 Found 1226 hits with Last Name = 'lamarche' and Initial = 'mj'
Found 1226 hits with Last Name = 'lamarche' and Initial = 'mj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183974
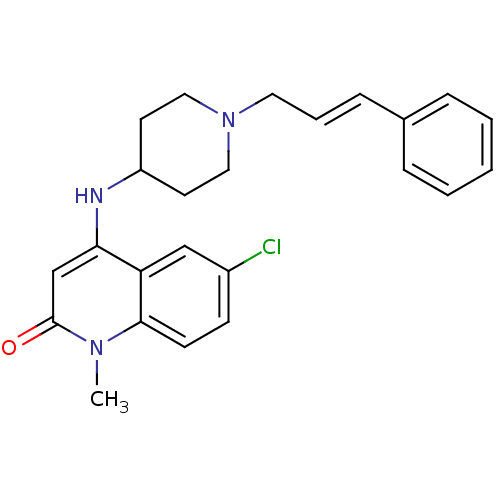
((E)-6-chloro-4-(1-cinnamylpiperidin-4-ylamino)-1-m...)Show SMILES Cn1c2ccc(Cl)cc2c(NC2CCN(C\C=C\c3ccccc3)CC2)cc1=O Show InChI InChI=1S/C24H26ClN3O/c1-27-23-10-9-19(25)16-21(23)22(17-24(27)29)26-20-11-14-28(15-12-20)13-5-8-18-6-3-2-4-7-18/h2-10,16-17,20,26H,11-15H2,1H3/b8-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183965
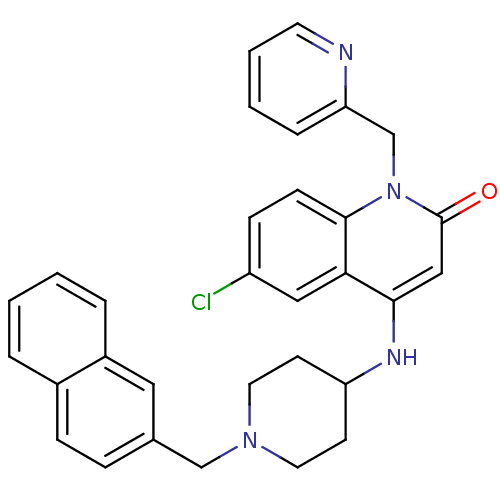
(6-chloro-4-(1-(naphthalen-2-ylmethyl)piperidin-4-y...)Show SMILES Clc1ccc2n(Cc3ccccn3)c(=O)cc(NC3CCN(Cc4ccc5ccccc5c4)CC3)c2c1 Show InChI InChI=1S/C31H29ClN4O/c32-25-10-11-30-28(18-25)29(19-31(37)36(30)21-27-7-3-4-14-33-27)34-26-12-15-35(16-13-26)20-22-8-9-23-5-1-2-6-24(23)17-22/h1-11,14,17-19,26,34H,12-13,15-16,20-21H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183969
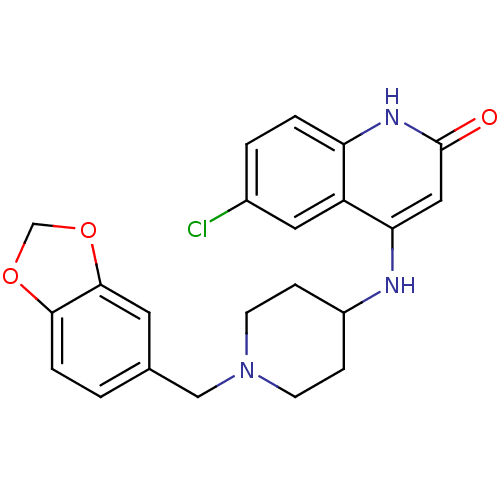
(4-(1-(benzo[d][1,3]dioxol-5-ylmethyl)piperidin-4-y...)Show SMILES Clc1ccc2[nH]c(=O)cc(NC3CCN(Cc4ccc5OCOc5c4)CC3)c2c1 Show InChI InChI=1S/C22H22ClN3O3/c23-15-2-3-18-17(10-15)19(11-22(27)25-18)24-16-5-7-26(8-6-16)12-14-1-4-20-21(9-14)29-13-28-20/h1-4,9-11,16H,5-8,12-13H2,(H2,24,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183966
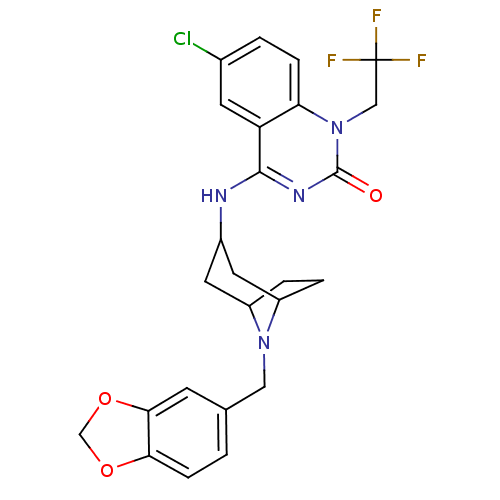
(4-(8-(benzo[d][1,3]dioxol-5-ylmethyl)-8-aza-bicycl...)Show SMILES FC(F)(F)Cn1c2ccc(Cl)cc2c(NC2CC3CCC(C2)N3Cc2ccc3OCOc3c2)nc1=O |TLB:14:15:22:18.19| Show InChI InChI=1S/C25H24ClF3N4O3/c26-15-2-5-20-19(8-15)23(31-24(34)33(20)12-25(27,28)29)30-16-9-17-3-4-18(10-16)32(17)11-14-1-6-21-22(7-14)36-13-35-21/h1-2,5-8,16-18H,3-4,9-13H2,(H,30,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183960
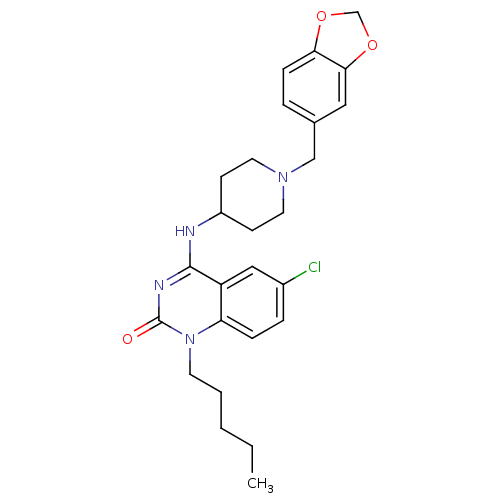
(4-(1-(benzo[d][1,3]dioxol-5-ylmethyl)piperidin-4-y...)Show SMILES CCCCCn1c2ccc(Cl)cc2c(NC2CCN(Cc3ccc4OCOc4c3)CC2)nc1=O Show InChI InChI=1S/C26H31ClN4O3/c1-2-3-4-11-31-22-7-6-19(27)15-21(22)25(29-26(31)32)28-20-9-12-30(13-10-20)16-18-5-8-23-24(14-18)34-17-33-23/h5-8,14-15,20H,2-4,9-13,16-17H2,1H3,(H,28,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 135 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183972
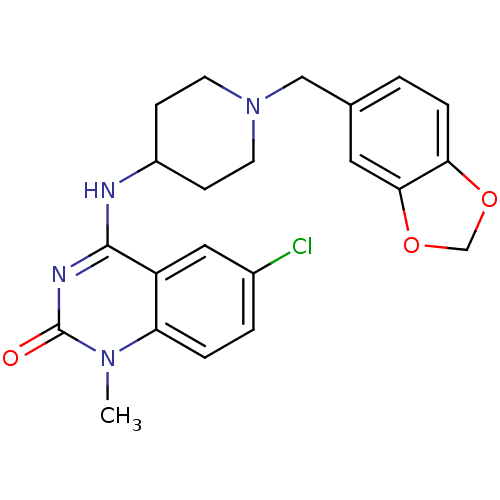
(4-(1-(benzo[d][1,3]dioxol-5-ylmethyl)piperidin-4-y...)Show SMILES Cn1c2ccc(Cl)cc2c(NC2CCN(Cc3ccc4OCOc4c3)CC2)nc1=O Show InChI InChI=1S/C22H23ClN4O3/c1-26-18-4-3-15(23)11-17(18)21(25-22(26)28)24-16-6-8-27(9-7-16)12-14-2-5-19-20(10-14)30-13-29-19/h2-5,10-11,16H,6-9,12-13H2,1H3,(H,24,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183971
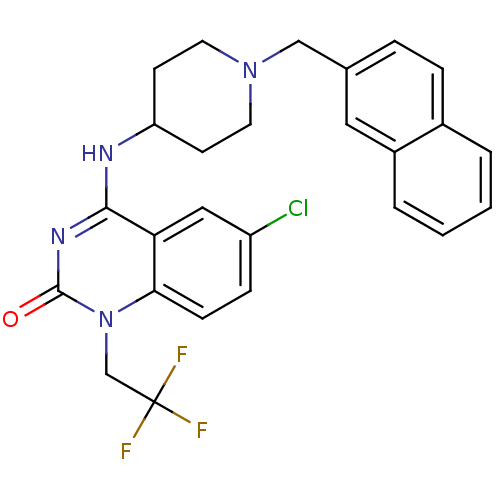
(6-chloro-4-(1-(naphthalen-2-ylmethyl)piperidin-4-y...)Show SMILES FC(F)(F)Cn1c2ccc(Cl)cc2c(NC2CCN(Cc3ccc4ccccc4c3)CC2)nc1=O Show InChI InChI=1S/C26H24ClF3N4O/c27-20-7-8-23-22(14-20)24(32-25(35)34(23)16-26(28,29)30)31-21-9-11-33(12-10-21)15-17-5-6-18-3-1-2-4-19(18)13-17/h1-8,13-14,21H,9-12,15-16H2,(H,31,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183968
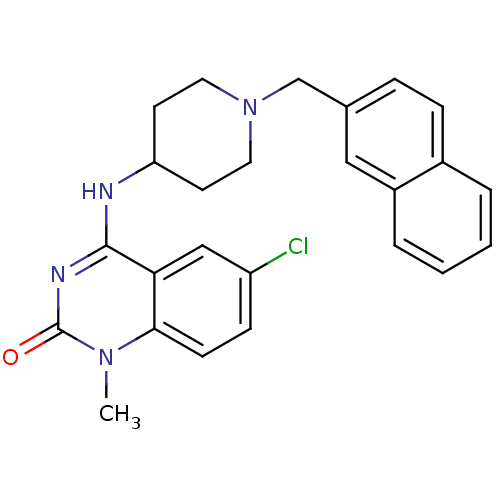
(6-chloro-1-methyl-4-(1-(naphthalen-2-ylmethyl)pipe...)Show SMILES Cn1c2ccc(Cl)cc2c(NC2CCN(Cc3ccc4ccccc4c3)CC2)nc1=O Show InChI InChI=1S/C25H25ClN4O/c1-29-23-9-8-20(26)15-22(23)24(28-25(29)31)27-21-10-12-30(13-11-21)16-17-6-7-18-4-2-3-5-19(18)14-17/h2-9,14-15,21H,10-13,16H2,1H3,(H,27,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183975
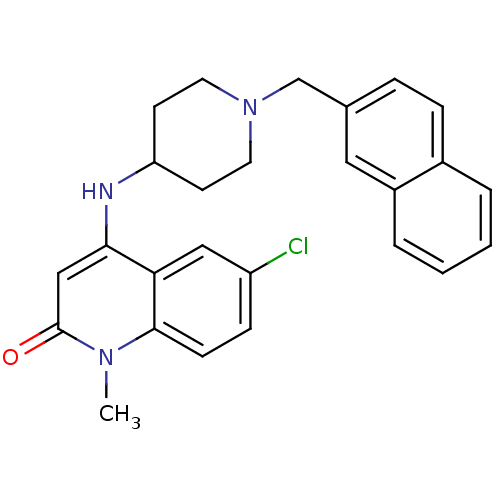
(6-chloro-1-methyl-4-(1-(naphthalen-2-ylmethyl)pipe...)Show SMILES Cn1c2ccc(Cl)cc2c(NC2CCN(Cc3ccc4ccccc4c3)CC2)cc1=O Show InChI InChI=1S/C26H26ClN3O/c1-29-25-9-8-21(27)15-23(25)24(16-26(29)31)28-22-10-12-30(13-11-22)17-18-6-7-19-4-2-3-5-20(19)14-18/h2-9,14-16,22,28H,10-13,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 215 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183964
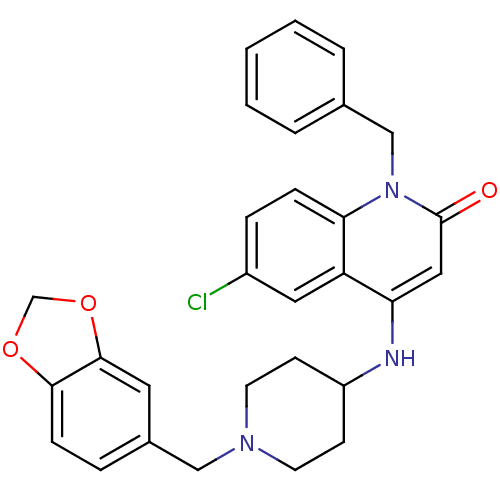
(4-(1-(benzo[d][1,3]dioxol-5-ylmethyl)piperidin-4-y...)Show SMILES Clc1ccc2n(Cc3ccccc3)c(=O)cc(NC3CCN(Cc4ccc5OCOc5c4)CC3)c2c1 Show InChI InChI=1S/C29H28ClN3O3/c30-22-7-8-26-24(15-22)25(16-29(34)33(26)18-20-4-2-1-3-5-20)31-23-10-12-32(13-11-23)17-21-6-9-27-28(14-21)36-19-35-27/h1-9,14-16,23,31H,10-13,17-19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183958
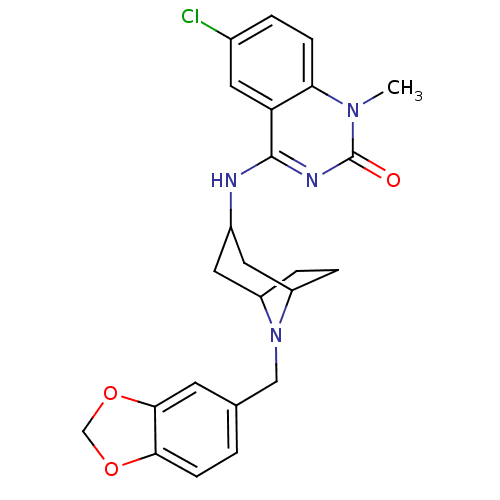
(4-(8-(benzo[d][1,3]dioxol-5-ylmethyl)-8-aza-bicycl...)Show SMILES Cn1c2ccc(Cl)cc2c(NC2CC3CCC(C2)N3Cc2ccc3OCOc3c2)nc1=O |TLB:10:11:18:14.15| Show InChI InChI=1S/C24H25ClN4O3/c1-28-20-6-3-15(25)9-19(20)23(27-24(28)30)26-16-10-17-4-5-18(11-16)29(17)12-14-2-7-21-22(8-14)32-13-31-21/h2-3,6-9,16-18H,4-5,10-13H2,1H3,(H,26,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183958

(4-(8-(benzo[d][1,3]dioxol-5-ylmethyl)-8-aza-bicycl...)Show SMILES Cn1c2ccc(Cl)cc2c(NC2CC3CCC(C2)N3Cc2ccc3OCOc3c2)nc1=O |TLB:10:11:18:14.15| Show InChI InChI=1S/C24H25ClN4O3/c1-28-20-6-3-15(25)9-19(20)23(27-24(28)30)26-16-10-17-4-5-18(11-16)29(17)12-14-2-7-21-22(8-14)32-13-31-21/h2-3,6-9,16-18H,4-5,10-13H2,1H3,(H,26,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183954
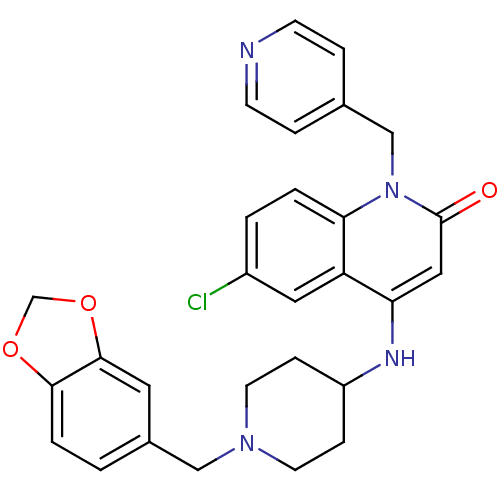
(4-(1-(benzo[d][1,3]dioxol-5-ylmethyl)piperidin-4-y...)Show SMILES Clc1ccc2n(Cc3ccncc3)c(=O)cc(NC3CCN(Cc4ccc5OCOc5c4)CC3)c2c1 Show InChI InChI=1S/C28H27ClN4O3/c29-21-2-3-25-23(14-21)24(15-28(34)33(25)17-19-5-9-30-10-6-19)31-22-7-11-32(12-8-22)16-20-1-4-26-27(13-20)36-18-35-26/h1-6,9-10,13-15,22,31H,7-8,11-12,16-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183973
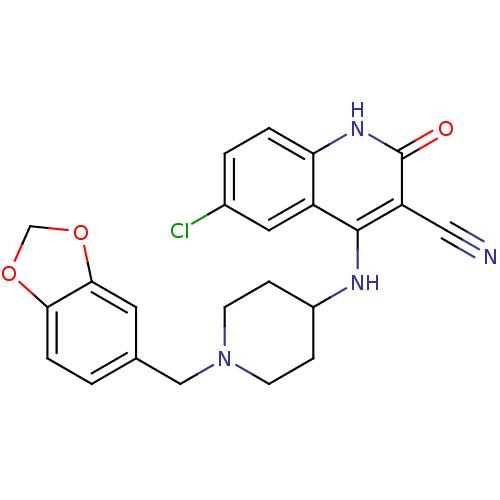
(4-(1-(benzo[d][1,3]dioxol-5-ylmethyl)piperidin-4-y...)Show SMILES Clc1ccc2[nH]c(=O)c(C#N)c(NC3CCN(Cc4ccc5OCOc5c4)CC3)c2c1 Show InChI InChI=1S/C23H21ClN4O3/c24-15-2-3-19-17(10-15)22(18(11-25)23(29)27-19)26-16-5-7-28(8-6-16)12-14-1-4-20-21(9-14)31-13-30-20/h1-4,9-10,16H,5-8,12-13H2,(H2,26,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183967
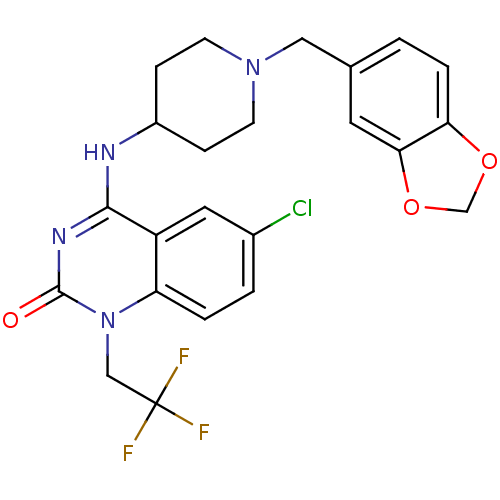
(4-(1-(benzo[d][1,3]dioxol-5-ylmethyl)piperidin-4-y...)Show SMILES FC(F)(F)Cn1c2ccc(Cl)cc2c(NC2CCN(Cc3ccc4OCOc4c3)CC2)nc1=O Show InChI InChI=1S/C23H22ClF3N4O3/c24-15-2-3-18-17(10-15)21(29-22(32)31(18)12-23(25,26)27)28-16-5-7-30(8-6-16)11-14-1-4-19-20(9-14)34-13-33-19/h1-4,9-10,16H,5-8,11-13H2,(H,28,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 445 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183953
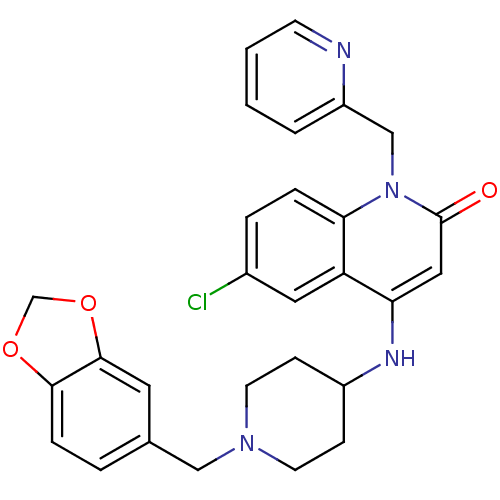
(4-(1-(benzo[d][1,3]dioxol-5-ylmethyl)piperidin-4-y...)Show SMILES Clc1ccc2n(Cc3ccccn3)c(=O)cc(NC3CCN(Cc4ccc5OCOc5c4)CC3)c2c1 Show InChI InChI=1S/C28H27ClN4O3/c29-20-5-6-25-23(14-20)24(15-28(34)33(25)17-22-3-1-2-10-30-22)31-21-8-11-32(12-9-21)16-19-4-7-26-27(13-19)36-18-35-26/h1-7,10,13-15,21,31H,8-9,11-12,16-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183957
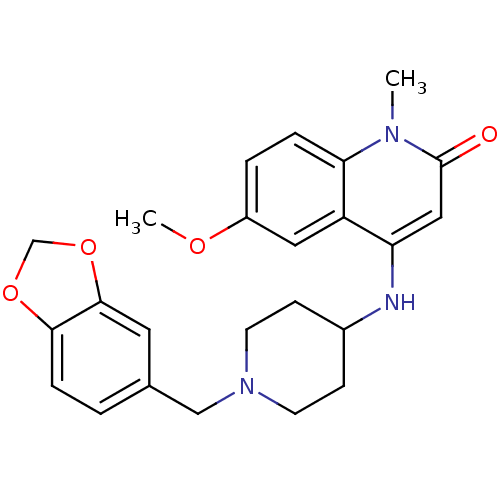
(4-(1-(benzo[d][1,3]dioxol-5-ylmethyl)piperidin-4-y...)Show SMILES COc1ccc2n(C)c(=O)cc(NC3CCN(Cc4ccc5OCOc5c4)CC3)c2c1 Show InChI InChI=1S/C24H27N3O4/c1-26-21-5-4-18(29-2)12-19(21)20(13-24(26)28)25-17-7-9-27(10-8-17)14-16-3-6-22-23(11-16)31-15-30-22/h3-6,11-13,17,25H,7-10,14-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 778 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183961
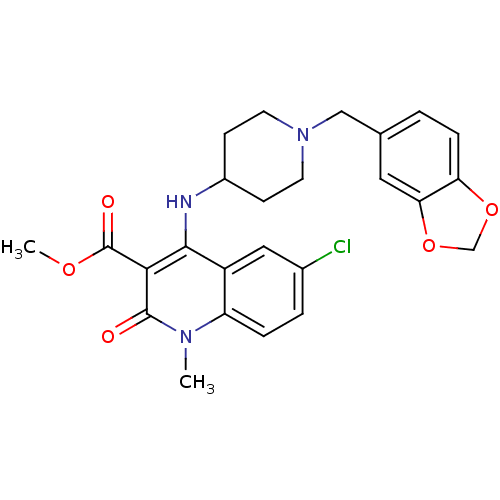
(CHEMBL205741 | methyl 4-(1-(benzo[d][1,3]dioxol-5-...)Show SMILES COC(=O)c1c(NC2CCN(Cc3ccc4OCOc4c3)CC2)c2cc(Cl)ccc2n(C)c1=O Show InChI InChI=1S/C25H26ClN3O5/c1-28-19-5-4-16(26)12-18(19)23(22(24(28)30)25(31)32-2)27-17-7-9-29(10-8-17)13-15-3-6-20-21(11-15)34-14-33-20/h3-6,11-12,17,27H,7-10,13-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183970
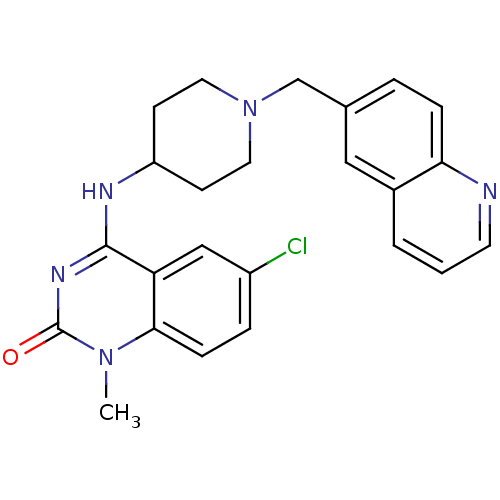
(6-chloro-1-methyl-4-(1-(quinolin-6-ylmethyl)piperi...)Show SMILES Cn1c2ccc(Cl)cc2c(NC2CCN(Cc3ccc4ncccc4c3)CC2)nc1=O Show InChI InChI=1S/C24H24ClN5O/c1-29-22-7-5-18(25)14-20(22)23(28-24(29)31)27-19-8-11-30(12-9-19)15-16-4-6-21-17(13-16)3-2-10-26-21/h2-7,10,13-14,19H,8-9,11-12,15H2,1H3,(H,27,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183963

(4-(1-(benzo[d][1,3]dioxol-5-ylmethyl)piperidin-4-y...)Show SMILES Cn1c2ccc(OC(F)(F)F)cc2c(NC2CCN(Cc3ccc4OCOc4c3)CC2)cc1=O Show InChI InChI=1S/C24H24F3N3O4/c1-29-20-4-3-17(34-24(25,26)27)11-18(20)19(12-23(29)31)28-16-6-8-30(9-7-16)13-15-2-5-21-22(10-15)33-14-32-21/h2-5,10-12,16,28H,6-9,13-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183959
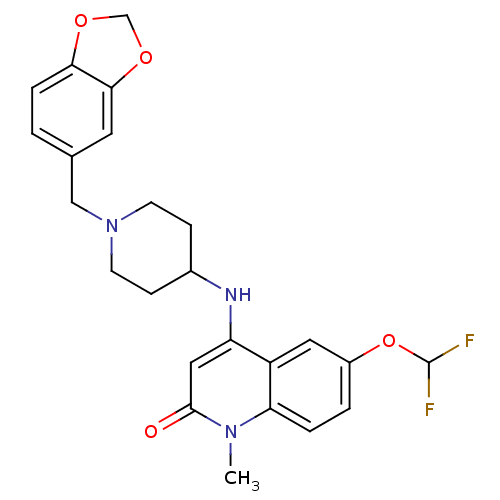
(4-(1-(benzo[d][1,3]dioxol-5-ylmethyl)piperidin-4-y...)Show SMILES Cn1c2ccc(OC(F)F)cc2c(NC2CCN(Cc3ccc4OCOc4c3)CC2)cc1=O Show InChI InChI=1S/C24H25F2N3O4/c1-28-20-4-3-17(33-24(25)26)11-18(20)19(12-23(28)30)27-16-6-8-29(9-7-16)13-15-2-5-21-22(10-15)32-14-31-21/h2-5,10-12,16,24,27H,6-9,13-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183962
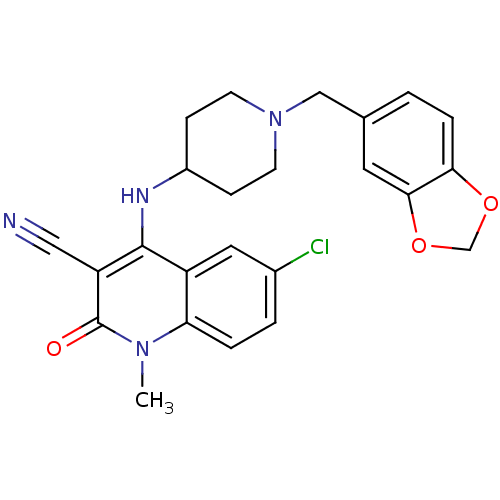
(4-(1-(benzo[d][1,3]dioxol-5-ylmethyl)piperidin-4-y...)Show SMILES Cn1c2ccc(Cl)cc2c(NC2CCN(Cc3ccc4OCOc4c3)CC2)c(C#N)c1=O Show InChI InChI=1S/C24H23ClN4O3/c1-28-20-4-3-16(25)11-18(20)23(19(12-26)24(28)30)27-17-6-8-29(9-7-17)13-15-2-5-21-22(10-15)32-14-31-21/h2-5,10-11,17,27H,6-9,13-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183955
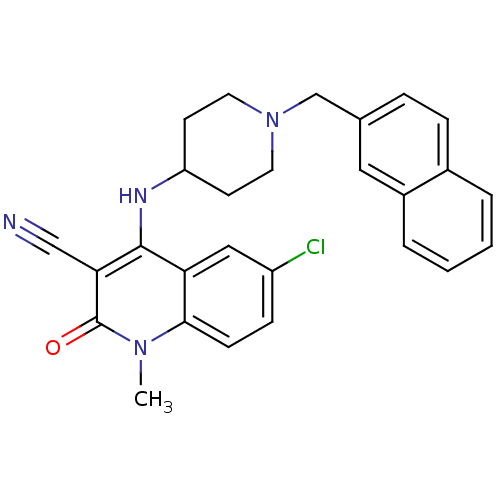
(6-chloro-1-methyl-4-(1-(naphthalen-2-ylmethyl)pipe...)Show SMILES Cn1c2ccc(Cl)cc2c(NC2CCN(Cc3ccc4ccccc4c3)CC2)c(C#N)c1=O Show InChI InChI=1S/C27H25ClN4O/c1-31-25-9-8-21(28)15-23(25)26(24(16-29)27(31)33)30-22-10-12-32(13-11-22)17-18-6-7-19-4-2-3-5-20(19)14-18/h2-9,14-15,22,30H,10-13,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183956
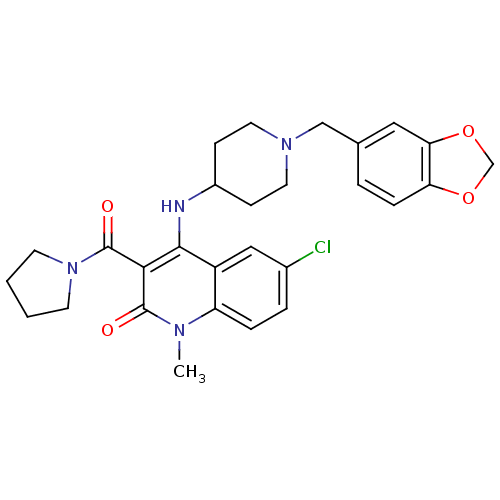
(4-(1-(benzo[d][1,3]dioxol-5-ylmethyl)piperidin-4-y...)Show SMILES Cn1c2ccc(Cl)cc2c(NC2CCN(Cc3ccc4OCOc4c3)CC2)c(C(=O)N2CCCC2)c1=O Show InChI InChI=1S/C28H31ClN4O4/c1-31-22-6-5-19(29)15-21(22)26(25(27(31)34)28(35)33-10-2-3-11-33)30-20-8-12-32(13-9-20)16-18-4-7-23-24(14-18)37-17-36-23/h4-7,14-15,20,30H,2-3,8-13,16-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50183964

(4-(1-(benzo[d][1,3]dioxol-5-ylmethyl)piperidin-4-y...)Show SMILES Clc1ccc2n(Cc3ccccc3)c(=O)cc(NC3CCN(Cc4ccc5OCOc5c4)CC3)c2c1 Show InChI InChI=1S/C29H28ClN3O3/c30-22-7-8-26-24(15-22)25(16-29(34)33(26)18-20-4-2-1-3-5-20)31-23-10-12-32(13-11-23)17-21-6-9-27-28(14-21)36-19-35-27/h1-9,14-16,23,31H,10-13,17-19H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from MCHr1 expressed in IMR32 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50183965

(6-chloro-4-(1-(naphthalen-2-ylmethyl)piperidin-4-y...)Show SMILES Clc1ccc2n(Cc3ccccn3)c(=O)cc(NC3CCN(Cc4ccc5ccccc5c4)CC3)c2c1 Show InChI InChI=1S/C31H29ClN4O/c32-25-10-11-30-28(18-25)29(19-31(37)36(30)21-27-7-3-4-14-33-27)34-26-12-15-35(16-13-26)20-22-8-9-23-5-1-2-6-24(23)17-22/h1-11,14,17-19,26,34H,12-13,15-16,20-21H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from MCHr1 expressed in IMR32 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
SH2B adapter protein 2
(Human) | BDBM484440
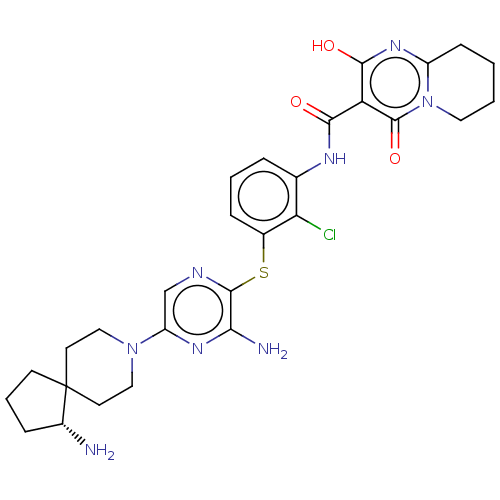
(US10934285, Example 24)Show SMILES N[C@@H]1CCCC11CCN(CC1)c1cnc(Sc2cccc(NC(=O)c3c(O)nc4CCCCn4c3=O)c2Cl)c(N)n1 |r| Show InChI InChI=1S/C28H33ClN8O3S/c29-22-16(33-24(38)21-25(39)35-19-8-1-2-12-37(19)27(21)40)5-3-6-17(22)41-26-23(31)34-20(15-32-26)36-13-10-28(11-14-36)9-4-7-18(28)30/h3,5-6,15,18,39H,1-2,4,7-14,30H2,(H2,31,34)(H,33,38)/t18-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| Assay Description
The inhibition of SHP2 by compounds of the invention (concentrations varying from 0.003-100 μM) was monitored using an assay in which 0.5 nM of ... |
US Patent US10934285 (2021)
BindingDB Entry DOI: 10.7270/Q2MK6H10 |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1/2
(Homo sapiens (Human)) | BDBM50531540
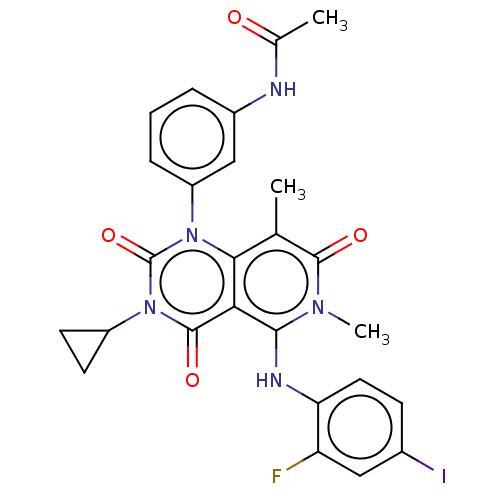
(CHEBI:75998 | GSK-1120212 | GSK1120212 | JTP 74057...)Show SMILES CC(=O)Nc1cccc(c1)-n1c2c(C)c(=O)n(C)c(Nc3ccc(I)cc3F)c2c(=O)n(C2CC2)c1=O Show InChI InChI=1S/C26H23FIN5O4/c1-13-22-21(23(31(3)24(13)35)30-20-10-7-15(28)11-19(20)27)25(36)33(17-8-9-17)26(37)32(22)18-6-4-5-16(12-18)29-14(2)34/h4-7,10-12,17,30H,8-9H2,1-3H3,(H,29,34) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MEK in human KYSE-520 cells assessed as reduction in p-ERK levels |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01170
BindingDB Entry DOI: 10.7270/Q2CC14BB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50183954

(4-(1-(benzo[d][1,3]dioxol-5-ylmethyl)piperidin-4-y...)Show SMILES Clc1ccc2n(Cc3ccncc3)c(=O)cc(NC3CCN(Cc4ccc5OCOc5c4)CC3)c2c1 Show InChI InChI=1S/C28H27ClN4O3/c29-21-2-3-25-23(14-21)24(15-28(34)33(25)17-19-5-9-30-10-6-19)31-22-7-11-32(12-8-22)16-20-1-4-26-27(13-20)36-18-35-26/h1-6,9-10,13-15,22,31H,7-8,11-12,16-18H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from MCHr1 expressed in IMR32 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50183953

(4-(1-(benzo[d][1,3]dioxol-5-ylmethyl)piperidin-4-y...)Show SMILES Clc1ccc2n(Cc3ccccn3)c(=O)cc(NC3CCN(Cc4ccc5OCOc5c4)CC3)c2c1 Show InChI InChI=1S/C28H27ClN4O3/c29-20-5-6-25-23(14-20)24(15-28(34)33(25)17-22-3-1-2-10-30-22)31-21-8-11-32(12-9-21)16-19-4-7-26-27(13-19)36-18-35-26/h1-7,10,13-15,21,31H,8-9,11-12,16-18H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from MCHr1 expressed in IMR32 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
SH2B adapter protein 2
(Human) | BDBM484457

(US10934285, Example 43)Show SMILES N[C@@H]1CCCC11CCN(CC1)c1nnc(Sc2cccc(NC(=O)c3c(O)nc4CCCCn4c3=O)c2Cl)c(N)n1 |r| Show InChI InChI=1S/C27H32ClN9O3S/c28-20-15(31-22(38)19-23(39)32-18-8-1-2-12-37(18)25(19)40)5-3-6-16(20)41-24-21(30)33-26(35-34-24)36-13-10-27(11-14-36)9-4-7-17(27)29/h3,5-6,17,39H,1-2,4,7-14,29H2,(H,31,38)(H2,30,33,35)/t17-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| Assay Description
The inhibition of SHP2 by compounds of the invention (concentrations varying from 0.003-100 μM) was monitored using an assay in which 0.5 nM of ... |
US Patent US10934285 (2021)
BindingDB Entry DOI: 10.7270/Q2MK6H10 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50183967

(4-(1-(benzo[d][1,3]dioxol-5-ylmethyl)piperidin-4-y...)Show SMILES FC(F)(F)Cn1c2ccc(Cl)cc2c(NC2CCN(Cc3ccc4OCOc4c3)CC2)nc1=O Show InChI InChI=1S/C23H22ClF3N4O3/c24-15-2-3-18-17(10-15)21(29-22(32)31(18)12-23(25,26)27)28-16-5-7-30(8-6-16)11-14-1-4-19-20(9-14)34-13-33-19/h1-4,9-10,16H,5-8,11-13H2,(H,28,29,32) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from MCHr1 expressed in IMR32 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50171590
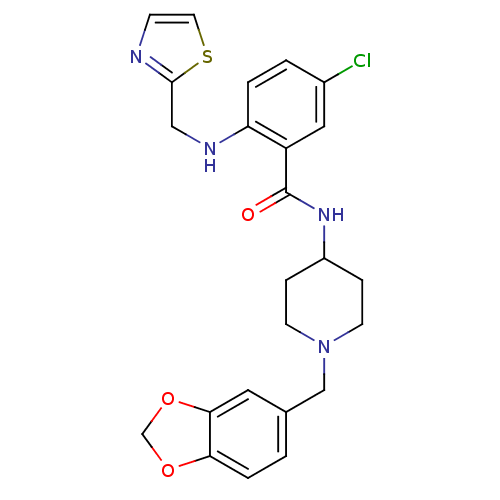
(CHEMBL194697 | N-(1-Benzo[1,3]dioxol-5-ylmethyl-pi...)Show SMILES Clc1ccc(NCc2nccs2)c(c1)C(=O)NC1CCN(Cc2ccc3OCOc3c2)CC1 Show InChI InChI=1S/C24H25ClN4O3S/c25-17-2-3-20(27-13-23-26-7-10-33-23)19(12-17)24(30)28-18-5-8-29(9-6-18)14-16-1-4-21-22(11-16)32-15-31-21/h1-4,7,10-12,18,27H,5-6,8-9,13-15H2,(H,28,30) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity towards melanin-concentrating hormone receptor 1 in IMR32 cells |
Bioorg Med Chem Lett 15: 4174-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.089
BindingDB Entry DOI: 10.7270/Q2N29WGN |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50173461

(4-(1-Benzo[1,3]dioxol-5-ylmethyl-piperidin-4-ylami...)Show SMILES Clc1ccc2oc(=O)cc(NC3CCN(Cc4ccc5OCOc5c4)CC3)c2c1 Show InChI InChI=1S/C22H21ClN2O4/c23-15-2-4-19-17(10-15)18(11-22(26)29-19)24-16-5-7-25(8-6-16)12-14-1-3-20-21(9-14)28-13-27-20/h1-4,9-11,16,24H,5-8,12-13H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration aganist melanin concentrating hormone receptor 1 expressed in IMR-32 cells using [125I]-MCH |
J Med Chem 48: 5888-91 (2005)
Article DOI: 10.1021/jm050598r
BindingDB Entry DOI: 10.7270/Q2QJ7GTV |
More data for this
Ligand-Target Pair | |
SH2B adapter protein 2
(Human) | BDBM484469
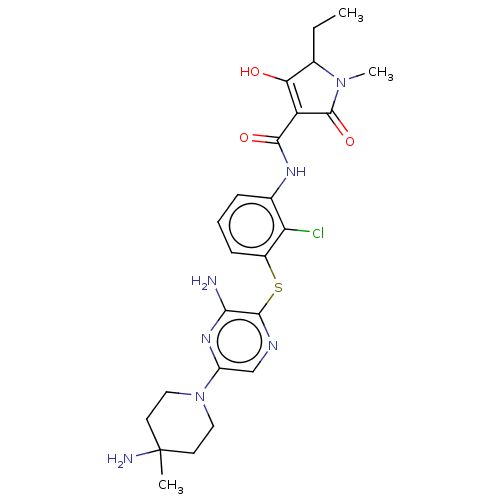
(US10934285, Example 55)Show SMILES CCC1N(C)C(=O)C(C(=O)Nc2cccc(Sc3ncc(nc3N)N3CCC(C)(N)CC3)c2Cl)=C1O |c:36| Show InChI InChI=1S/C24H30ClN7O3S/c1-4-14-19(33)17(23(35)31(14)3)21(34)29-13-6-5-7-15(18(13)25)36-22-20(26)30-16(12-28-22)32-10-8-24(2,27)9-11-32/h5-7,12,14,33H,4,8-11,27H2,1-3H3,(H2,26,30)(H,29,34) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| Assay Description
The inhibition of SHP2 by compounds of the invention (concentrations varying from 0.003-100 μM) was monitored using an assay in which 0.5 nM of ... |
US Patent US10934285 (2021)
BindingDB Entry DOI: 10.7270/Q2MK6H10 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50173454

(4-(1-Benzooxazol-5-ylmethyl-piperidin-4-ylamino)-6...)Show SMILES Clc1ccc2oc(=O)cc(NC3CCN(Cc4ccc5ocnc5c4)CC3)c2c1 Show InChI InChI=1S/C22H20ClN3O3/c23-15-2-4-20-17(10-15)18(11-22(27)29-20)25-16-5-7-26(8-6-16)12-14-1-3-21-19(9-14)24-13-28-21/h1-4,9-11,13,16,25H,5-8,12H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration aganist melanin concentrating hormone receptor 1 expressed in IMR-32 cells using [125I]-MCH |
J Med Chem 48: 5888-91 (2005)
Article DOI: 10.1021/jm050598r
BindingDB Entry DOI: 10.7270/Q2QJ7GTV |
More data for this
Ligand-Target Pair | |
SH2B adapter protein 2
(Human) | BDBM484461
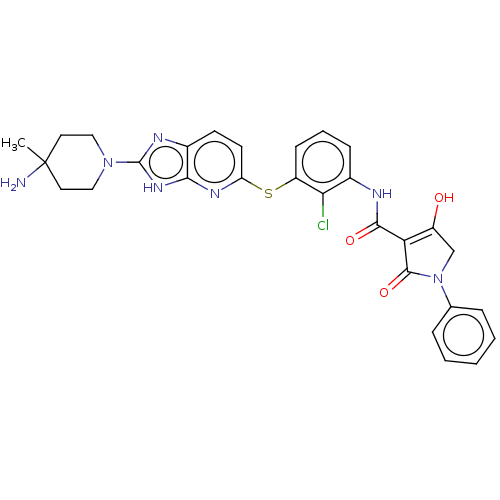
(US10934285, Example 47)Show SMILES CC1(N)CCN(CC1)c1nc2ccc(Sc3cccc(NC(=O)C4=C(O)CN(C4=O)c4ccccc4)c3Cl)nc2[nH]1 |c:24| Show InChI InChI=1S/C29H28ClN7O3S/c1-29(31)12-14-36(15-13-29)28-33-19-10-11-22(34-25(19)35-28)41-21-9-5-8-18(24(21)30)32-26(39)23-20(38)16-37(27(23)40)17-6-3-2-4-7-17/h2-11,38H,12-16,31H2,1H3,(H,32,39)(H,33,34,35) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| Assay Description
The inhibition of SHP2 by compounds of the invention (concentrations varying from 0.003-100 μM) was monitored using an assay in which 0.5 nM of ... |
US Patent US10934285 (2021)
BindingDB Entry DOI: 10.7270/Q2MK6H10 |
More data for this
Ligand-Target Pair | |
SH2B adapter protein 2
(Human) | BDBM484466
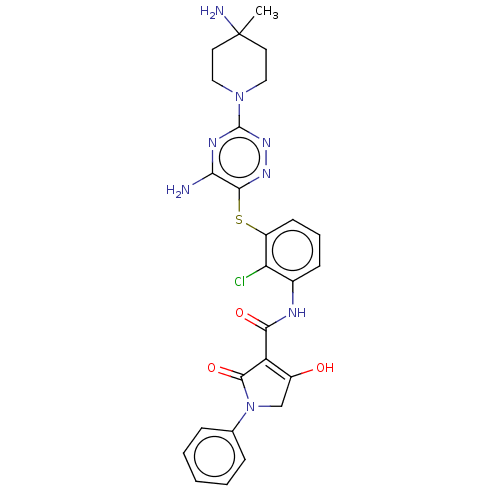
(US10934285, Example 52)Show SMILES CC1(N)CCN(CC1)c1nnc(Sc2cccc(NC(=O)C3=C(O)CN(C3=O)c3ccccc3)c2Cl)c(N)n1 |c:22| Show InChI InChI=1S/C26H27ClN8O3S/c1-26(29)10-12-34(13-11-26)25-31-21(28)23(32-33-25)39-18-9-5-8-16(20(18)27)30-22(37)19-17(36)14-35(24(19)38)15-6-3-2-4-7-15/h2-9,36H,10-14,29H2,1H3,(H,30,37)(H2,28,31,33) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| Assay Description
The inhibition of SHP2 by compounds of the invention (concentrations varying from 0.003-100 μM) was monitored using an assay in which 0.5 nM of ... |
US Patent US10934285 (2021)
BindingDB Entry DOI: 10.7270/Q2MK6H10 |
More data for this
Ligand-Target Pair | |
SH2B adapter protein 2
(Human) | BDBM484472

(US10934285, Example 59)Show SMILES CN1C(=O)C(C(=O)Nc2cccc(Sc3ncc(nc3N)N3CCC(C)(N)CC3)c2Cl)=C(O)C1(C)C |t:33| Show InChI InChI=1S/C24H30ClN7O3S/c1-23(2)18(33)16(22(35)31(23)4)20(34)29-13-6-5-7-14(17(13)25)36-21-19(26)30-15(12-28-21)32-10-8-24(3,27)9-11-32/h5-7,12,33H,8-11,27H2,1-4H3,(H2,26,30)(H,29,34) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| Assay Description
The inhibition of SHP2 by compounds of the invention (concentrations varying from 0.003-100 μM) was monitored using an assay in which 0.5 nM of ... |
US Patent US10934285 (2021)
BindingDB Entry DOI: 10.7270/Q2MK6H10 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM408072
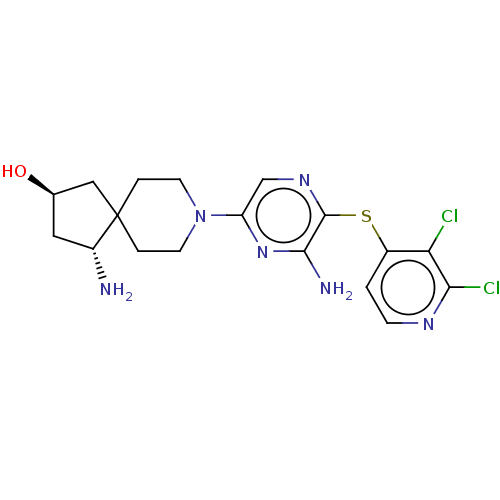
(US10336774, Example 57)Show SMILES N[C@@H]1C[C@@H](O)CC11CCN(CC1)c1cnc(Sc2ccnc(Cl)c2Cl)c(N)n1 |r| Show InChI InChI=1S/C18H22Cl2N6OS/c19-14-11(1-4-23-15(14)20)28-17-16(22)25-13(9-24-17)26-5-2-18(3-6-26)8-10(27)7-12(18)21/h1,4,9-10,12,27H,2-3,5-8,21H2,(H2,22,25)/t10-,12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
SHP2 is allosterically activated through binding of bis-tyrosyl-phorphorylated peptides to its Src Homology 2 (SH2) domains. The latter activation st... |
US Patent US10336774 (2019)
BindingDB Entry DOI: 10.7270/Q2BR8VJV |
More data for this
Ligand-Target Pair | |
SH2B adapter protein 2
(Human) | BDBM484421
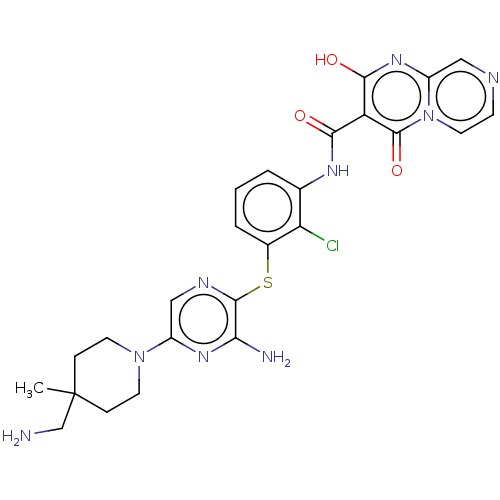
(US10934285, Example 5)Show SMILES CC1(CN)CCN(CC1)c1cnc(Sc2cccc(NC(=O)c3c(O)nc4cnccn4c3=O)c2Cl)c(N)n1 Show InChI InChI=1S/C25H26ClN9O3S/c1-25(13-27)5-8-34(9-6-25)16-12-30-23(20(28)32-16)39-15-4-2-3-14(19(15)26)31-21(36)18-22(37)33-17-11-29-7-10-35(17)24(18)38/h2-4,7,10-12,37H,5-6,8-9,13,27H2,1H3,(H2,28,32)(H,31,36) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| Assay Description
The inhibition of SHP2 by compounds of the invention (concentrations varying from 0.003-100 μM) was monitored using an assay in which 0.5 nM of ... |
US Patent US10934285 (2021)
BindingDB Entry DOI: 10.7270/Q2MK6H10 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50183957

(4-(1-(benzo[d][1,3]dioxol-5-ylmethyl)piperidin-4-y...)Show SMILES COc1ccc2n(C)c(=O)cc(NC3CCN(Cc4ccc5OCOc5c4)CC3)c2c1 Show InChI InChI=1S/C24H27N3O4/c1-26-21-5-4-18(29-2)12-19(21)20(13-24(26)28)25-17-7-9-27(10-8-17)14-16-3-6-22-23(11-16)31-15-30-22/h3-6,11-13,17,25H,7-10,14-15H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from MCHr1 expressed in IMR32 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50173465
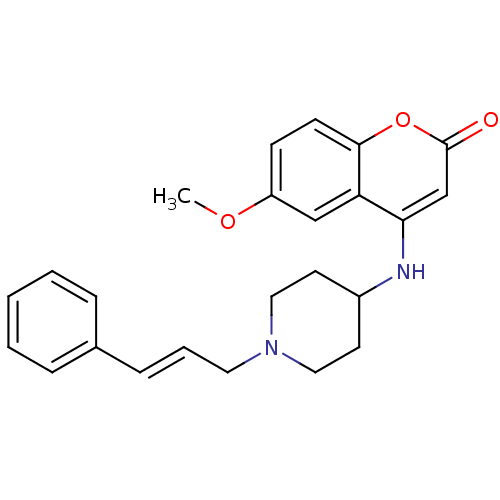
(6-Methoxy-4-[1-(3-phenyl-allyl)-piperidin-4-ylamin...)Show SMILES COc1ccc2oc(=O)cc(NC3CCN(C\C=C\c4ccccc4)CC3)c2c1 Show InChI InChI=1S/C24H26N2O3/c1-28-20-9-10-23-21(16-20)22(17-24(27)29-23)25-19-11-14-26(15-12-19)13-5-8-18-6-3-2-4-7-18/h2-10,16-17,19,25H,11-15H2,1H3/b8-5+ | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration aganist melanin concentrating hormone receptor 1 expressed in IMR-32 cells using [125I]-MCH |
J Med Chem 48: 5888-91 (2005)
Article DOI: 10.1021/jm050598r
BindingDB Entry DOI: 10.7270/Q2QJ7GTV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM408096
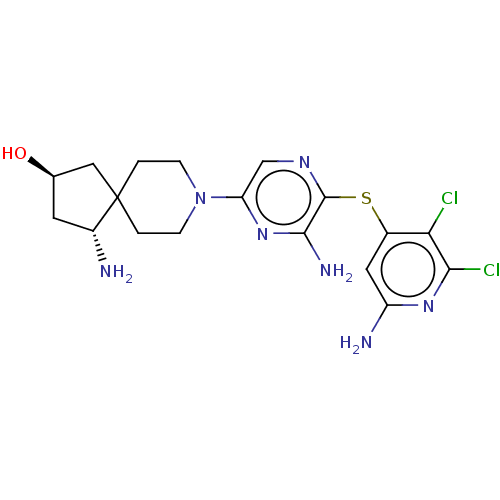
(US10336774, Example 90)Show SMILES N[C@@H]1C[C@@H](O)CC11CCN(CC1)c1cnc(Sc2cc(N)nc(Cl)c2Cl)c(N)n1 |r| Show InChI InChI=1S/C18H23Cl2N7OS/c19-14-10(6-12(22)25-15(14)20)29-17-16(23)26-13(8-24-17)27-3-1-18(2-4-27)7-9(28)5-11(18)21/h6,8-9,11,28H,1-5,7,21H2,(H2,22,25)(H2,23,26)/t9-,11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
SHP2 is allosterically activated through binding of bis-tyrosyl-phorphorylated peptides to its Src Homology 2 (SH2) domains. The latter activation st... |
US Patent US10336774 (2019)
BindingDB Entry DOI: 10.7270/Q2BR8VJV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM408094

(US10336774, Example 88)Show SMILES C[C@@H]1OCC2(CCN(CC2)c2cnc(Sc3cc(N)nc(Cl)c3Cl)c(N)n2)[C@@H]1N |r| Show InChI InChI=1S/C18H23Cl2N7OS/c1-9-14(22)18(8-28-9)2-4-27(5-3-18)12-7-24-17(16(23)26-12)29-10-6-11(21)25-15(20)13(10)19/h6-7,9,14H,2-5,8,22H2,1H3,(H2,21,25)(H2,23,26)/t9-,14+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
SHP2 is allosterically activated through binding of bis-tyrosyl-phorphorylated peptides to its Src Homology 2 (SH2) domains. The latter activation st... |
US Patent US10336774 (2019)
BindingDB Entry DOI: 10.7270/Q2BR8VJV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM408075

(US10336774, Example 60)Show SMILES CO[C@H]1C[C@@H](N)C2(C1)CCN(CC2)c1cnc(Sc2ccnc(Cl)c2Cl)c(N)n1 |r| Show InChI InChI=1S/C19H24Cl2N6OS/c1-28-11-8-13(22)19(9-11)3-6-27(7-4-19)14-10-25-18(17(23)26-14)29-12-2-5-24-16(21)15(12)20/h2,5,10-11,13H,3-4,6-9,22H2,1H3,(H2,23,26)/t11-,13+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
SHP2 is allosterically activated through binding of bis-tyrosyl-phorphorylated peptides to its Src Homology 2 (SH2) domains. The latter activation st... |
US Patent US10336774 (2019)
BindingDB Entry DOI: 10.7270/Q2BR8VJV |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50171580
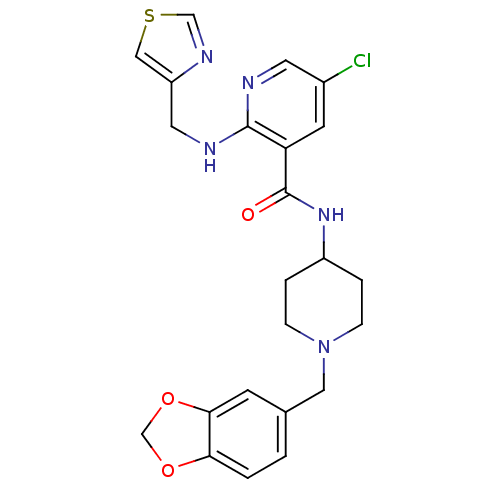
(CHEMBL196277 | N-(1-Benzo[1,3]dioxol-5-ylmethyl-pi...)Show SMILES Clc1cnc(NCc2cscn2)c(c1)C(=O)NC1CCN(Cc2ccc3OCOc3c2)CC1 Show InChI InChI=1S/C23H24ClN5O3S/c24-16-8-19(22(25-9-16)26-10-18-12-33-13-27-18)23(30)28-17-3-5-29(6-4-17)11-15-1-2-20-21(7-15)32-14-31-20/h1-2,7-9,12-13,17H,3-6,10-11,14H2,(H,25,26)(H,28,30) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity towards melanin-concentrating hormone receptor 1 in IMR32 cells |
Bioorg Med Chem Lett 15: 4174-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.089
BindingDB Entry DOI: 10.7270/Q2N29WGN |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50183966

(4-(8-(benzo[d][1,3]dioxol-5-ylmethyl)-8-aza-bicycl...)Show SMILES FC(F)(F)Cn1c2ccc(Cl)cc2c(NC2CC3CCC(C2)N3Cc2ccc3OCOc3c2)nc1=O |TLB:14:15:22:18.19| Show InChI InChI=1S/C25H24ClF3N4O3/c26-15-2-5-20-19(8-15)23(31-24(34)33(20)12-25(27,28)29)30-16-9-17-3-4-18(10-16)32(17)11-14-1-6-21-22(7-14)36-13-35-21/h1-2,5-8,16-18H,3-4,9-13H2,(H,30,31,34) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from MCHr1 expressed in IMR32 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50183969

(4-(1-(benzo[d][1,3]dioxol-5-ylmethyl)piperidin-4-y...)Show SMILES Clc1ccc2[nH]c(=O)cc(NC3CCN(Cc4ccc5OCOc5c4)CC3)c2c1 Show InChI InChI=1S/C22H22ClN3O3/c23-15-2-3-18-17(10-15)19(11-22(27)25-18)24-16-5-7-26(8-6-16)12-14-1-4-20-21(9-14)29-13-28-20/h1-4,9-11,16H,5-8,12-13H2,(H2,24,25,27) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from MCHr1 expressed in IMR32 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50530254
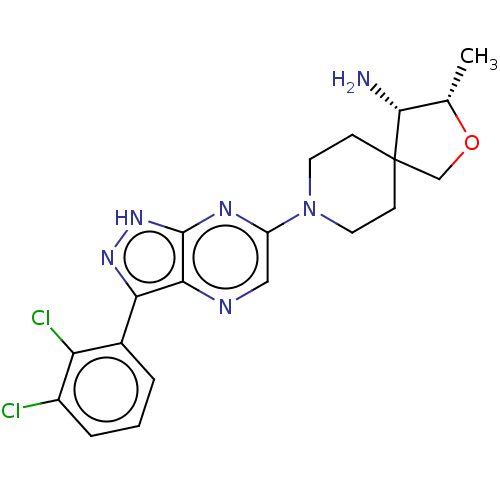
(CHEMBL4475043)Show SMILES C[C@@H]1OCC2(CCN(CC2)c2cnc3c(n[nH]c3n2)-c2cccc(Cl)c2Cl)[C@@H]1N |r| Show InChI InChI=1S/C20H22Cl2N6O/c1-11-18(23)20(10-29-11)5-7-28(8-6-20)14-9-24-17-16(26-27-19(17)25-14)12-3-2-4-13(21)15(12)22/h2-4,9,11,18H,5-8,10,23H2,1H3,(H,25,26,27)/t11-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by Q-patch assay |
J Med Chem 62: 1781-1792 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01725
BindingDB Entry DOI: 10.7270/Q25T3PZD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data