

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50145684 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against C-C chemokine receptor type 5 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50145681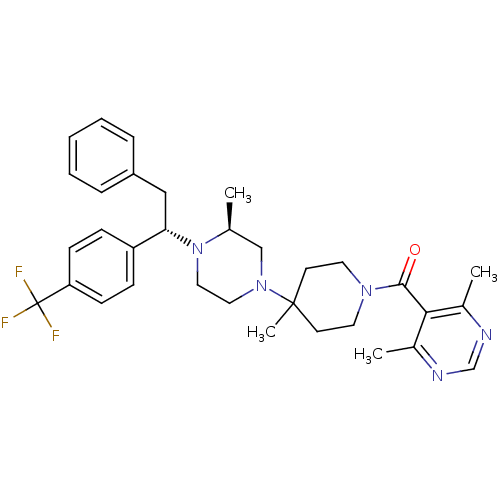 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against C-C chemokine receptor type 5 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50145686 ((4-{(S)-4-[(S)-2-Cyclopropyl-1-(4-trifluoromethyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against C-C chemokine receptor type 5 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50145685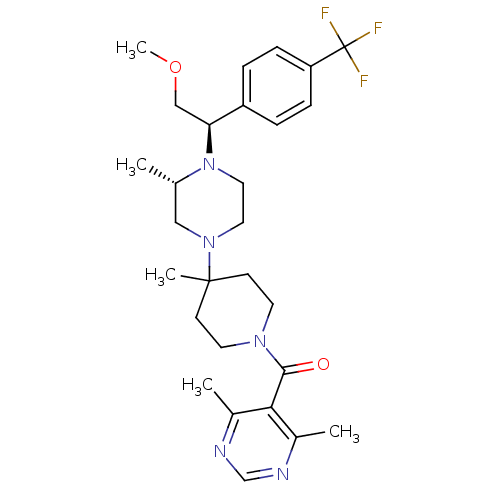 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-{(S)-4-[(R)-2-met...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against C-C chemokine receptor type 5 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50123435 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against C-C chemokine receptor type 5 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50145682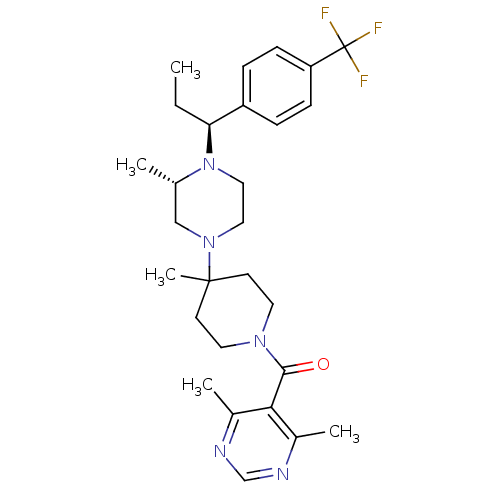 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against C-C chemokine receptor type 5 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50123435 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 456 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50123435 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 575 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic M1 receptor | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50123435 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 716 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic M3 receptor | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50145686 ((4-{(S)-4-[(S)-2-Cyclopropyl-1-(4-trifluoromethyl-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic M1 receptor | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50145686 ((4-{(S)-4-[(S)-2-Cyclopropyl-1-(4-trifluoromethyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50145683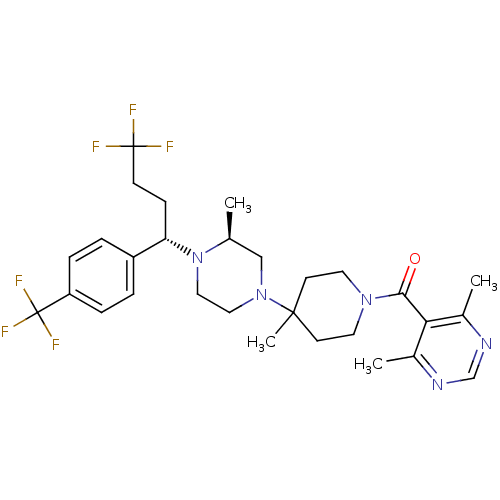 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic M1 receptor | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50145681 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic M1 receptor | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50145682 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic M1 receptor | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50145682 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50145684 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50145681 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic M3 receptor | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50145684 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic M3 receptor | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50145681 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50145684 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic M1 receptor | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50145683 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50145682 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic M3 receptor | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50145685 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-{(S)-4-[(R)-2-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50145685 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-{(S)-4-[(R)-2-met...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic M1 receptor | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acetylglucosamine-1-phosphotransferase subunits alpha/beta (Human) | BDBM518926 (N-cyclohexyl-N-ethyl-3-(2-(trans-4-ethylcyclohexyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The TarO biochemical enzymatic assay is a liquid chromatography-mass spectroscopy (LC-MS) based end point assay that measures C55-P-P-GlcNAc (LIPID I... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XK8JQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acetylglucosamine-1-phosphotransferase subunits alpha/beta (Human) | BDBM519420 ((4S,5R)-5-[3-chloro-5- (trifluoromethyl)phenyl]- 4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The TarO biochemical enzymatic assay is a liquid chromatography-mass spectroscopy (LC-MS) based end point assay that measures C55-P-P-GlcNAc (LIPID I... | Citation and Details BindingDB Entry DOI: 10.7270/Q2ST7T0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50223567 (3-bromo-5-(2-chlorophenyl)-N-(pyridin-3-ylmethyl)p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description CDK2 kinase assays (either cyclin A or cyclin E-dependent) were performed in low protein binding 96-well plates (Corning Inc., Corning, N.Y.). | US Patent US8580782 (2013) BindingDB Entry DOI: 10.7270/Q2VM49WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM105239 (US8580782, 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description CDK2 kinase assays (either cyclin A or cyclin E-dependent) were performed in low protein binding 96-well plates (Corning Inc., Corning, N.Y.). | US Patent US8580782 (2013) BindingDB Entry DOI: 10.7270/Q2VM49WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acetylglucosamine-1-phosphotransferase subunits alpha/beta (Human) | BDBM519070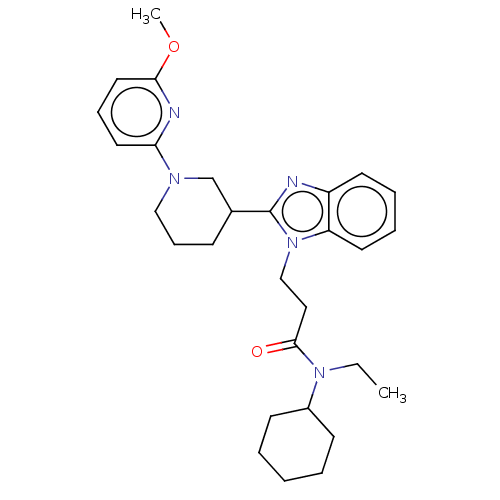 (N-cyclohexyl-N-ethyl- 3-{2-[1-(6- methoxypyridin-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The TarO biochemical enzymatic assay is a liquid chromatography-mass spectroscopy (LC-MS) based end point assay that measures C55-P-P-GlcNAc (LIPID I... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XK8JQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acetylglucosamine-1-phosphotransferase subunits alpha/beta (Human) | BDBM518864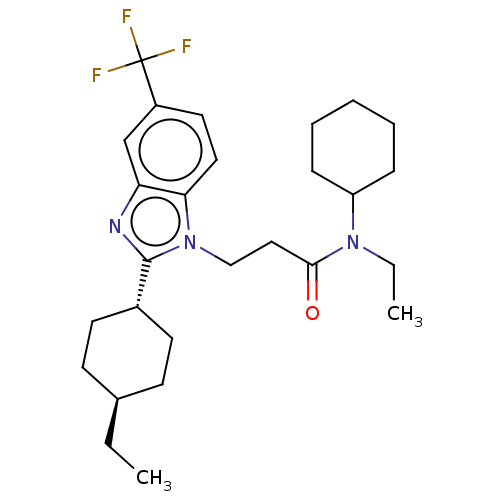 (N-cyclohexyl-N-ethyl- 3-[2-(trans-4- ethylcyclohex...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The TarO biochemical enzymatic assay is a liquid chromatography-mass spectroscopy (LC-MS) based end point assay that measures C55-P-P-GlcNAc (LIPID I... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XK8JQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acetylglucosamine-1-phosphotransferase subunits alpha/beta (Human) | BDBM518851 (3-[5-bromo-2-(trans-4- ethylcyclohexyl)-1H- benzim...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The TarO biochemical enzymatic assay is a liquid chromatography-mass spectroscopy (LC-MS) based end point assay that measures C55-P-P-GlcNAc (LIPID I... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XK8JQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acetylglucosamine-1-phosphotransferase subunits alpha/beta (Human) | BDBM519422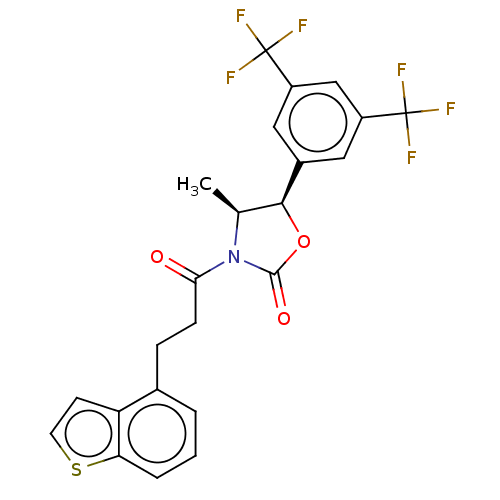 ((4S,5R)-3-[3-(1- benzothiophen-4- yl)propanoyl]-5-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The TarO biochemical enzymatic assay is a liquid chromatography-mass spectroscopy (LC-MS) based end point assay that measures C55-P-P-GlcNAc (LIPID I... | Citation and Details BindingDB Entry DOI: 10.7270/Q2ST7T0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acetylglucosamine-1-phosphotransferase subunits alpha/beta (Human) | BDBM519421 ((4S,5R)-5-[3,5- bis(trifluoromethyl) phenyl]-4-met...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The TarO biochemical enzymatic assay is a liquid chromatography-mass spectroscopy (LC-MS) based end point assay that measures C55-P-P-GlcNAc (LIPID I... | Citation and Details BindingDB Entry DOI: 10.7270/Q2ST7T0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acetylglucosamine-1-phosphotransferase subunits alpha/beta (Human) | BDBM519419 ((4S,5R)-5-(3,5-bis(trifluoromethyl)phenyl)-3-(3-(i...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The TarO biochemical enzymatic assay is a liquid chromatography-mass spectroscopy (LC-MS) based end point assay that measures C55-P-P-GlcNAc (LIPID I... | Citation and Details BindingDB Entry DOI: 10.7270/Q2ST7T0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acetylglucosamine-1-phosphotransferase subunits alpha/beta (Human) | BDBM519416 ((4S,5S)-5-[6-ethoxy- 4-(trifluoromethyl) pyridin-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The TarO biochemical enzymatic assay is a liquid chromatography-mass spectroscopy (LC-MS) based end point assay that measures C55-P-P-GlcNAc (LIPID I... | Citation and Details BindingDB Entry DOI: 10.7270/Q2ST7T0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acetylglucosamine-1-phosphotransferase subunits alpha/beta (Human) | BDBM519403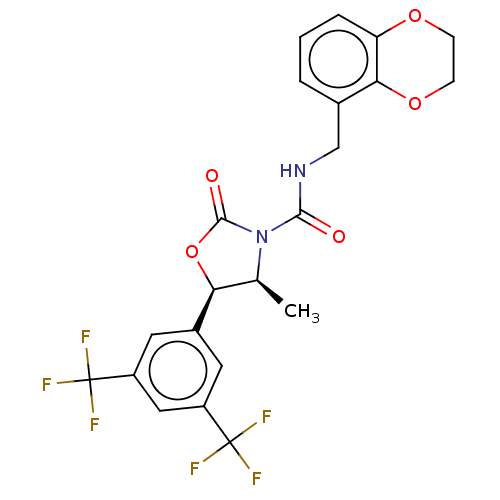 ((4S,5R)-5-[3,5- bis(trifluoromethyl) phenyl]-N-(2,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The TarO biochemical enzymatic assay is a liquid chromatography-mass spectroscopy (LC-MS) based end point assay that measures C55-P-P-GlcNAc (LIPID I... | Citation and Details BindingDB Entry DOI: 10.7270/Q2ST7T0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acetylglucosamine-1-phosphotransferase subunits alpha/beta (Human) | BDBM519402 ((4S,5R)-5-[3-fluoro-5- (trifluoromethyl)phenyl]- N...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The TarO biochemical enzymatic assay is a liquid chromatography-mass spectroscopy (LC-MS) based end point assay that measures C55-P-P-GlcNAc (LIPID I... | Citation and Details BindingDB Entry DOI: 10.7270/Q2ST7T0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acetylglucosamine-1-phosphotransferase subunits alpha/beta (Human) | BDBM519343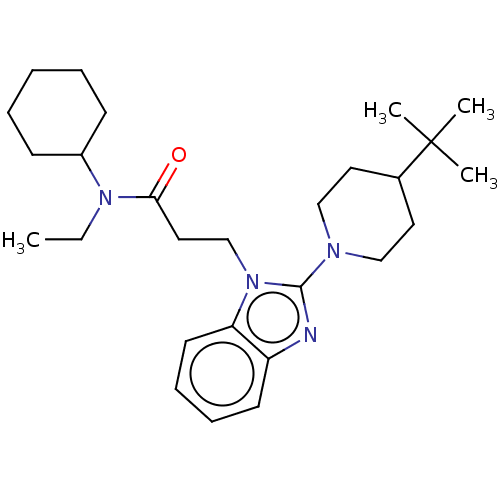 (3-[2-(4-tert- butylpiperidin-1-yl)- 1H-benzimidazo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The TarO biochemical enzymatic assay is a liquid chromatography-mass spectroscopy (LC-MS) based end point assay that measures C55-P-P-GlcNAc (LIPID I... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XK8JQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acetylglucosamine-1-phosphotransferase subunits alpha/beta (Human) | BDBM519352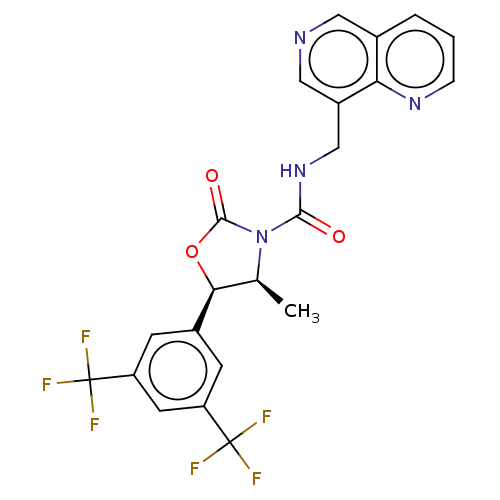 ((4S,5R)-5-[3,5- bis(trifluoromethyl)phenyl]- 4-met...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The TarO biochemical enzymatic assay is a liquid chromatography-mass spectroscopy (LC-MS) based end point assay that measures C55-P-P-GlcNAc (LIPID I... | Citation and Details BindingDB Entry DOI: 10.7270/Q2ST7T0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acetylglucosamine-1-phosphotransferase subunits alpha/beta (Human) | BDBM519351 ((4S,5R)-5-[3-fluoro-5- (trifluoromethyl)phenyl]- 4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The TarO biochemical enzymatic assay is a liquid chromatography-mass spectroscopy (LC-MS) based end point assay that measures C55-P-P-GlcNAc (LIPID I... | Citation and Details BindingDB Entry DOI: 10.7270/Q2ST7T0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acetylglucosamine-1-phosphotransferase subunits alpha/beta (Human) | BDBM519350 ((4S,5R)-5-[3,5- bis(trifluoromethyl)phenyl]- 4-met...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The TarO biochemical enzymatic assay is a liquid chromatography-mass spectroscopy (LC-MS) based end point assay that measures C55-P-P-GlcNAc (LIPID I... | Citation and Details BindingDB Entry DOI: 10.7270/Q2ST7T0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM105239 (US8580782, 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description CDK2 kinase assays (either cyclin A or cyclin E-dependent) were performed in low protein binding 96-well plates (Corning Inc., Corning, N.Y.). | US Patent US8580782 (2013) BindingDB Entry DOI: 10.7270/Q2VM49WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM105236 (US8580782, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description CDK2 kinase assays (either cyclin A or cyclin E-dependent) were performed in low protein binding 96-well plates (Corning Inc., Corning, N.Y.). | US Patent US8580782 (2013) BindingDB Entry DOI: 10.7270/Q2VM49WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM105234 (US8580782, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description CDK2 kinase assays (either cyclin A or cyclin E-dependent) were performed in low protein binding 96-well plates (Corning Inc., Corning, N.Y.). | US Patent US8580782 (2013) BindingDB Entry DOI: 10.7270/Q2VM49WG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| N-acetylglucosamine-1-phosphotransferase subunits alpha/beta (Human) | BDBM519354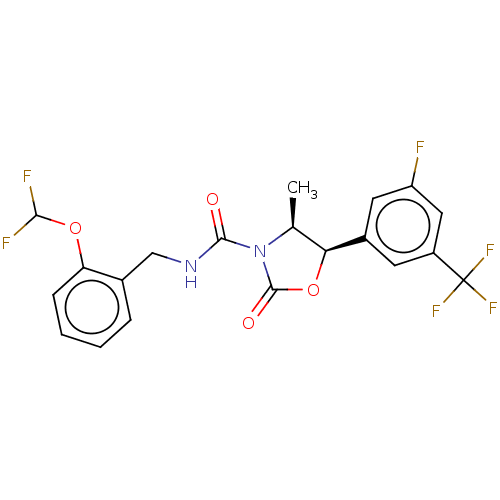 ((4S,5R)-N-[2- (difluoromethoxy) benzyl]-5-[3-fluor...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The TarO biochemical enzymatic assay is a liquid chromatography-mass spectroscopy (LC-MS) based end point assay that measures C55-P-P-GlcNAc (LIPID I... | Citation and Details BindingDB Entry DOI: 10.7270/Q2ST7T0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acetylglucosamine-1-phosphotransferase subunits alpha/beta (Human) | BDBM519355 ((4S,5R)-5-[3-fluoro-5- (trifluoromethyl)phenyl]- 4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The TarO biochemical enzymatic assay is a liquid chromatography-mass spectroscopy (LC-MS) based end point assay that measures C55-P-P-GlcNAc (LIPID I... | Citation and Details BindingDB Entry DOI: 10.7270/Q2ST7T0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acetylglucosamine-1-phosphotransferase subunits alpha/beta (Human) | BDBM519033 (N-cyclohexyl-N-ethyl- 3-{2-[1-(6- methoxypyridin-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The TarO biochemical enzymatic assay is a liquid chromatography-mass spectroscopy (LC-MS) based end point assay that measures C55-P-P-GlcNAc (LIPID I... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XK8JQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acetylglucosamine-1-phosphotransferase subunits alpha/beta (Human) | BDBM518860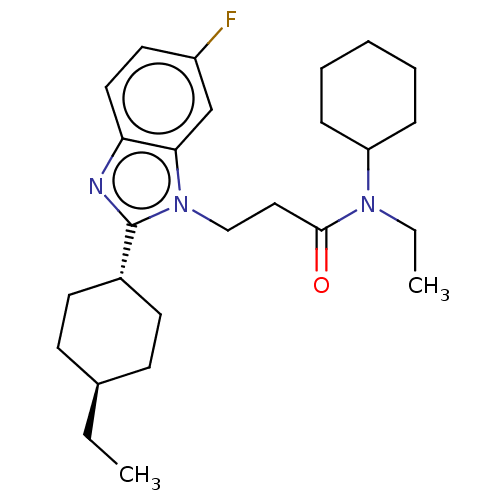 (N-cyclohexyl-N-ethyl- 3-(2-(trans-4- ethvlcyclohex...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The TarO biochemical enzymatic assay is a liquid chromatography-mass spectroscopy (LC-MS) based end point assay that measures C55-P-P-GlcNAc (LIPID I... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XK8JQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM105237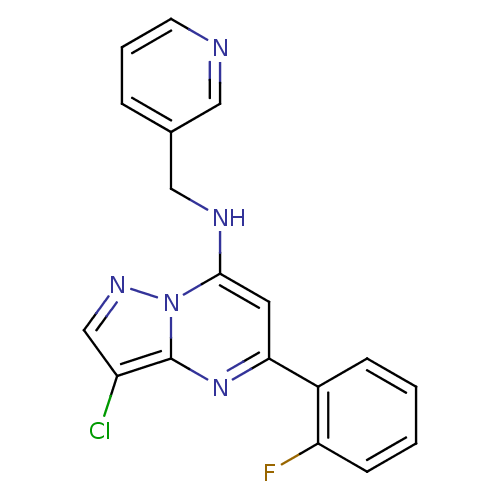 (US8575203, I-4 | US8580782, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 21 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description CDK2 kinase assays (either cyclin A or cyclin E-dependent) were performed in low protein binding 96-well plates (Corning Inc., Corning, N.Y.). | US Patent US8580782 (2013) BindingDB Entry DOI: 10.7270/Q2VM49WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM105235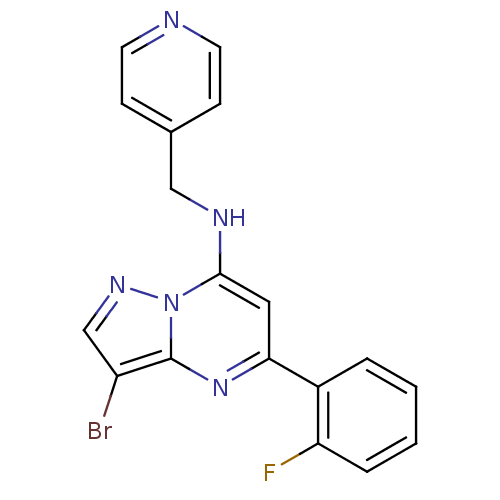 (US8580782, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description CDK2 kinase assays (either cyclin A or cyclin E-dependent) were performed in low protein binding 96-well plates (Corning Inc., Corning, N.Y.). | US Patent US8580782 (2013) BindingDB Entry DOI: 10.7270/Q2VM49WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 202 total ) | Next | Last >> |