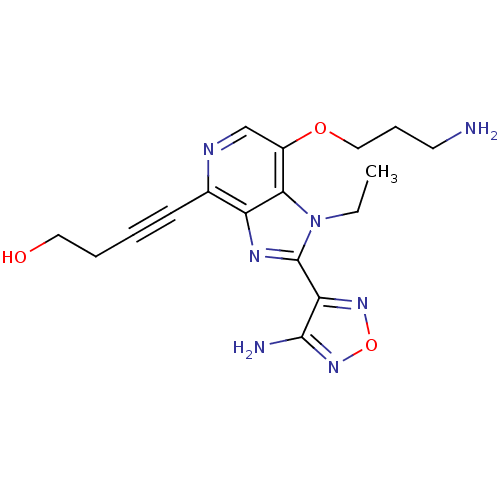 (4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-7-(3-aminoprop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Rattus norvegicus (Rat)) | BDBM24995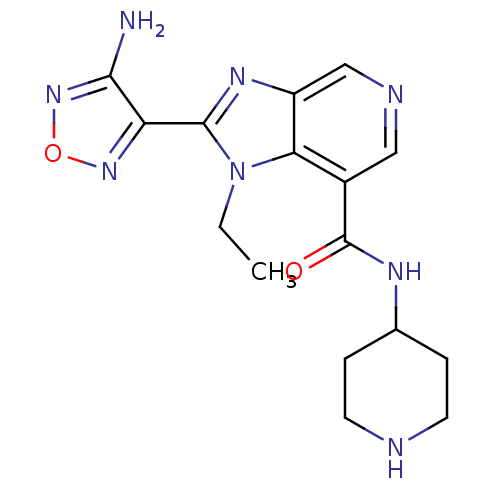 (2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-N-(piperi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM25015 (4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-(3-{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM25011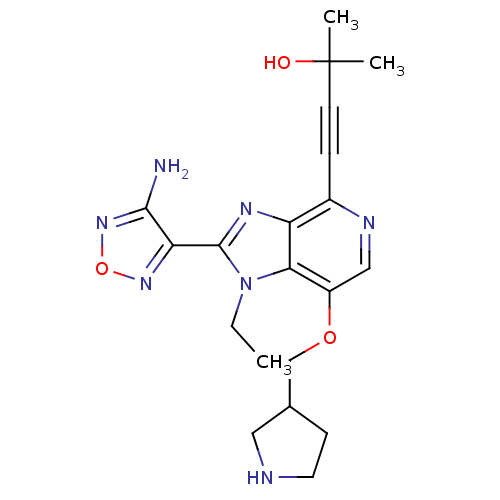 (4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-(pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.5 | 22 |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM25012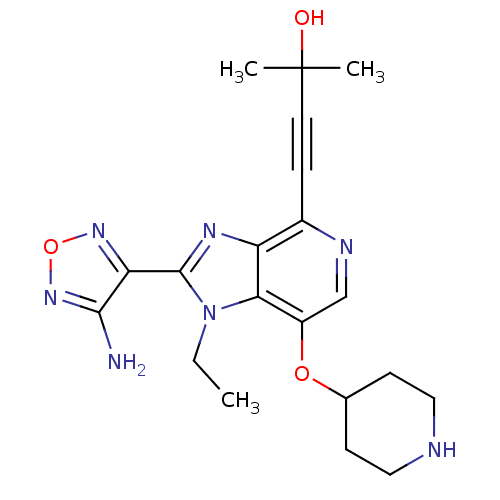 (4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-(pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.5 | 22 |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM24989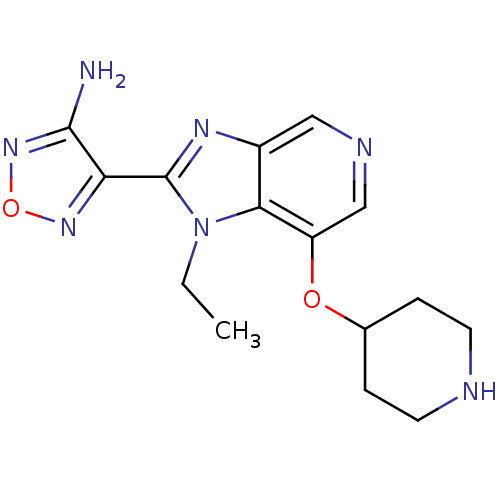 (4-[1-ethyl-7-(piperidin-4-yloxy)-1H-imidazo[4,5-c]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM25012 (4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-(pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-5 (Homo sapiens (Human)) | BDBM24996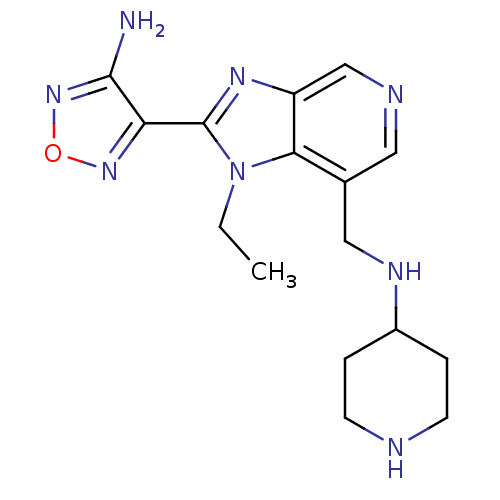 (4-{1-ethyl-7-[(piperidin-4-ylamino)methyl]-1H-imid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM25008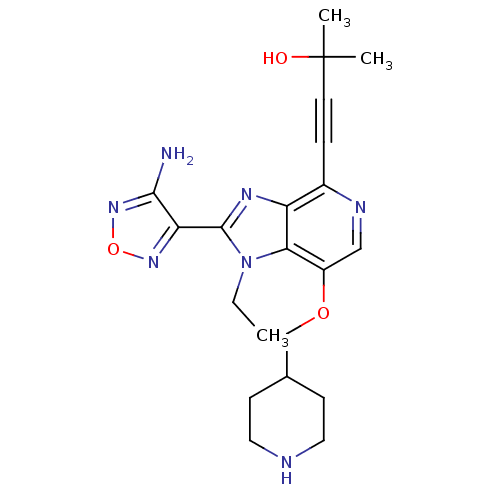 (4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-(pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM25007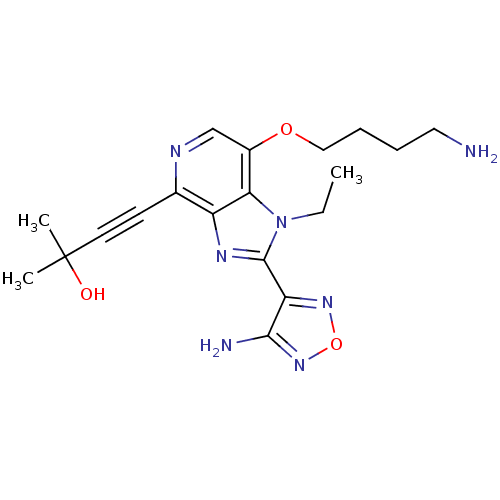 (4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-7-(4-aminobuto...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.5 | 22 |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-gamma serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM25013 (4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-[(3S...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM25007 (4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-7-(4-aminobuto...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2 (Homo sapiens (Human)) | BDBM259367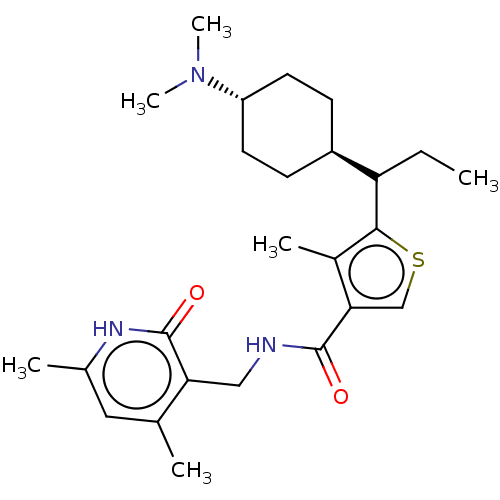 (US9505745, 44 | US9505745, 45 | US9790212, Example...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property (No. 2) Limited US Patent | Assay Description Protocol 2: Compounds contained herein were evaluated for their ability to inhibit the methyltransferase activity of EZH2 within the PRC2 complex. Hu... | US Patent US9505745 (2016) BindingDB Entry DOI: 10.7270/Q23B5Z3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2 (Homo sapiens (Human)) | BDBM259356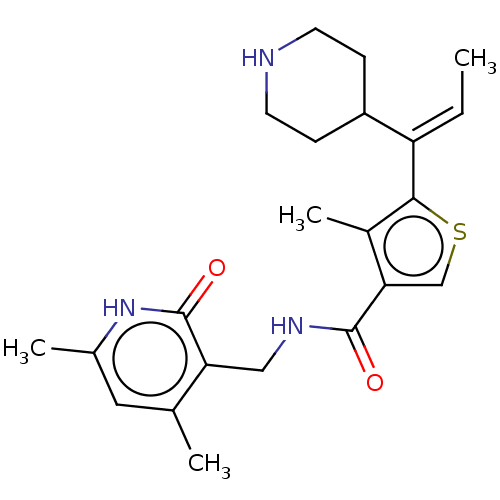 (US9505745, 33 | US9790212, Example 33) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property (No. 2) Limited US Patent | Assay Description Protocol 2: Compounds contained herein were evaluated for their ability to inhibit the methyltransferase activity of EZH2 within the PRC2 complex. Hu... | US Patent US9505745 (2016) BindingDB Entry DOI: 10.7270/Q23B5Z3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2 (Homo sapiens (Human)) | BDBM347682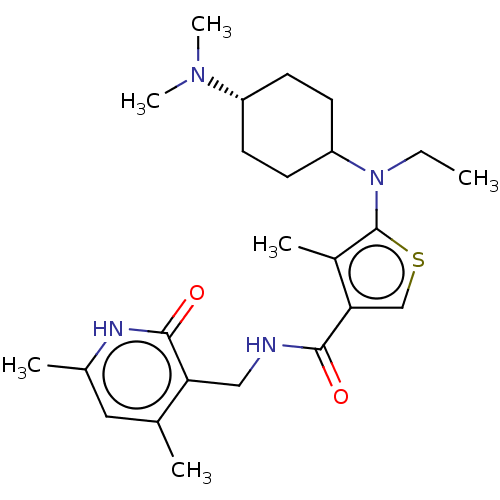 (N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)met...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE INTELLECTUAL PROPERTY (NO. 2) LIMITED US Patent | Assay Description Compounds contained herein were evaluated for their ability to inhibit the methyltransferase activity of EZH2 within the PRC2 complex. Human PRC2 com... | US Patent US9790212 (2017) BindingDB Entry DOI: 10.7270/Q2PZ5BZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2 (Homo sapiens (Human)) | BDBM259335 (US9505745, 12 | US9790212, Example 12) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE INTELLECTUAL PROPERTY (NO. 2) LIMITED US Patent | Assay Description Compounds contained herein were evaluated for their ability to inhibit the methyltransferase activity of EZH2 within the PRC2 complex. Human PRC2 com... | US Patent US9790212 (2017) BindingDB Entry DOI: 10.7270/Q2PZ5BZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2 (Homo sapiens (Human)) | BDBM259342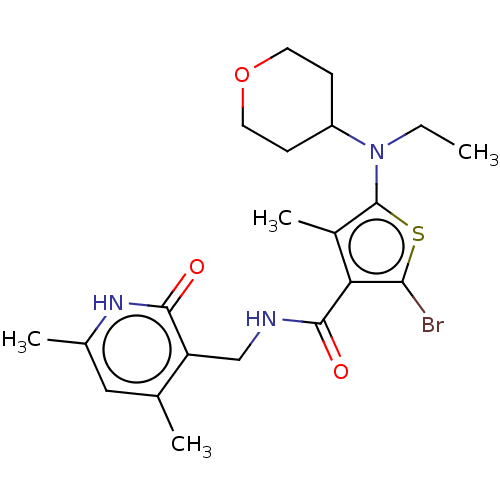 (US9505745, 19 | US9790212, Example 19) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE INTELLECTUAL PROPERTY (NO. 2) LIMITED US Patent | Assay Description Compounds contained herein were evaluated for their ability to inhibit the methyltransferase activity of EZH2 within the PRC2 complex. Human PRC2 com... | US Patent US9790212 (2017) BindingDB Entry DOI: 10.7270/Q2PZ5BZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2 (Homo sapiens (Human)) | BDBM259355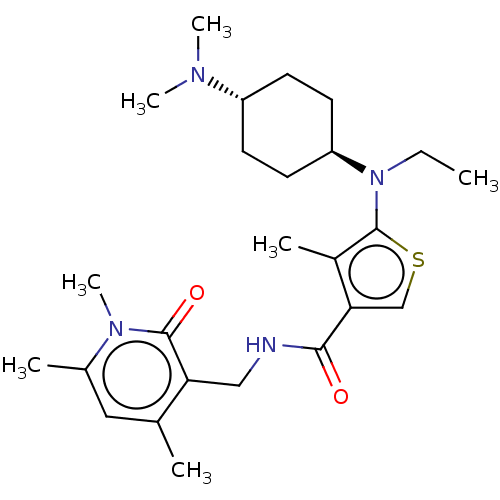 (US9505745, 32 | US9790212, Example 32) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE INTELLECTUAL PROPERTY (NO. 2) LIMITED US Patent | Assay Description Compounds contained herein were evaluated for their ability to inhibit the methyltransferase activity of EZH2 within the PRC2 complex. Human PRC2 com... | US Patent US9790212 (2017) BindingDB Entry DOI: 10.7270/Q2PZ5BZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2 (Homo sapiens (Human)) | BDBM259356 (US9505745, 33 | US9790212, Example 33) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE INTELLECTUAL PROPERTY (NO. 2) LIMITED US Patent | Assay Description Compounds contained herein were evaluated for their ability to inhibit the methyltransferase activity of EZH2 within the PRC2 complex. Human PRC2 com... | US Patent US9790212 (2017) BindingDB Entry DOI: 10.7270/Q2PZ5BZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2 (Homo sapiens (Human)) | BDBM259359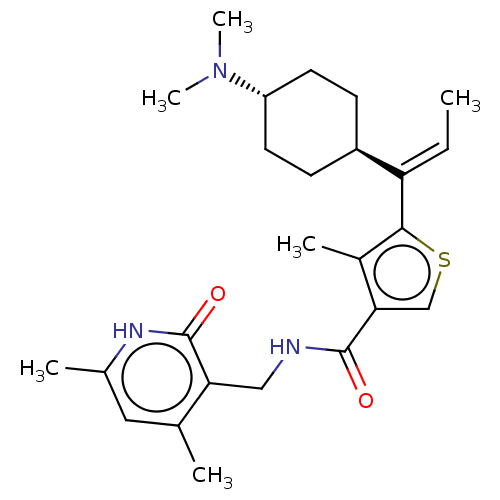 (US9505745, 36 | US9790212, Example 36) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE INTELLECTUAL PROPERTY (NO. 2) LIMITED US Patent | Assay Description Compounds contained herein were evaluated for their ability to inhibit the methyltransferase activity of EZH2 within the PRC2 complex. Human PRC2 com... | US Patent US9790212 (2017) BindingDB Entry DOI: 10.7270/Q2PZ5BZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2 (Homo sapiens (Human)) | BDBM259367 (US9505745, 44 | US9505745, 45 | US9790212, Example...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE INTELLECTUAL PROPERTY (NO. 2) LIMITED US Patent | Assay Description Compounds contained herein were evaluated for their ability to inhibit the methyltransferase activity of EZH2 within the PRC2 complex. Human PRC2 com... | US Patent US9790212 (2017) BindingDB Entry DOI: 10.7270/Q2PZ5BZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2 (Homo sapiens (Human)) | BDBM347682 (N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)met...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE INTELLECTUAL PROPERTY (NO. 2) LIMITED US Patent | Assay Description Compounds contained herein were evaluated for their ability to inhibit the methyltransferase activity of EZH2 within the PRC2 complex. Human PRC2 com... | US Patent US9790212 (2017) BindingDB Entry DOI: 10.7270/Q2PZ5BZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2 (Homo sapiens (Human)) | BDBM259339 (US9505745, 16 | US9790212, Example 16) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE INTELLECTUAL PROPERTY (NO. 2) LIMITED US Patent | Assay Description Compounds contained herein were evaluated for their ability to inhibit the methyltransferase activity of EZH2 within the PRC2 complex. Human PRC2 com... | US Patent US9790212 (2017) BindingDB Entry DOI: 10.7270/Q2PZ5BZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2 (Homo sapiens (Human)) | BDBM259341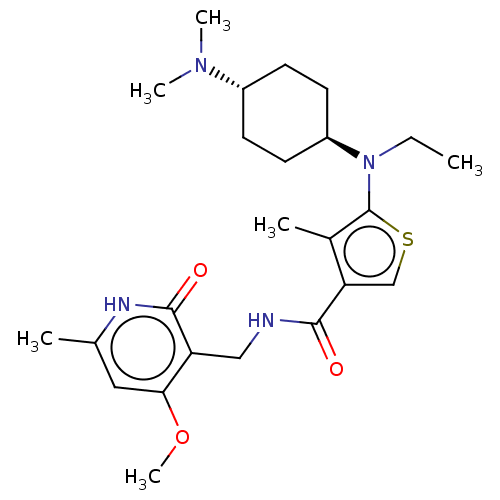 (US9505745, 18 | US9790212, Example 18) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE INTELLECTUAL PROPERTY (NO. 2) LIMITED US Patent | Assay Description Compounds contained herein were evaluated for their ability to inhibit the methyltransferase activity of EZH2 within the PRC2 complex. Human PRC2 com... | US Patent US9790212 (2017) BindingDB Entry DOI: 10.7270/Q2PZ5BZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM439129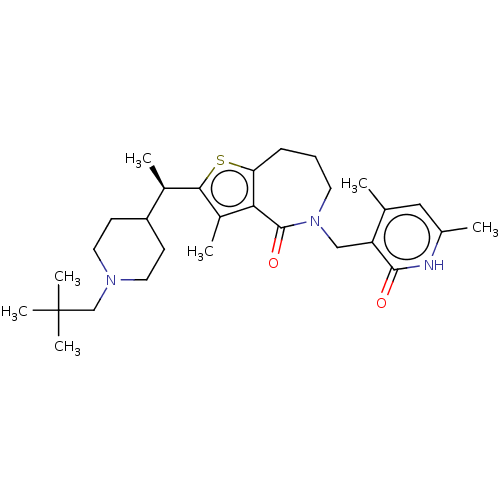 ((R)-5-((4,6-Dimethyl-2-oxo-1,2-dihydropyridin-3-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE INTELLECTUAL PROPERTY (NO.2) LIMITED US Patent | Assay Description 1. Prepare 10 mM stock of compounds from solid in 100% DMSO.2. Set up an 11-point serial dilution (1:4 dilution, top concentration 10 mM) in 100% DMS... | US Patent US10604531 (2020) BindingDB Entry DOI: 10.7270/Q23X89N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM439130 ((R)-2-(1-(1-(Cyclobutylmethyl)piperidin-4-yl)ethyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE INTELLECTUAL PROPERTY (NO.2) LIMITED US Patent | Assay Description 1. Prepare 10 mM stock of compounds from solid in 100% DMSO.2. Set up an 11-point serial dilution (1:4 dilution, top concentration 10 mM) in 100% DMS... | US Patent US10604531 (2020) BindingDB Entry DOI: 10.7270/Q23X89N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM439132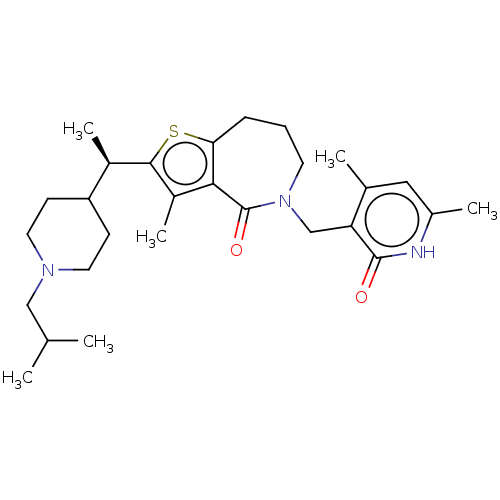 ((R)-5-((4,6-Dimethyl-2-oxo-1,2-dihydropyridin-3-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE INTELLECTUAL PROPERTY (NO.2) LIMITED US Patent | Assay Description 1. Prepare 10 mM stock of compounds from solid in 100% DMSO.2. Set up an 11-point serial dilution (1:4 dilution, top concentration 10 mM) in 100% DMS... | US Patent US10604531 (2020) BindingDB Entry DOI: 10.7270/Q23X89N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM439133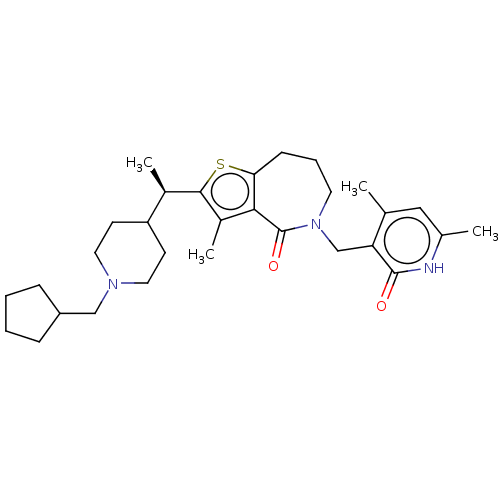 ((R)-2-(1-(1-(Cyclopentylmethyl)piperidin-4-yl)ethy...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE INTELLECTUAL PROPERTY (NO.2) LIMITED US Patent | Assay Description 1. Prepare 10 mM stock of compounds from solid in 100% DMSO.2. Set up an 11-point serial dilution (1:4 dilution, top concentration 10 mM) in 100% DMS... | US Patent US10604531 (2020) BindingDB Entry DOI: 10.7270/Q23X89N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM439163 ((R)-5-((4,6-Dimethyl-2-oxo-1,2-dihydropyridin-3-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE INTELLECTUAL PROPERTY (NO.2) LIMITED US Patent | Assay Description 1. Prepare 10 mM stock of compounds from solid in 100% DMSO.2. Set up an 11-point serial dilution (1:4 dilution, top concentration 10 mM) in 100% DMS... | US Patent US10604531 (2020) BindingDB Entry DOI: 10.7270/Q23X89N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM439164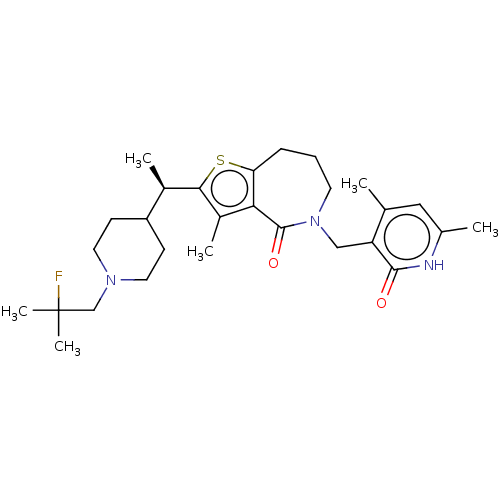 ((R)-5-((4,6-Dimethyl-2-oxo-1,2-dihydropyridin-3-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE INTELLECTUAL PROPERTY (NO.2) LIMITED US Patent | Assay Description 1. Prepare 10 mM stock of compounds from solid in 100% DMSO.2. Set up an 11-point serial dilution (1:4 dilution, top concentration 10 mM) in 100% DMS... | US Patent US10604531 (2020) BindingDB Entry DOI: 10.7270/Q23X89N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM439165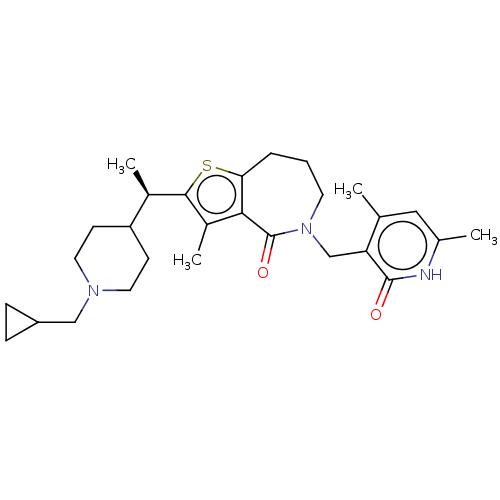 ((R)-2-(1-(1-(Cyclopropylmethyl)piperidin-4-yl)ethy...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE INTELLECTUAL PROPERTY (NO.2) LIMITED US Patent | Assay Description 1. Prepare 10 mM stock of compounds from solid in 100% DMSO.2. Set up an 11-point serial dilution (1:4 dilution, top concentration 10 mM) in 100% DMS... | US Patent US10604531 (2020) BindingDB Entry DOI: 10.7270/Q23X89N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM439169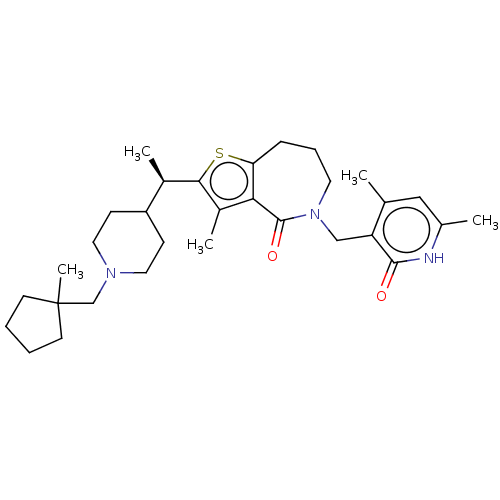 ((R)-5-((4,6-Dimethyl-2-oxo-1,2-dihydropyridin-3-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE INTELLECTUAL PROPERTY (NO.2) LIMITED US Patent | Assay Description 1. Prepare 10 mM stock of compounds from solid in 100% DMSO.2. Set up an 11-point serial dilution (1:4 dilution, top concentration 10 mM) in 100% DMS... | US Patent US10604531 (2020) BindingDB Entry DOI: 10.7270/Q23X89N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM439190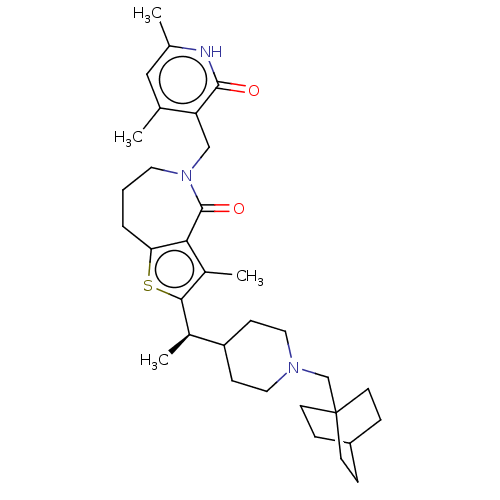 ((R)-2-(1-(1-(Bicyclo[2.2.2]octan-1-ylmethyl)piperi...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE INTELLECTUAL PROPERTY (NO.2) LIMITED US Patent | Assay Description 1. Prepare 10 mM stock of compounds from solid in 100% DMSO.2. Set up an 11-point serial dilution (1:4 dilution, top concentration 10 mM) in 100% DMS... | US Patent US10604531 (2020) BindingDB Entry DOI: 10.7270/Q23X89N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM439192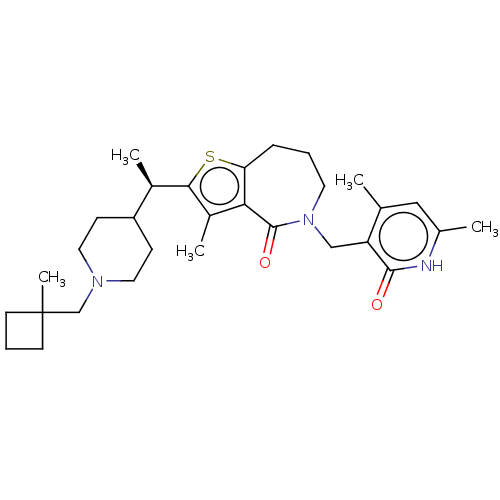 ((R)-5-((4,6-Dimethyl-2-oxo-1,2-dihydropyridin-3-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE INTELLECTUAL PROPERTY (NO.2) LIMITED US Patent | Assay Description 1. Prepare 10 mM stock of compounds from solid in 100% DMSO.2. Set up an 11-point serial dilution (1:4 dilution, top concentration 10 mM) in 100% DMS... | US Patent US10604531 (2020) BindingDB Entry DOI: 10.7270/Q23X89N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 406 total ) | Next | Last >> |