

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin K (Homo sapiens (Human)) | BDBM50066650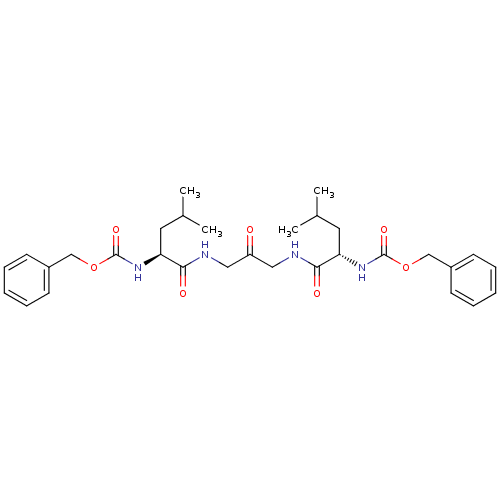 (1,3-BIS[[N-[(PHENYLMETHOXY)CARBONYL]-L-LEUCYL]AMIN...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity measured against cathepsin K. | J Med Chem 41: 4567-76 (1998) Article DOI: 10.1021/jm980249f BindingDB Entry DOI: 10.7270/Q26974TN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50146461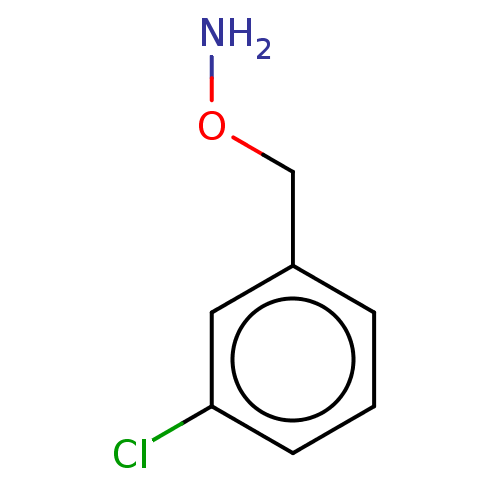 (CHEMBL3765807) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 154 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 60 mins by Michaelis-Menton... | Eur J Med Chem 108: 564-76 (2016) Article DOI: 10.1016/j.ejmech.2015.12.028 BindingDB Entry DOI: 10.7270/Q26Q203T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50146460 (CHEMBL3763688) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 164 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 60 mins by Michaelis-Menton... | Eur J Med Chem 108: 564-76 (2016) Article DOI: 10.1016/j.ejmech.2015.12.028 BindingDB Entry DOI: 10.7270/Q26Q203T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24666 (4-(1H-imidazol-4-yl)benzene-1-thiol | 4-(1H-imidaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.80E+3 | -31.6 | 7.70E+3 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 51: 4968-77 (2008) Article DOI: 10.1021/jm800512z BindingDB Entry DOI: 10.7270/Q2154FB1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24665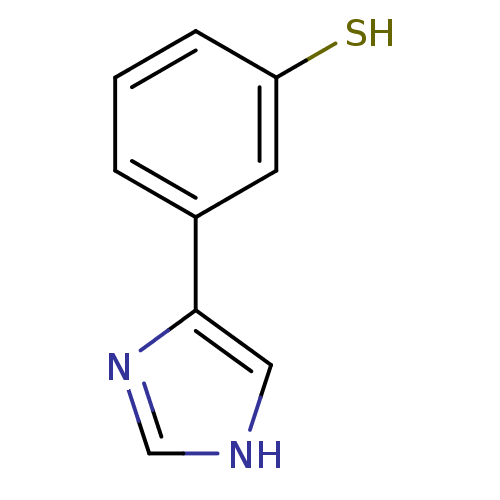 (3-(1H-imidazol-4-yl)benzene-1-thiol | 3-(1H-imidaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.30E+3 | -31.3 | 7.60E+3 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 51: 4968-77 (2008) Article DOI: 10.1021/jm800512z BindingDB Entry DOI: 10.7270/Q2154FB1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24663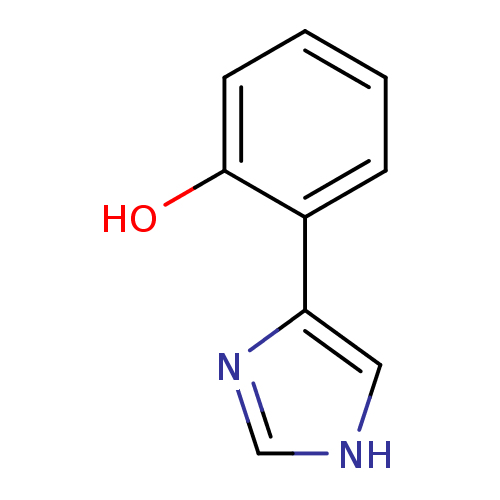 (2-(1H-imidazol-4-yl)phenol | 2-(1H-imidazol-4-yl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 8.90E+3 | -30.0 | 4.80E+3 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 51: 4968-77 (2008) Article DOI: 10.1021/jm800512z BindingDB Entry DOI: 10.7270/Q2154FB1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Papain (Carica papaya) | BDBM50066650 (1,3-BIS[[N-[(PHENYLMETHOXY)CARBONYL]-L-LEUCYL]AMIN...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity measured against papain. | J Med Chem 41: 4567-76 (1998) Article DOI: 10.1021/jm980249f BindingDB Entry DOI: 10.7270/Q26974TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24797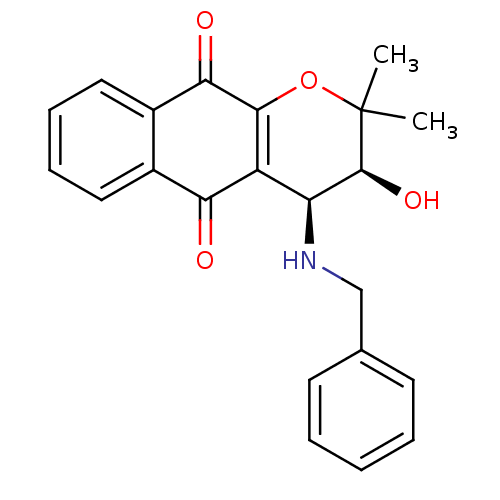 ((3S,4S)-4-(benzylamino)-3-hydroxy-2,2-dimethyl-2H,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 51: 1706-18 (2008) Article DOI: 10.1021/jm7014155 BindingDB Entry DOI: 10.7270/Q2VD6WSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50146461 (CHEMBL3765807) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of doxycycline-induced human IDO1 expressed in Trex cells assessed as reduction in kynurenine production treated with 5-fold serial diluti... | Eur J Med Chem 108: 564-76 (2016) Article DOI: 10.1016/j.ejmech.2015.12.028 BindingDB Entry DOI: 10.7270/Q26Q203T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24802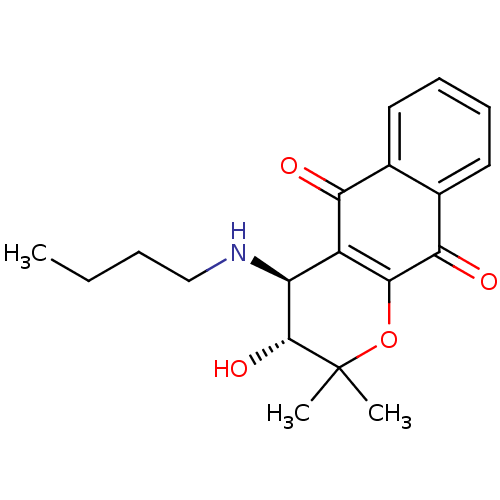 ((3R,4S)-4-(butylamino)-3-hydroxy-2,2-dimethyl-2H,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 51: 1706-18 (2008) Article DOI: 10.1021/jm7014155 BindingDB Entry DOI: 10.7270/Q2VD6WSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50146460 (CHEMBL3763688) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of IFN gamma induced human IDO1 in HeLa cells assessed as reduction in kynurenine production treated with 5-fold serial dilution beginning... | Eur J Med Chem 108: 564-76 (2016) Article DOI: 10.1016/j.ejmech.2015.12.028 BindingDB Entry DOI: 10.7270/Q26Q203T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50146460 (CHEMBL3763688) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of IFN gamma induced human IDO1 in HeLa cells assessed as reduction in kynurenine production treated with 3-fold serial dilution beginning... | Eur J Med Chem 108: 564-76 (2016) Article DOI: 10.1016/j.ejmech.2015.12.028 BindingDB Entry DOI: 10.7270/Q26Q203T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50146461 (CHEMBL3765807) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of IFN gamma induced human IDO1 in HeLa cells assessed as reduction in kynurenine production treated with 5-fold serial dilution beginning... | Eur J Med Chem 108: 564-76 (2016) Article DOI: 10.1016/j.ejmech.2015.12.028 BindingDB Entry DOI: 10.7270/Q26Q203T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24794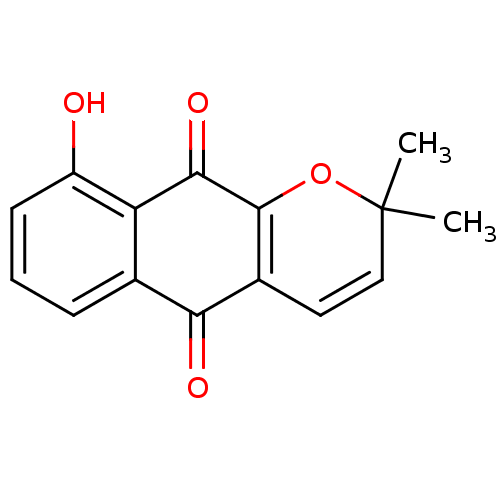 (9-hydroxy-2,2-dimethyl-2H,5H,10H-benzo[g]chromene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 51: 1706-18 (2008) Article DOI: 10.1021/jm7014155 BindingDB Entry DOI: 10.7270/Q2VD6WSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24801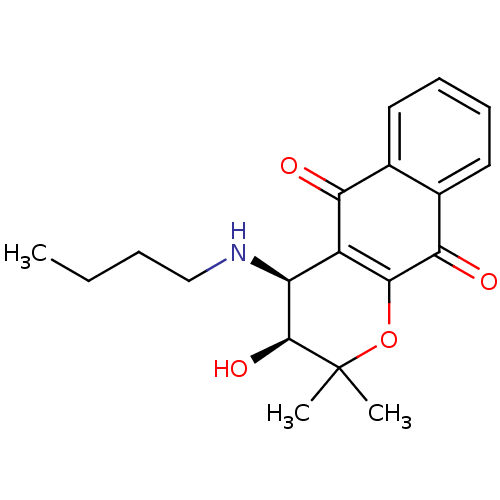 ((3S,4S)-4-(butylamino)-3-hydroxy-2,2-dimethyl-2H,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 51: 1706-18 (2008) Article DOI: 10.1021/jm7014155 BindingDB Entry DOI: 10.7270/Q2VD6WSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50146461 (CHEMBL3765807) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 134 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of IFN gamma induced human IDO1 in HeLa cells assessed as reduction in kynurenine production treated with 3-fold serial dilution beginning... | Eur J Med Chem 108: 564-76 (2016) Article DOI: 10.1016/j.ejmech.2015.12.028 BindingDB Entry DOI: 10.7270/Q26Q203T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50146460 (CHEMBL3763688) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of doxycycline-induced human IDO1 expressed in Trex cells assessed as reduction in kynurenine production treated with 5-fold serial diluti... | Eur J Med Chem 108: 564-76 (2016) Article DOI: 10.1016/j.ejmech.2015.12.028 BindingDB Entry DOI: 10.7270/Q26Q203T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24800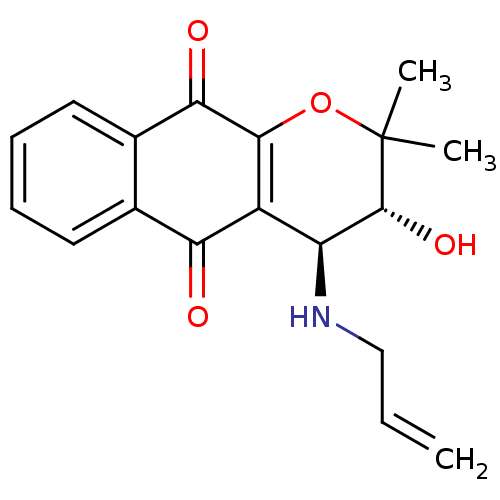 ((3R,4S)-3-hydroxy-2,2-dimethyl-4-(prop-2-en-1-ylam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 183 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 51: 1706-18 (2008) Article DOI: 10.1021/jm7014155 BindingDB Entry DOI: 10.7270/Q2VD6WSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24799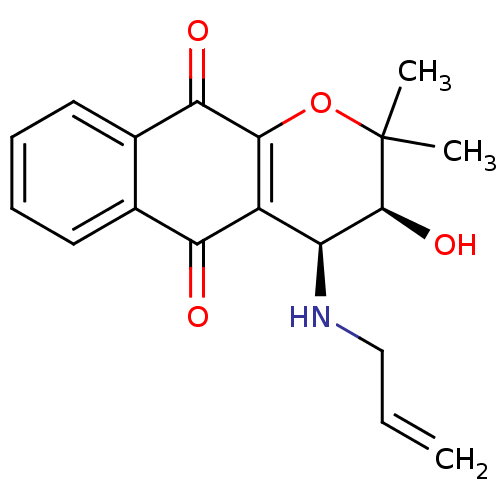 ((3S,4S)-3-hydroxy-2,2-dimethyl-4-(prop-2-en-1-ylam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 186 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 51: 1706-18 (2008) Article DOI: 10.1021/jm7014155 BindingDB Entry DOI: 10.7270/Q2VD6WSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24788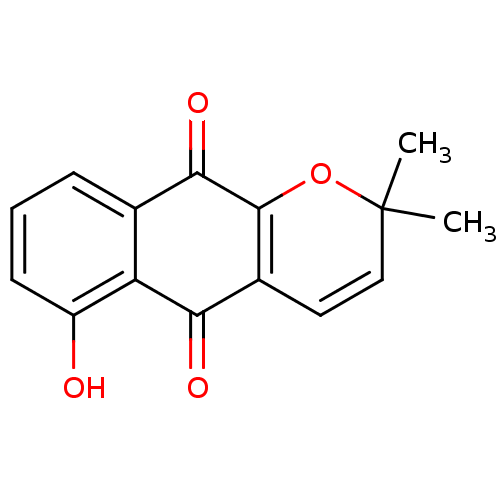 (6-hydroxy-2,2-dimethyl-2H,5H,10H-benzo[g]chromene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 51: 1706-18 (2008) Article DOI: 10.1021/jm7014155 BindingDB Entry DOI: 10.7270/Q2VD6WSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50146460 (CHEMBL3763688) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 60 mins by microplate reade... | Eur J Med Chem 108: 564-76 (2016) Article DOI: 10.1016/j.ejmech.2015.12.028 BindingDB Entry DOI: 10.7270/Q26Q203T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24786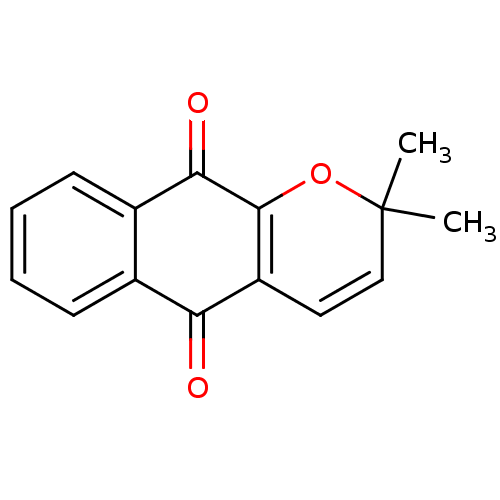 (2,2-dimethyl-2H,5H,10H-benzo[g]chromene-5,10-dione...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 214 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 51: 1706-18 (2008) Article DOI: 10.1021/jm7014155 BindingDB Entry DOI: 10.7270/Q2VD6WSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50444455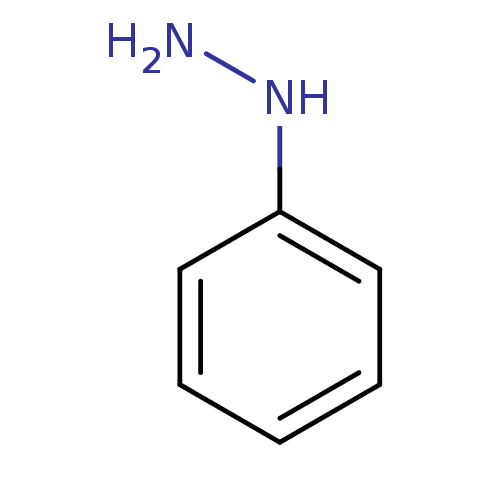 (CHEBI:27924 | Phenylhydrazine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 60 mins by microplate reade... | Eur J Med Chem 108: 564-76 (2016) Article DOI: 10.1016/j.ejmech.2015.12.028 BindingDB Entry DOI: 10.7270/Q26Q203T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24787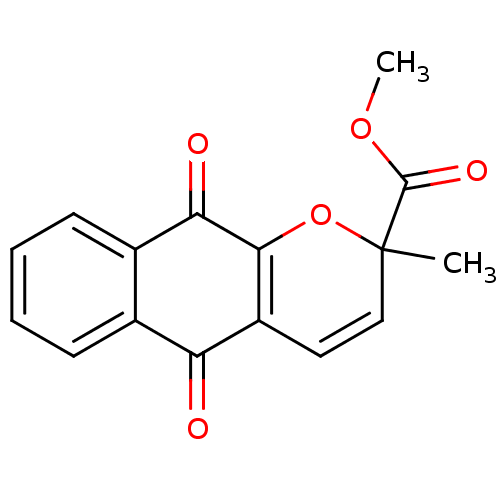 (Pyranonaphthoquinone derivative, 24 | methyl 2-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 247 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 51: 1706-18 (2008) Article DOI: 10.1021/jm7014155 BindingDB Entry DOI: 10.7270/Q2VD6WSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24798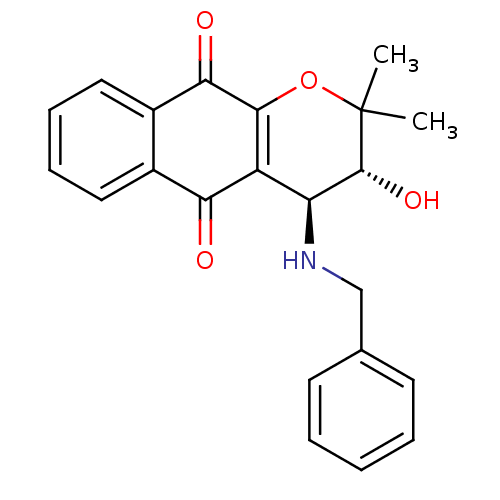 ((3R,4S)-4-(benzylamino)-3-hydroxy-2,2-dimethyl-2H,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 252 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 51: 1706-18 (2008) Article DOI: 10.1021/jm7014155 BindingDB Entry DOI: 10.7270/Q2VD6WSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24774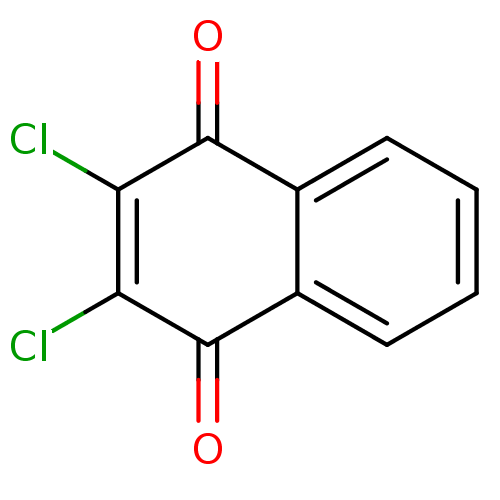 (2,3-dichloro-1,4-dihydronaphthalene-1,4-dione | 2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 51: 1706-18 (2008) Article DOI: 10.1021/jm7014155 BindingDB Entry DOI: 10.7270/Q2VD6WSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50146461 (CHEMBL3765807) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 60 mins by microplate reade... | Eur J Med Chem 108: 564-76 (2016) Article DOI: 10.1016/j.ejmech.2015.12.028 BindingDB Entry DOI: 10.7270/Q26Q203T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50146407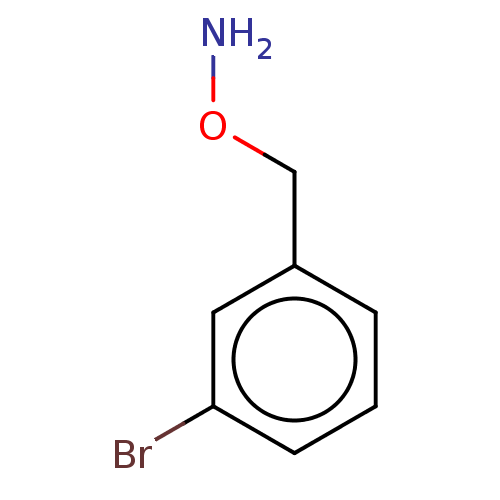 (CHEMBL3763469) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 60 mins by microplate reade... | Eur J Med Chem 108: 564-76 (2016) Article DOI: 10.1016/j.ejmech.2015.12.028 BindingDB Entry DOI: 10.7270/Q26Q203T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24785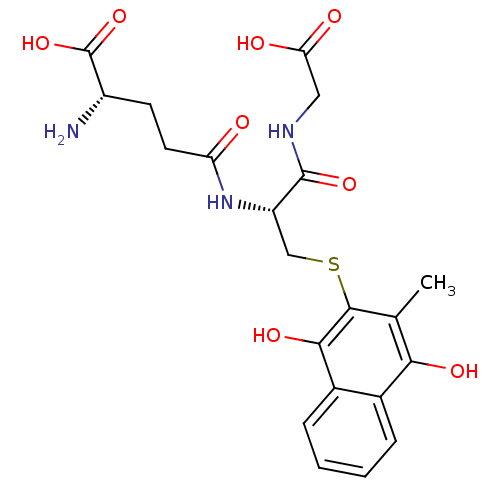 ((2S)-2-amino-4-{[(1R)-1-[(carboxymethyl)carbamoyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG MMDB PC cid PC sid UniChem | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 51: 1706-18 (2008) Article DOI: 10.1021/jm7014155 BindingDB Entry DOI: 10.7270/Q2VD6WSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50146409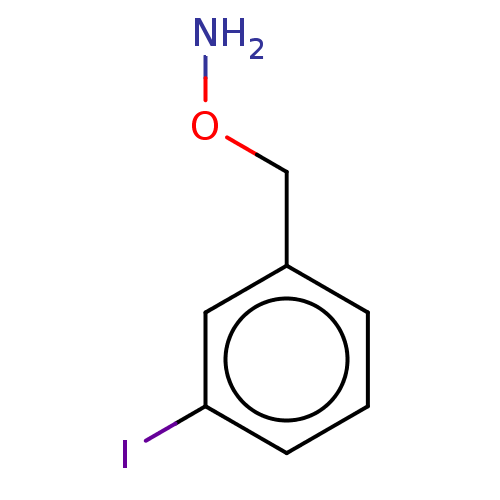 (CHEMBL3764760) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 60 mins by microplate reade... | Eur J Med Chem 108: 564-76 (2016) Article DOI: 10.1016/j.ejmech.2015.12.028 BindingDB Entry DOI: 10.7270/Q26Q203T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24804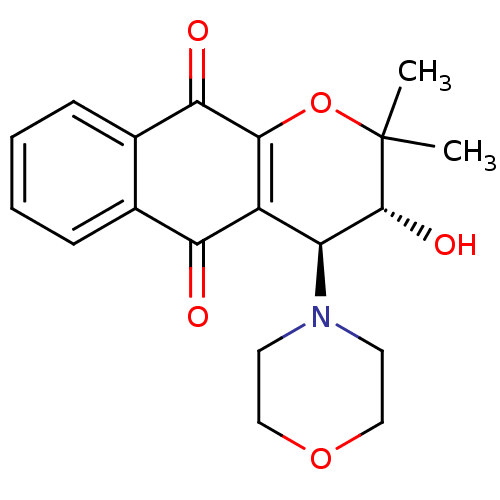 ((3R,4S)-3-hydroxy-2,2-dimethyl-4-(morpholin-4-yl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 361 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 51: 1706-18 (2008) Article DOI: 10.1021/jm7014155 BindingDB Entry DOI: 10.7270/Q2VD6WSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50146522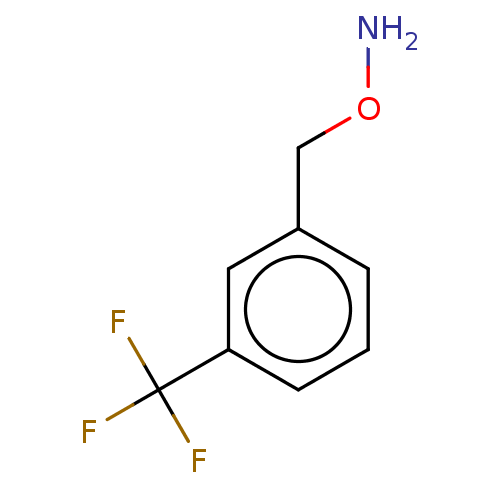 (CHEMBL3764803) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 60 mins by microplate reade... | Eur J Med Chem 108: 564-76 (2016) Article DOI: 10.1016/j.ejmech.2015.12.028 BindingDB Entry DOI: 10.7270/Q26Q203T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50146450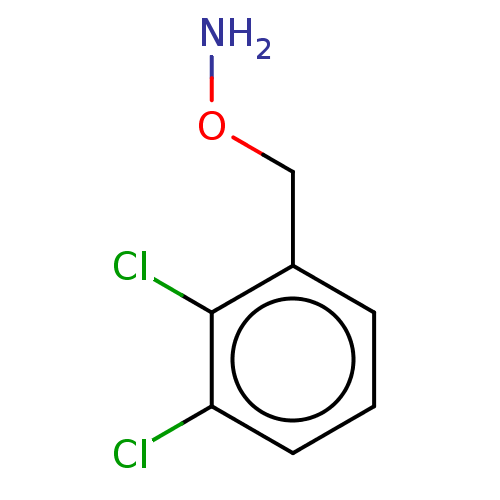 (CHEMBL3763307) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 60 mins by microplate reade... | Eur J Med Chem 108: 564-76 (2016) Article DOI: 10.1016/j.ejmech.2015.12.028 BindingDB Entry DOI: 10.7270/Q26Q203T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50146451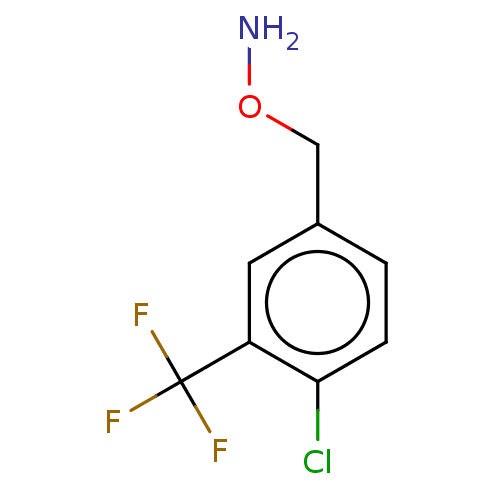 (CHEMBL3763639) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 60 mins by microplate reade... | Eur J Med Chem 108: 564-76 (2016) Article DOI: 10.1016/j.ejmech.2015.12.028 BindingDB Entry DOI: 10.7270/Q26Q203T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24811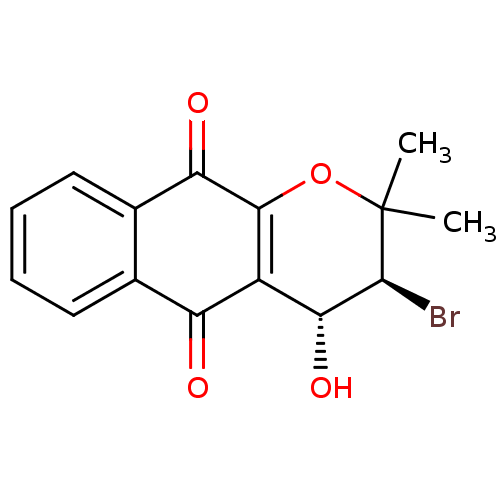 ((3S,4R)-3-bromo-4-hydroxy-2,2-dimethyl-2H,3H,4H,5H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 512 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 51: 1706-18 (2008) Article DOI: 10.1021/jm7014155 BindingDB Entry DOI: 10.7270/Q2VD6WSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50146452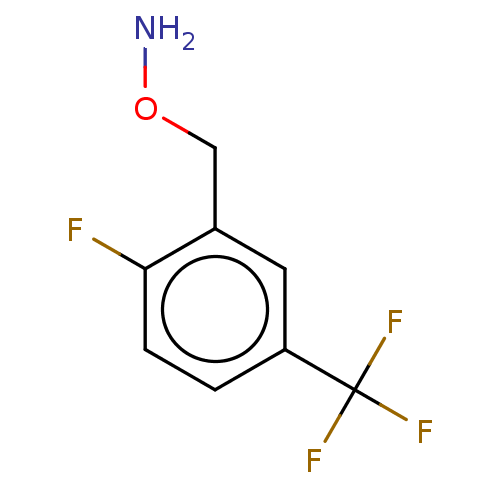 (CHEMBL3763481) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 60 mins by microplate reade... | Eur J Med Chem 108: 564-76 (2016) Article DOI: 10.1016/j.ejmech.2015.12.028 BindingDB Entry DOI: 10.7270/Q26Q203T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50146523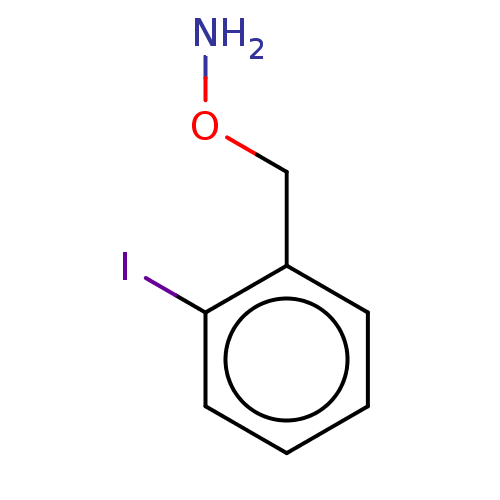 (CHEMBL3765088) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 60 mins by microplate reade... | Eur J Med Chem 108: 564-76 (2016) Article DOI: 10.1016/j.ejmech.2015.12.028 BindingDB Entry DOI: 10.7270/Q26Q203T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50146525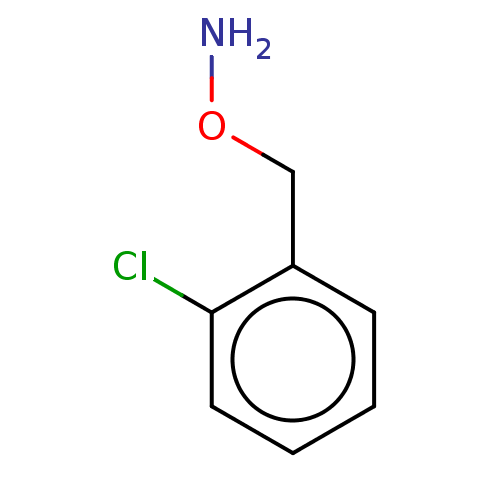 (CHEMBL3765142) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 60 mins by microplate reade... | Eur J Med Chem 108: 564-76 (2016) Article DOI: 10.1016/j.ejmech.2015.12.028 BindingDB Entry DOI: 10.7270/Q26Q203T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50146521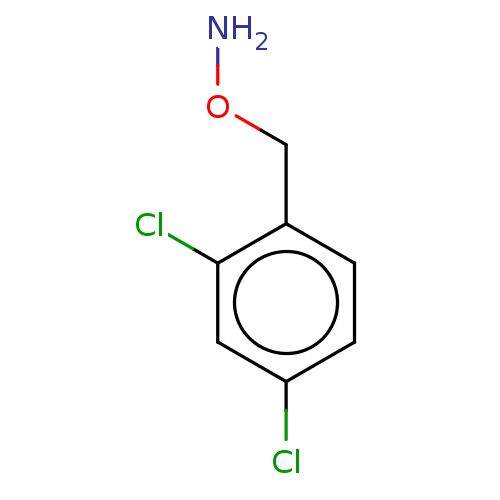 (CHEMBL3764703) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 60 mins by microplate reade... | Eur J Med Chem 108: 564-76 (2016) Article DOI: 10.1016/j.ejmech.2015.12.028 BindingDB Entry DOI: 10.7270/Q26Q203T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24775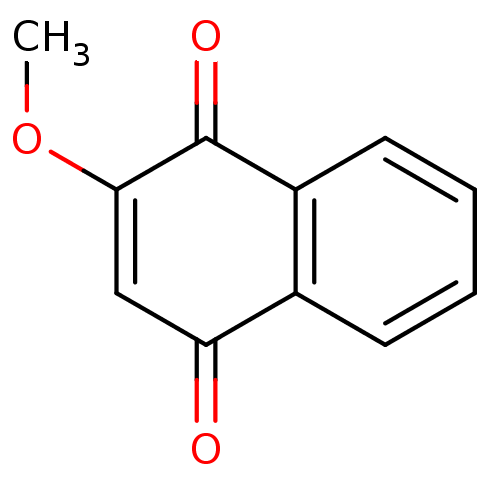 (2-methoxy-1,4-dihydronaphthalene-1,4-dione | 2-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 51: 1706-18 (2008) Article DOI: 10.1021/jm7014155 BindingDB Entry DOI: 10.7270/Q2VD6WSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50146520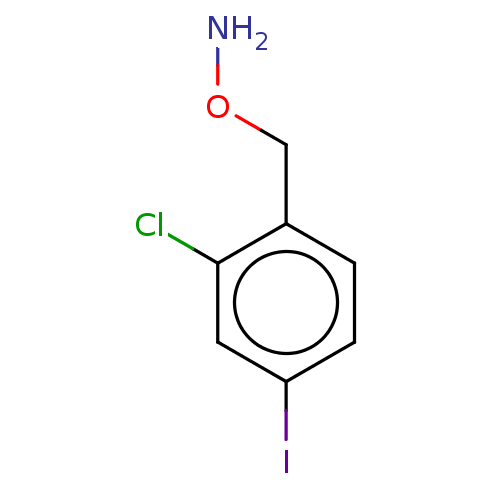 (CHEMBL3763690) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 60 mins by microplate reade... | Eur J Med Chem 108: 564-76 (2016) Article DOI: 10.1016/j.ejmech.2015.12.028 BindingDB Entry DOI: 10.7270/Q26Q203T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50146579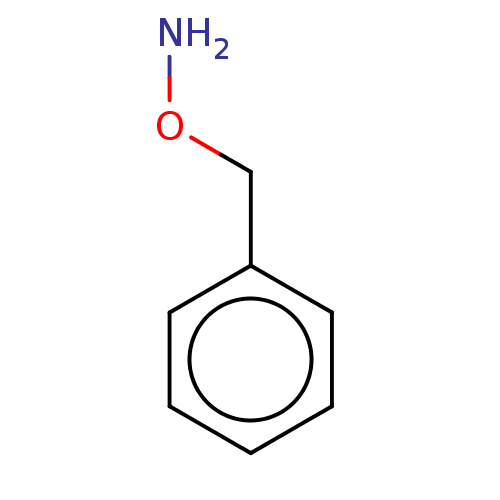 (Benzyl-O-Hydroxylamine | CHEMBL443652 | O-Benzylhy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 60 mins by microplate reade... | Eur J Med Chem 108: 564-76 (2016) Article DOI: 10.1016/j.ejmech.2015.12.028 BindingDB Entry DOI: 10.7270/Q26Q203T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24784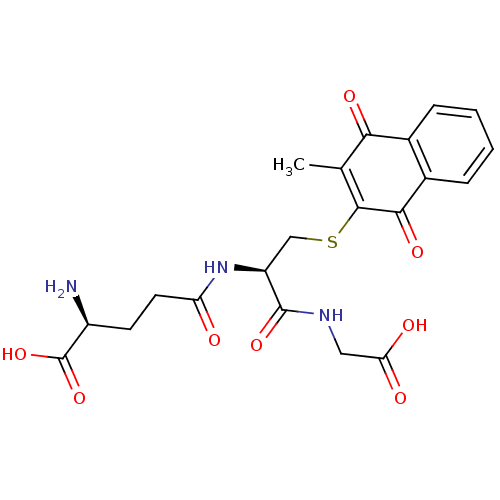 ((2S)-2-amino-4-{[(1R)-1-[(carboxymethyl)carbamoyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 51: 1706-18 (2008) Article DOI: 10.1021/jm7014155 BindingDB Entry DOI: 10.7270/Q2VD6WSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50146519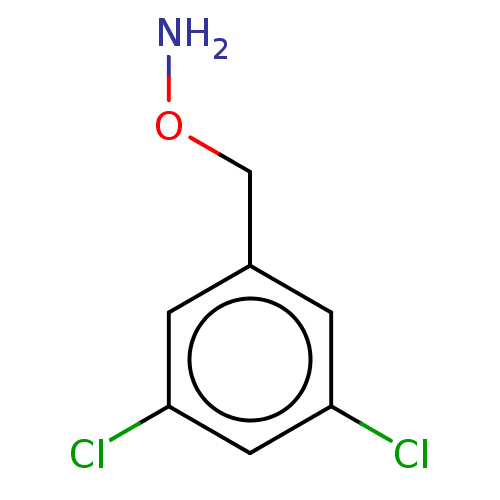 (CHEMBL3764853) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 60 mins by microplate reade... | Eur J Med Chem 108: 564-76 (2016) Article DOI: 10.1016/j.ejmech.2015.12.028 BindingDB Entry DOI: 10.7270/Q26Q203T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24793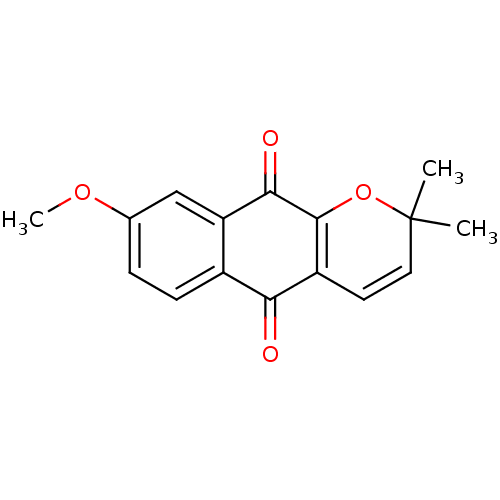 (8-methoxy-2,2-dimethyl-2H,5H,10H-benzo[g]chromene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 933 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 51: 1706-18 (2008) Article DOI: 10.1021/jm7014155 BindingDB Entry DOI: 10.7270/Q2VD6WSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24805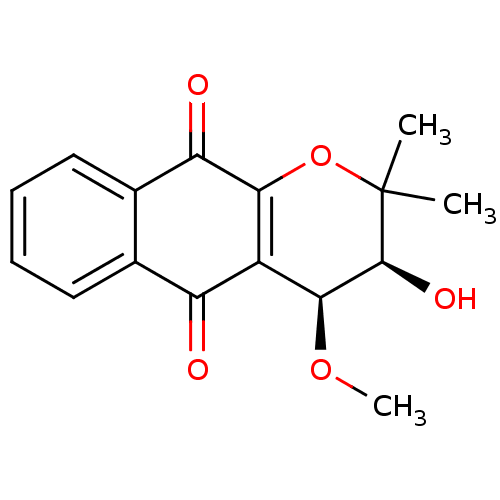 ((3S,4S)-3-hydroxy-4-methoxy-2,2-dimethyl-2H,3H,4H,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 976 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 51: 1706-18 (2008) Article DOI: 10.1021/jm7014155 BindingDB Entry DOI: 10.7270/Q2VD6WSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50146526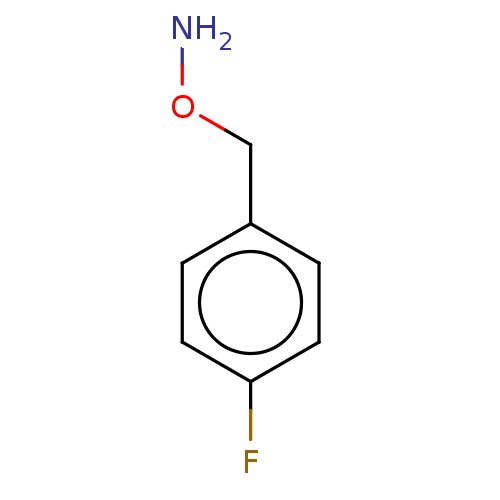 (CHEMBL3765396) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 60 mins by microplate reade... | Eur J Med Chem 108: 564-76 (2016) Article DOI: 10.1016/j.ejmech.2015.12.028 BindingDB Entry DOI: 10.7270/Q26Q203T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24776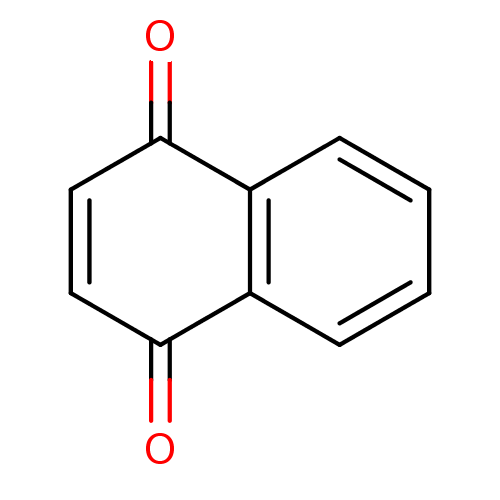 (1,4-Naphthoquinone | 1,4-Naphthoquinone (5a) | 1,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 990 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 51: 1706-18 (2008) Article DOI: 10.1021/jm7014155 BindingDB Entry DOI: 10.7270/Q2VD6WSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50146408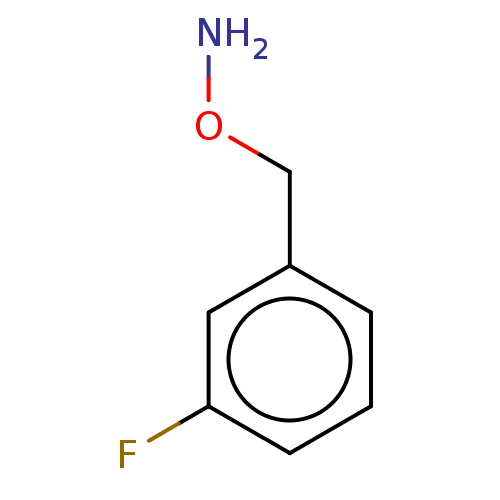 (CHEMBL3764504) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 60 mins by microplate reade... | Eur J Med Chem 108: 564-76 (2016) Article DOI: 10.1016/j.ejmech.2015.12.028 BindingDB Entry DOI: 10.7270/Q26Q203T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24777 (5-hydroxy-1,4-dihydronaphthalene-1,4-dione | 5-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 51: 1706-18 (2008) Article DOI: 10.1021/jm7014155 BindingDB Entry DOI: 10.7270/Q2VD6WSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 305 total ) | Next | Last >> |