 Found 1011 hits with Last Name = 'lacombe' and Initial = 'p'
Found 1011 hits with Last Name = 'lacombe' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50081444
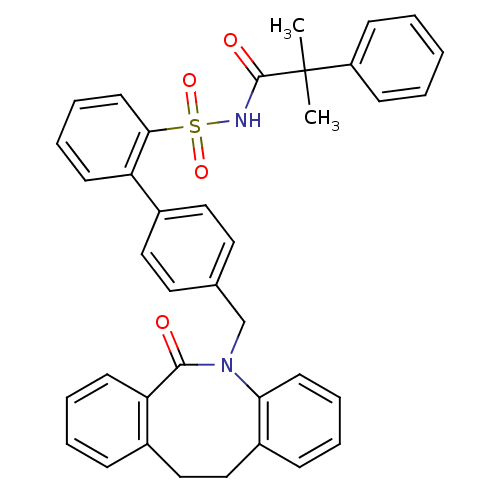
(4'-(6-Oxo-11,12-dihydro-6H-dibenzo[b,f]azocin-5-yl...)Show SMILES CC(C)(C(=O)NS(=O)(=O)c1ccccc1-c1ccc(CN2c3ccccc3CCc3ccccc3C2=O)cc1)c1ccccc1 Show InChI InChI=1S/C38H34N2O4S/c1-38(2,31-14-4-3-5-15-31)37(42)39-45(43,44)35-19-11-9-16-32(35)29-22-20-27(21-23-29)26-40-34-18-10-7-13-30(34)25-24-28-12-6-8-17-33(28)36(40)41/h3-23H,24-26H2,1-2H3,(H,39,42) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Affinity at human EP1 receptor. |
Bioorg Med Chem Lett 9: 2699-704 (1999)
BindingDB Entry DOI: 10.7270/Q2HX1BV9 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50081440

(4'-(6-Oxo-11,12-dihydro-6H-dibenzo[b,f]azocin-5-yl...)Show SMILES CO[C@](C(=O)NS(=O)(=O)c1ccccc1-c1ccc(CN2c3ccccc3CCc3ccccc3C2=O)cc1)(c1ccccc1)C(F)(F)F Show InChI InChI=1S/C38H31F3N2O5S/c1-48-37(38(39,40)41,30-13-3-2-4-14-30)36(45)42-49(46,47)34-18-10-8-15-31(34)28-21-19-26(20-22-28)25-43-33-17-9-6-12-29(33)24-23-27-11-5-7-16-32(27)35(43)44/h2-22H,23-25H2,1H3,(H,42,45)/t37-/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Affinity at human EP1 receptor. |
Bioorg Med Chem Lett 9: 2699-704 (1999)
BindingDB Entry DOI: 10.7270/Q2HX1BV9 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50081453
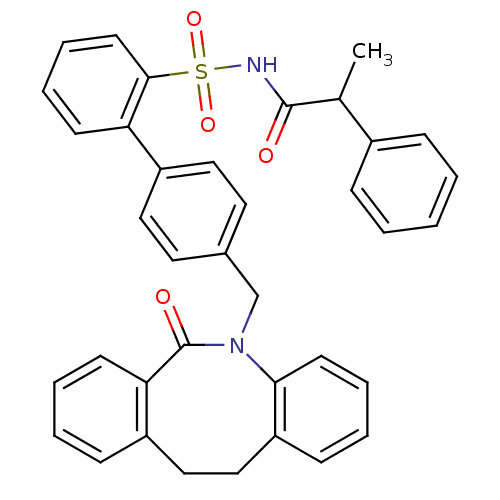
(4'-(6-Oxo-11,12-dihydro-6H-dibenzo[b,f]azocin-5-yl...)Show SMILES CC(C(=O)NS(=O)(=O)c1ccccc1-c1ccc(CN2c3ccccc3CCc3ccccc3C2=O)cc1)c1ccccc1 Show InChI InChI=1S/C37H32N2O4S/c1-26(28-11-3-2-4-12-28)36(40)38-44(42,43)35-18-10-8-15-32(35)30-21-19-27(20-22-30)25-39-34-17-9-6-14-31(34)24-23-29-13-5-7-16-33(29)37(39)41/h2-22,26H,23-25H2,1H3,(H,38,40) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Affinity at human EP1 receptor. |
Bioorg Med Chem Lett 9: 2699-704 (1999)
BindingDB Entry DOI: 10.7270/Q2HX1BV9 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50117700
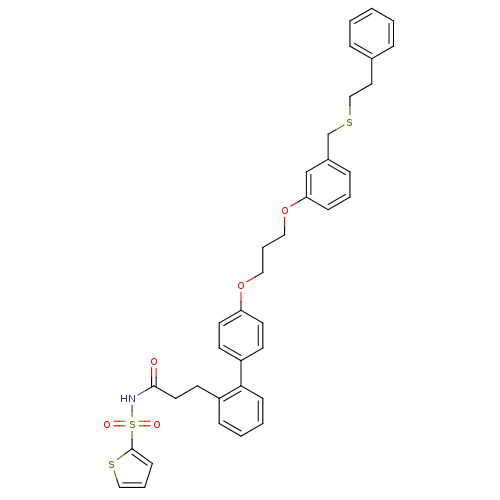
(CHEMBL87366 | Thiophene-2-sulfonic acid (3-{4'-[3-...)Show SMILES O=C(CCc1ccccc1-c1ccc(OCCCOc2cccc(CSCCc3ccccc3)c2)cc1)NS(=O)(=O)c1cccs1 Show InChI InChI=1S/C37H37NO5S3/c39-36(38-46(40,41)37-15-7-25-45-37)21-18-31-12-4-5-14-35(31)32-16-19-33(20-17-32)42-23-8-24-43-34-13-6-11-30(27-34)28-44-26-22-29-9-2-1-3-10-29/h1-7,9-17,19-20,25,27H,8,18,21-24,26,28H2,(H,38,39) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity at human Prostanoid EP3 receptor. |
Bioorg Med Chem Lett 12: 2583-6 (2002)
BindingDB Entry DOI: 10.7270/Q2QN663S |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50081439
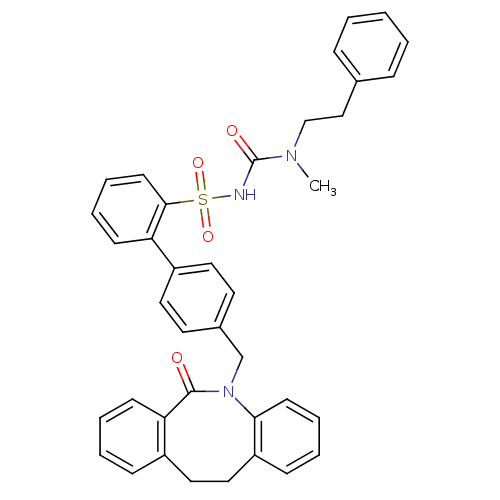
(CHEMBL92539 | sulfonylurea analogue)Show SMILES CN(CCc1ccccc1)C(=O)NS(=O)(=O)c1ccccc1-c1ccc(CN2c3ccccc3CCc3ccccc3C2=O)cc1 Show InChI InChI=1S/C38H35N3O4S/c1-40(26-25-28-11-3-2-4-12-28)38(43)39-46(44,45)36-18-10-8-15-33(36)31-21-19-29(20-22-31)27-41-35-17-9-6-14-32(35)24-23-30-13-5-7-16-34(30)37(41)42/h2-22H,23-27H2,1H3,(H,39,43) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Affinity at human EP1 receptor. |
Bioorg Med Chem Lett 9: 2699-704 (1999)
BindingDB Entry DOI: 10.7270/Q2HX1BV9 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50081445
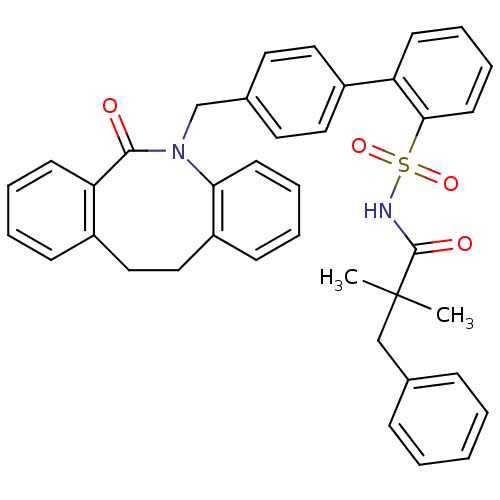
(4'-(6-Oxo-11,12-dihydro-6H-dibenzo[b,f]azocin-5-yl...)Show SMILES CC(C)(Cc1ccccc1)C(=O)NS(=O)(=O)c1ccccc1-c1ccc(CN2c3ccccc3CCc3ccccc3C2=O)cc1 Show InChI InChI=1S/C39H36N2O4S/c1-39(2,26-28-12-4-3-5-13-28)38(43)40-46(44,45)36-19-11-9-16-33(36)31-22-20-29(21-23-31)27-41-35-18-10-7-15-32(35)25-24-30-14-6-8-17-34(30)37(41)42/h3-23H,24-27H2,1-2H3,(H,40,43) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Affinity at human EP1 receptor. |
Bioorg Med Chem Lett 9: 2699-704 (1999)
BindingDB Entry DOI: 10.7270/Q2HX1BV9 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50117689
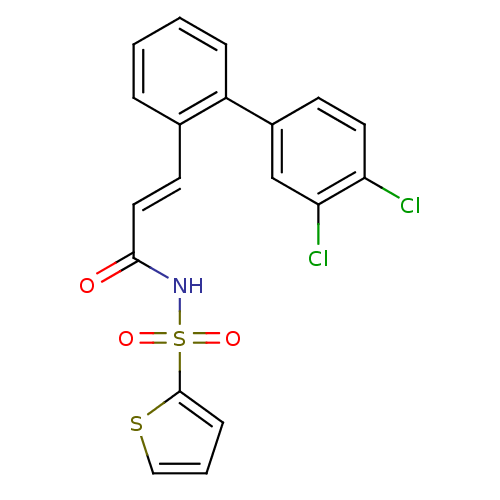
(CHEMBL87371 | Thiophene-2-sulfonic acid [(E)-3-(3'...)Show SMILES Clc1ccc(cc1Cl)-c1ccccc1\C=C\C(=O)NS(=O)(=O)c1cccs1 Show InChI InChI=1S/C19H13Cl2NO3S2/c20-16-9-7-14(12-17(16)21)15-5-2-1-4-13(15)8-10-18(23)22-27(24,25)19-6-3-11-26-19/h1-12H,(H,22,23)/b10-8+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity at human Prostanoid EP3 receptor. |
Bioorg Med Chem Lett 12: 2583-6 (2002)
BindingDB Entry DOI: 10.7270/Q2QN663S |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50117690

(CHEMBL86933 | Thiophene-2-sulfonic acid [3-(3'-phe...)Show SMILES O=C(CCc1ccccc1-c1cccc(CSCCc2ccccc2)c1)NS(=O)(=O)c1cccs1 Show InChI InChI=1S/C28H27NO3S3/c30-27(29-35(31,32)28-14-7-18-34-28)16-15-24-11-4-5-13-26(24)25-12-6-10-23(20-25)21-33-19-17-22-8-2-1-3-9-22/h1-14,18,20H,15-17,19,21H2,(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity at human Prostanoid EP3 receptor. |
Bioorg Med Chem Lett 12: 2583-6 (2002)
BindingDB Entry DOI: 10.7270/Q2QN663S |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50340424
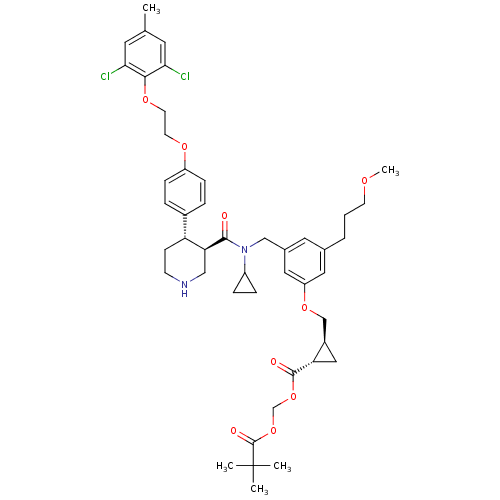
((1S,2S)-pivaloyloxymethyl 2-((3-(((3R,4S)-N-cyclop...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OC[C@H]2C[C@@H]2C(=O)OCOC(=O)C(C)(C)C)c1 |r| Show InChI InChI=1S/C46H58Cl2N2O9/c1-29-19-40(47)42(41(48)20-29)56-18-17-55-35-12-8-32(9-13-35)37-14-15-49-25-39(37)43(51)50(34-10-11-34)26-31-21-30(7-6-16-54-5)22-36(23-31)57-27-33-24-38(33)44(52)58-28-59-45(53)46(2,3)4/h8-9,12-13,19-23,33-34,37-39,49H,6-7,10-11,14-18,24-28H2,1-5H3/t33-,37-,38+,39+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50117699

(CHEMBL83518 | Thiophene-2-sulfonic acid [3-(4'-phe...)Show SMILES O=C(CCc1ccccc1-c1ccc(CSCCc2ccccc2)cc1)NS(=O)(=O)c1cccs1 Show InChI InChI=1S/C28H27NO3S3/c30-27(29-35(31,32)28-11-6-19-34-28)17-16-24-9-4-5-10-26(24)25-14-12-23(13-15-25)21-33-20-18-22-7-2-1-3-8-22/h1-15,19H,16-18,20-21H2,(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity at human Prostanoid EP3 receptor. |
Bioorg Med Chem Lett 12: 2583-6 (2002)
BindingDB Entry DOI: 10.7270/Q2QN663S |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50039916

(4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...)Show SMILES CCCCc1nn(-c2ccccc2C(F)(F)F)c(=O)n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)c1cccs1 Show InChI InChI=1S/C31H27F3N4O4S2/c1-2-3-14-28-35-38(25-11-6-5-10-24(25)31(32,33)34)30(40)37(28)20-21-15-17-22(18-16-21)23-9-4-7-13-27(23)44(41,42)36-29(39)26-12-8-19-43-26/h4-13,15-19H,2-3,14,20H2,1H3,(H,36,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity at human Prostanoid EP4 receptor. |
Bioorg Med Chem Lett 12: 2583-6 (2002)
BindingDB Entry DOI: 10.7270/Q2QN663S |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50081436

(4'-(6-Oxo-11,12-dihydro-6H-dibenzo[b,f]azocin-5-yl...)Show SMILES O=C(CCc1ccccc1)NS(=O)(=O)c1ccccc1-c1ccc(CN2c3ccccc3CCc3ccccc3C2=O)cc1 Show InChI InChI=1S/C37H32N2O4S/c40-36(25-20-27-10-2-1-3-11-27)38-44(42,43)35-17-9-7-14-32(35)30-21-18-28(19-22-30)26-39-34-16-8-5-13-31(34)24-23-29-12-4-6-15-33(29)37(39)41/h1-19,21-22H,20,23-26H2,(H,38,40) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Affinity at human EP1 receptor. |
Bioorg Med Chem Lett 9: 2699-704 (1999)
BindingDB Entry DOI: 10.7270/Q2HX1BV9 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50081438
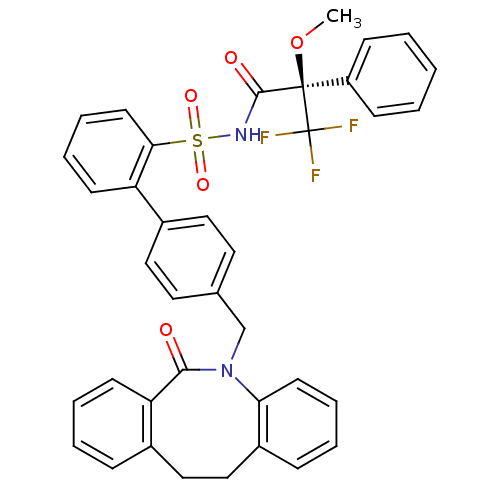
(4'-(6-Oxo-11,12-dihydro-6H-dibenzo[b,f]azocin-5-yl...)Show SMILES CO[C@@](C(=O)NS(=O)(=O)c1ccccc1-c1ccc(CN2c3ccccc3CCc3ccccc3C2=O)cc1)(c1ccccc1)C(F)(F)F Show InChI InChI=1S/C38H31F3N2O5S/c1-48-37(38(39,40)41,30-13-3-2-4-14-30)36(45)42-49(46,47)34-18-10-8-15-31(34)28-21-19-26(20-22-28)25-43-33-17-9-6-12-29(33)24-23-27-11-5-7-16-32(27)35(43)44/h2-22H,23-25H2,1H3,(H,42,45)/t37-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Affinity at human EP1 receptor. |
Bioorg Med Chem Lett 9: 2699-704 (1999)
BindingDB Entry DOI: 10.7270/Q2HX1BV9 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50117695

(CHEMBL87263 | Thiophene-2-sulfonic acid {3-[4'-(3-...)Show SMILES Cc1cccc(OCCCOc2ccc(cc2)-c2ccccc2CCC(=O)NS(=O)(=O)c2cccs2)c1 Show InChI InChI=1S/C29H29NO5S2/c1-22-7-4-9-26(21-22)35-19-6-18-34-25-15-12-24(13-16-25)27-10-3-2-8-23(27)14-17-28(31)30-37(32,33)29-11-5-20-36-29/h2-5,7-13,15-16,20-21H,6,14,17-19H2,1H3,(H,30,31) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity at human Prostanoid EP3 receptor. |
Bioorg Med Chem Lett 12: 2583-6 (2002)
BindingDB Entry DOI: 10.7270/Q2QN663S |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50081452
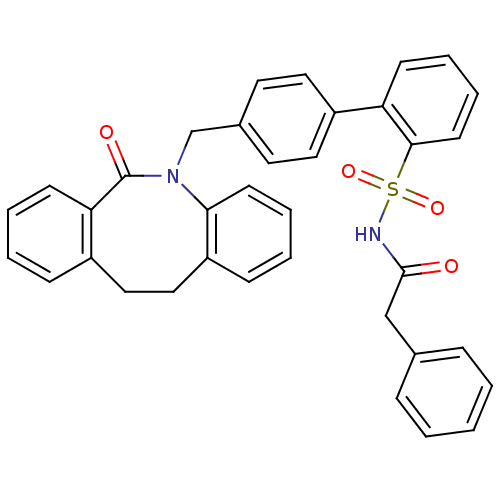
(4'-(6-Oxo-11,12-dihydro-6H-dibenzo[b,f]azocin-5-yl...)Show SMILES O=C(Cc1ccccc1)NS(=O)(=O)c1ccccc1-c1ccc(CN2c3ccccc3CCc3ccccc3C2=O)cc1 Show InChI InChI=1S/C36H30N2O4S/c39-35(24-26-10-2-1-3-11-26)37-43(41,42)34-17-9-7-14-31(34)29-20-18-27(19-21-29)25-38-33-16-8-5-13-30(33)23-22-28-12-4-6-15-32(28)36(38)40/h1-21H,22-25H2,(H,37,39) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Affinity at human EP1 receptor. |
Bioorg Med Chem Lett 9: 2699-704 (1999)
BindingDB Entry DOI: 10.7270/Q2HX1BV9 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50340406
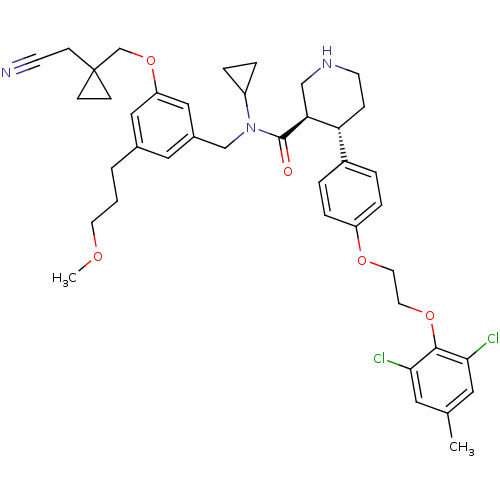
((3R,4S)-N-(3-((1-(cyanomethyl)cyclopropyl)methoxy)...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OCC2(CC#N)CC2)c1 |r| Show InChI InChI=1S/C41H49Cl2N3O5/c1-28-20-37(42)39(38(43)21-28)50-19-18-49-33-9-5-31(6-10-33)35-11-16-45-25-36(35)40(47)46(32-7-8-32)26-30-22-29(4-3-17-48-2)23-34(24-30)51-27-41(12-13-41)14-15-44/h5-6,9-10,20-24,32,35-36,45H,3-4,7-8,11-14,16-19,25-27H2,1-2H3/t35-,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50403949

(CHEMBL2112332)Show SMILES C(Cc1ccccc1)SCc1ccc(cc1)-c1ccccc1CCc1nnn[nH]1 Show InChI InChI=1S/C24H24N4S/c1-2-6-19(7-3-1)16-17-29-18-20-10-12-22(13-11-20)23-9-5-4-8-21(23)14-15-24-25-27-28-26-24/h1-13H,14-18H2,(H,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity at human Prostanoid EP2 receptor. |
Bioorg Med Chem Lett 12: 2583-6 (2002)
BindingDB Entry DOI: 10.7270/Q2QN663S |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50340407
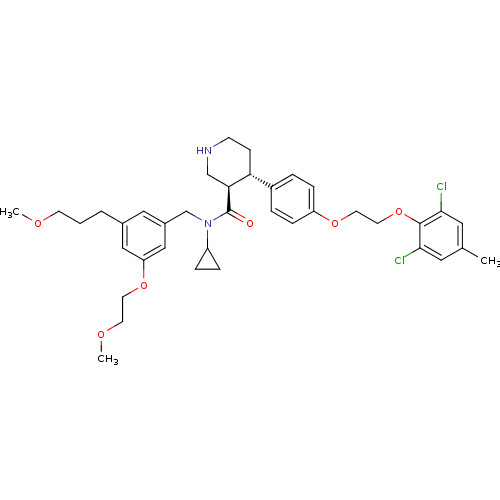
((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro-4-meth...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OCCOC)c1 |r| Show InChI InChI=1S/C38H48Cl2N2O6/c1-26-19-35(39)37(36(40)20-26)48-18-17-46-31-10-6-29(7-11-31)33-12-13-41-24-34(33)38(43)42(30-8-9-30)25-28-21-27(5-4-14-44-2)22-32(23-28)47-16-15-45-3/h6-7,10-11,19-23,30,33-34,41H,4-5,8-9,12-18,24-25H2,1-3H3/t33-,34+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50340409
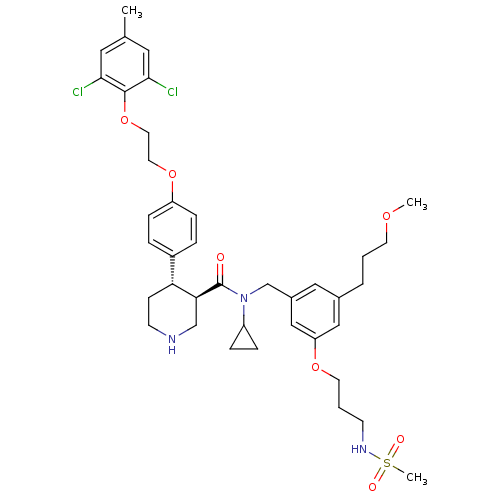
((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro-4-meth...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OCCCNS(C)(=O)=O)c1 |r| Show InChI InChI=1S/C39H51Cl2N3O7S/c1-27-20-36(40)38(37(41)21-27)51-19-18-50-32-11-7-30(8-12-32)34-13-15-42-25-35(34)39(45)44(31-9-10-31)26-29-22-28(6-4-16-48-2)23-33(24-29)49-17-5-14-43-52(3,46)47/h7-8,11-12,20-24,31,34-35,42-43H,4-6,9-10,13-19,25-26H2,1-3H3/t34-,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50340412
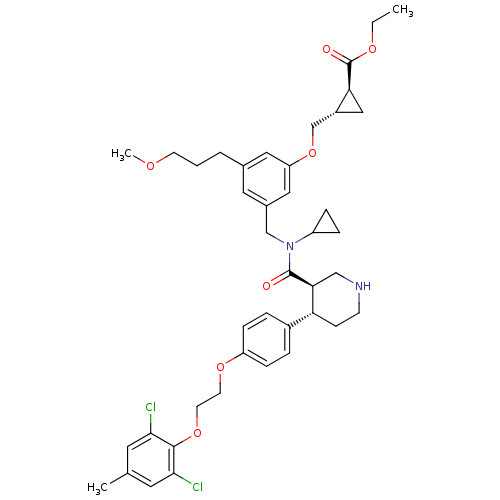
((1S,2S)-ethyl 2-((3-(((3R,4S)-N-cyclopropyl-4-(4-(...)Show SMILES CCOC(=O)[C@H]1C[C@@H]1COc1cc(CCCOC)cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)c1 |r| Show InChI InChI=1S/C42H52Cl2N2O7/c1-4-50-42(48)36-23-31(36)26-53-34-21-28(6-5-15-49-3)20-29(22-34)25-46(32-9-10-32)41(47)37-24-45-14-13-35(37)30-7-11-33(12-8-30)51-16-17-52-40-38(43)18-27(2)19-39(40)44/h7-8,11-12,18-22,31-32,35-37,45H,4-6,9-10,13-17,23-26H2,1-3H3/t31-,35-,36+,37+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50117697
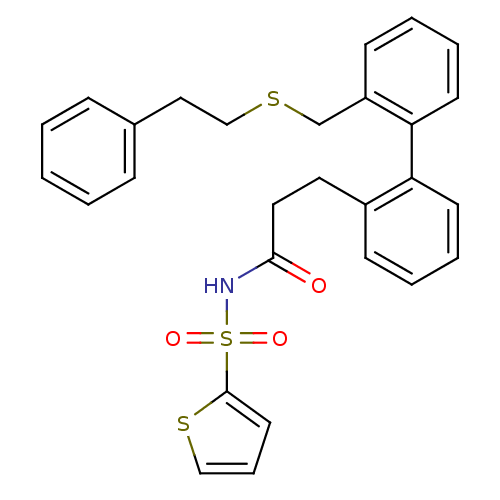
(CHEMBL431612 | Thiophene-2-sulfonic acid [3-(2'-ph...)Show SMILES O=C(CCc1ccccc1-c1ccccc1CSCCc1ccccc1)NS(=O)(=O)c1cccs1 Show InChI InChI=1S/C28H27NO3S3/c30-27(29-35(31,32)28-15-8-19-34-28)17-16-23-11-4-6-13-25(23)26-14-7-5-12-24(26)21-33-20-18-22-9-2-1-3-10-22/h1-15,19H,16-18,20-21H2,(H,29,30) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity at human Prostanoid EP1 receptor. |
Bioorg Med Chem Lett 12: 2583-6 (2002)
BindingDB Entry DOI: 10.7270/Q2QN663S |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50081435
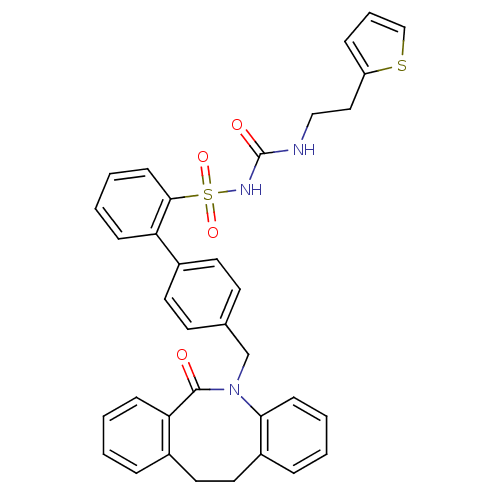
(CHEMBL328697 | sulfonylurea analogue)Show SMILES O=C(NCCc1cccs1)NS(=O)(=O)c1ccccc1-c1ccc(CN2c3ccccc3CCc3ccccc3C2=O)cc1 Show InChI InChI=1S/C35H31N3O4S2/c39-34-31-12-3-1-8-26(31)19-20-28-9-2-5-13-32(28)38(34)24-25-15-17-27(18-16-25)30-11-4-6-14-33(30)44(41,42)37-35(40)36-22-21-29-10-7-23-43-29/h1-18,23H,19-22,24H2,(H2,36,37,40) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Affinity at human EP1 receptor. |
Bioorg Med Chem Lett 9: 2699-704 (1999)
BindingDB Entry DOI: 10.7270/Q2HX1BV9 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50117683

(CHEMBL314616 | Thiophene-2-sulfonic acid ((E)-3-{3...)Show SMILES Clc1ccc2ccc(\C=C\c3cccc(c3)-c3ccccc3\C=C\C(=O)NS(=O)(=O)c3cccs3)nc2c1 Show InChI InChI=1S/C30H21ClN2O3S2/c31-25-14-11-23-12-16-26(32-28(23)20-25)15-10-21-5-3-7-24(19-21)27-8-2-1-6-22(27)13-17-29(34)33-38(35,36)30-9-4-18-37-30/h1-20H,(H,33,34)/b15-10+,17-13+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity at human Prostanoid EP3 receptor. |
Bioorg Med Chem Lett 12: 2583-6 (2002)
BindingDB Entry DOI: 10.7270/Q2QN663S |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50117684

((4'-(3-[3-methylphenyl]-propoxy)-1,1'-biphenyl-2-y...)Show SMILES Cc1cccc(OCCCOc2ccc(cc2)-c2ccccc2COC(=O)NS(=O)(=O)c2cccs2)c1 Show InChI InChI=1S/C28H27NO6S2/c1-21-7-4-9-25(19-21)34-17-6-16-33-24-14-12-22(13-15-24)26-10-3-2-8-23(26)20-35-28(30)29-37(31,32)27-11-5-18-36-27/h2-5,7-15,18-19H,6,16-17,20H2,1H3,(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity at human Prostanoid EP3 receptor. |
Bioorg Med Chem Lett 12: 2583-6 (2002)
BindingDB Entry DOI: 10.7270/Q2QN663S |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50081450

(CHEMBL90269 | sulfonylurea analogue)Show SMILES O=C(NCCc1ccccc1)NS(=O)(=O)c1ccccc1-c1ccc(CN2c3ccccc3CCc3ccccc3C2=O)cc1 Show InChI InChI=1S/C37H33N3O4S/c41-36-33-15-6-4-12-29(33)22-23-31-13-5-8-16-34(31)40(36)26-28-18-20-30(21-19-28)32-14-7-9-17-35(32)45(43,44)39-37(42)38-25-24-27-10-2-1-3-11-27/h1-21H,22-26H2,(H2,38,39,42) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Affinity at human EP1 receptor. |
Bioorg Med Chem Lett 9: 2699-704 (1999)
BindingDB Entry DOI: 10.7270/Q2HX1BV9 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50117694

(CHEMBL83450 | Thiophene-2-sulfonic acid (3-{3'-[(E...)Show SMILES Clc1ccc2ccc(\C=C\c3cccc(c3)-c3ccccc3CCC(=O)NS(=O)(=O)c3cccs3)nc2c1 Show InChI InChI=1S/C30H23ClN2O3S2/c31-25-14-11-23-12-16-26(32-28(23)20-25)15-10-21-5-3-7-24(19-21)27-8-2-1-6-22(27)13-17-29(34)33-38(35,36)30-9-4-18-37-30/h1-12,14-16,18-20H,13,17H2,(H,33,34)/b15-10+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity at human Prostanoid EP3 receptor. |
Bioorg Med Chem Lett 12: 2583-6 (2002)
BindingDB Entry DOI: 10.7270/Q2QN663S |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50340411
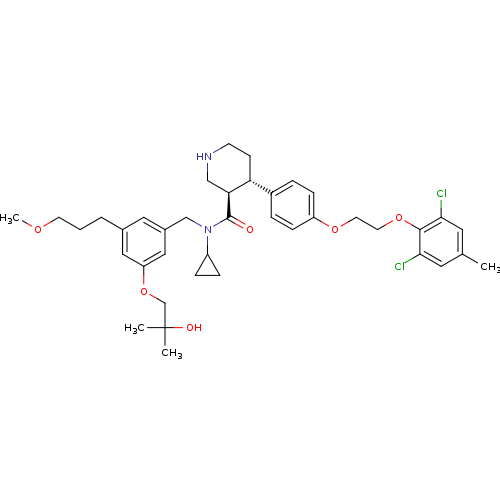
((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro-4-meth...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OCC(C)(C)O)c1 |r| Show InChI InChI=1S/C39H50Cl2N2O6/c1-26-18-35(40)37(36(41)19-26)48-17-16-47-31-11-7-29(8-12-31)33-13-14-42-23-34(33)38(44)43(30-9-10-30)24-28-20-27(6-5-15-46-4)21-32(22-28)49-25-39(2,3)45/h7-8,11-12,18-22,30,33-34,42,45H,5-6,9-10,13-17,23-25H2,1-4H3/t33-,34+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50117681

(3-(4'-Phenethylsulfanylmethyl-biphenyl-2-yl)-propi...)Show InChI InChI=1S/C24H24O2S/c25-24(26)15-14-21-8-4-5-9-23(21)22-12-10-20(11-13-22)18-27-17-16-19-6-2-1-3-7-19/h1-13H,14-18H2,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity at human Prostanoid EP2 receptor. |
Bioorg Med Chem Lett 12: 2583-6 (2002)
BindingDB Entry DOI: 10.7270/Q2QN663S |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50081448
(4'-(6-Oxo-11,12-dihydro-6H-dibenzo[b,f]azocin-5-yl...)Show SMILES O=C(COc1ccccc1)NS(=O)(=O)c1ccccc1-c1ccc(CN2c3ccccc3CCc3ccccc3C2=O)cc1 Show InChI InChI=1S/C36H30N2O5S/c39-35(25-43-30-12-2-1-3-13-30)37-44(41,42)34-17-9-7-14-31(34)28-20-18-26(19-21-28)24-38-33-16-8-5-11-29(33)23-22-27-10-4-6-15-32(27)36(38)40/h1-21H,22-25H2,(H,37,39) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Affinity at human EP1 receptor. |
Bioorg Med Chem Lett 9: 2699-704 (1999)
BindingDB Entry DOI: 10.7270/Q2HX1BV9 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50081434
(CHEMBL327597 | sulfonylurea analogue)Show SMILES C[C@H](NC(=O)NS(=O)(=O)c1ccccc1-c1ccc(CN2c3ccccc3CCc3ccccc3C2=O)cc1)c1ccccc1 Show InChI InChI=1S/C37H33N3O4S/c1-26(28-11-3-2-4-12-28)38-37(42)39-45(43,44)35-18-10-8-15-32(35)30-21-19-27(20-22-30)25-40-34-17-9-6-14-31(34)24-23-29-13-5-7-16-33(29)36(40)41/h2-22,26H,23-25H2,1H3,(H2,38,39,42)/t26-/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Affinity at human EP1 receptor. |
Bioorg Med Chem Lett 9: 2699-704 (1999)
BindingDB Entry DOI: 10.7270/Q2HX1BV9 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50117698
(CHEMBL87797 | N-({[(4'-(3-[3-methylphenyl]-propoxy...)Show SMILES Cc1cccc(OCCCOc2ccc(cc2)-c2ccccc2CNC(=O)NS(=O)(=O)c2cccs2)c1 Show InChI InChI=1S/C28H28N2O5S2/c1-21-7-4-9-25(19-21)35-17-6-16-34-24-14-12-22(13-15-24)26-10-3-2-8-23(26)20-29-28(31)30-37(32,33)27-11-5-18-36-27/h2-5,7-15,18-19H,6,16-17,20H2,1H3,(H2,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity at human Prostanoid EP3 receptor. |
Bioorg Med Chem Lett 12: 2583-6 (2002)
BindingDB Entry DOI: 10.7270/Q2QN663S |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50081447
(CHEMBL91063 | sulfonylurea analogue)Show SMILES O=C(NS(=O)(=O)c1ccccc1-c1ccc(CN2c3ccccc3CCc3ccccc3C2=O)cc1)OCc1ccccc1 Show InChI InChI=1S/C36H30N2O5S/c39-35-32-15-6-4-12-28(32)22-23-30-13-5-8-16-33(30)38(35)24-26-18-20-29(21-19-26)31-14-7-9-17-34(31)44(41,42)37-36(40)43-25-27-10-2-1-3-11-27/h1-21H,22-25H2,(H,37,40) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Affinity at human EP1 receptor. |
Bioorg Med Chem Lett 9: 2699-704 (1999)
BindingDB Entry DOI: 10.7270/Q2HX1BV9 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50340410
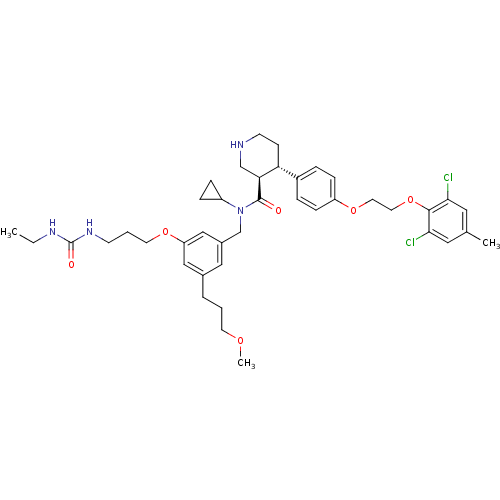
((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro-4-meth...)Show SMILES CCNC(=O)NCCCOc1cc(CCCOC)cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)c1 |r| Show InChI InChI=1S/C41H54Cl2N4O6/c1-4-45-41(49)46-15-6-18-51-34-24-29(7-5-17-50-3)23-30(25-34)27-47(32-10-11-32)40(48)36-26-44-16-14-35(36)31-8-12-33(13-9-31)52-19-20-53-39-37(42)21-28(2)22-38(39)43/h8-9,12-13,21-25,32,35-36,44H,4-7,10-11,14-20,26-27H2,1-3H3,(H2,45,46,49)/t35-,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50355516
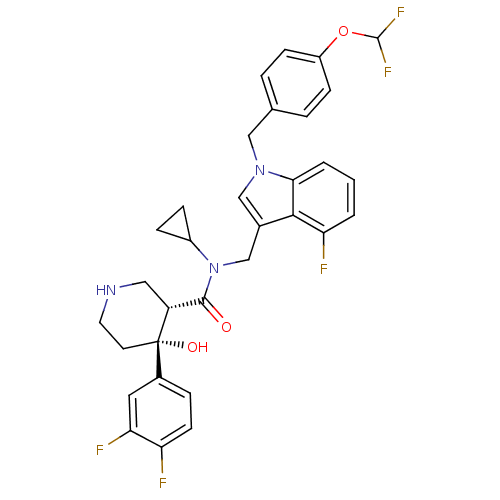
(CHEMBL1910318)Show SMILES O[C@@]1(CCNC[C@@H]1C(=O)N(Cc1cn(Cc2ccc(OC(F)F)cc2)c2cccc(F)c12)C1CC1)c1ccc(F)c(F)c1 |r| Show InChI InChI=1S/C32H30F5N3O3/c33-25-11-6-21(14-27(25)35)32(42)12-13-38-15-24(32)30(41)40(22-7-8-22)18-20-17-39(28-3-1-2-26(34)29(20)28)16-19-4-9-23(10-5-19)43-31(36)37/h1-6,9-11,14,17,22,24,31,38,42H,7-8,12-13,15-16,18H2/t24-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 3976-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.014
BindingDB Entry DOI: 10.7270/Q21N81HN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50340408
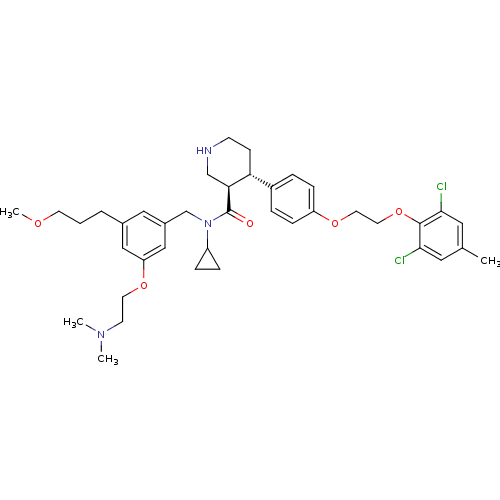
((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro-4-meth...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OCCN(C)C)c1 |r| Show InChI InChI=1S/C39H51Cl2N3O5/c1-27-20-36(40)38(37(41)21-27)49-19-18-48-32-11-7-30(8-12-32)34-13-14-42-25-35(34)39(45)44(31-9-10-31)26-29-22-28(6-5-16-46-4)23-33(24-29)47-17-15-43(2)3/h7-8,11-12,20-24,31,34-35,42H,5-6,9-10,13-19,25-26H2,1-4H3/t34-,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50117686
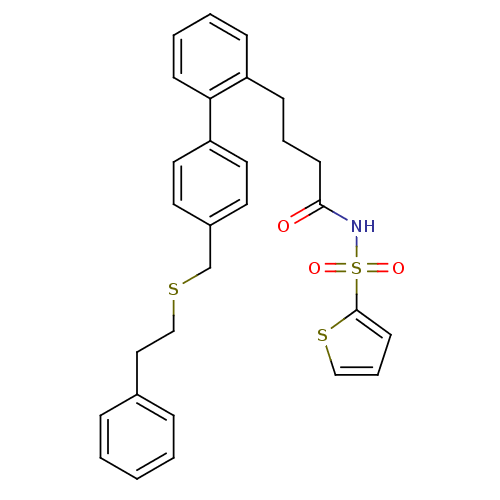
(CHEMBL445895 | Thiophene-2-sulfonic acid [4-(4'-ph...)Show SMILES O=C(CCCc1ccccc1-c1ccc(CSCCc2ccccc2)cc1)NS(=O)(=O)c1cccs1 Show InChI InChI=1S/C29H29NO3S3/c31-28(30-36(32,33)29-14-7-20-35-29)13-6-11-25-10-4-5-12-27(25)26-17-15-24(16-18-26)22-34-21-19-23-8-2-1-3-9-23/h1-5,7-10,12,14-18,20H,6,11,13,19,21-22H2,(H,30,31) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity at human Prostanoid EP3 receptor. |
Bioorg Med Chem Lett 12: 2583-6 (2002)
BindingDB Entry DOI: 10.7270/Q2QN663S |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50340423
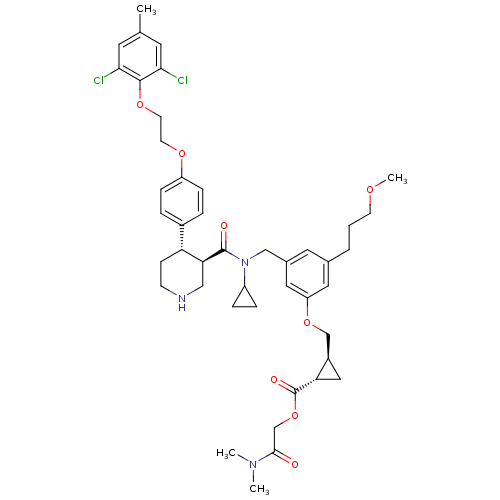
((1S,2S)-2-(dimethylamino)-2-oxoethyl 2-((3-(((3R,4...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OC[C@H]2C[C@@H]2C(=O)OCC(=O)N(C)C)c1 |r| Show InChI InChI=1S/C44H55Cl2N3O8/c1-28-18-39(45)42(40(46)19-28)55-17-16-54-34-11-7-31(8-12-34)36-13-14-47-24-38(36)43(51)49(33-9-10-33)25-30-20-29(6-5-15-53-4)21-35(22-30)56-26-32-23-37(32)44(52)57-27-41(50)48(2)3/h7-8,11-12,18-22,32-33,36-38,47H,5-6,9-10,13-17,23-27H2,1-4H3/t32-,36-,37+,38+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50081442
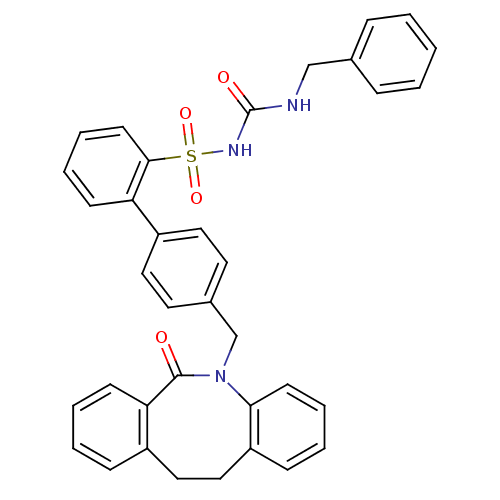
(CHEMBL90226 | sulfonylurea analogue)Show SMILES O=C(NCc1ccccc1)NS(=O)(=O)c1ccccc1-c1ccc(CN2c3ccccc3CCc3ccccc3C2=O)cc1 Show InChI InChI=1S/C36H31N3O4S/c40-35-32-15-6-4-12-28(32)22-23-30-13-5-8-16-33(30)39(35)25-27-18-20-29(21-19-27)31-14-7-9-17-34(31)44(42,43)38-36(41)37-24-26-10-2-1-3-11-26/h1-21H,22-25H2,(H2,37,38,41) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Affinity at human EP1 receptor. |
Bioorg Med Chem Lett 9: 2699-704 (1999)
BindingDB Entry DOI: 10.7270/Q2HX1BV9 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50340417
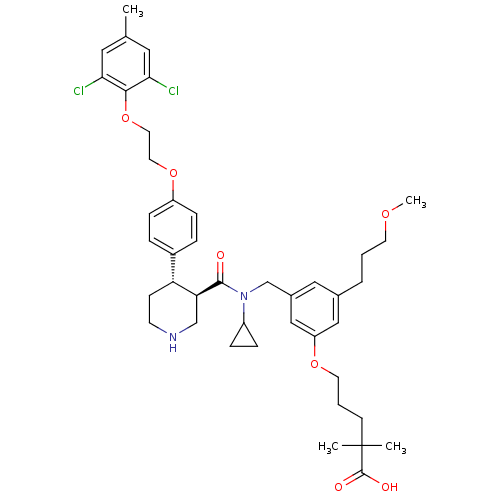
(5-(3-(((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OCCCC(C)(C)C(O)=O)c1 |r| Show InChI InChI=1S/C42H54Cl2N2O7/c1-28-21-37(43)39(38(44)22-28)53-20-19-52-33-12-8-31(9-13-33)35-14-16-45-26-36(35)40(47)46(32-10-11-32)27-30-23-29(7-5-17-50-4)24-34(25-30)51-18-6-15-42(2,3)41(48)49/h8-9,12-13,21-25,32,35-36,45H,5-7,10-11,14-20,26-27H2,1-4H3,(H,48,49)/t35-,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50081446

(CHEMBL91270 | sulfonylurea analogue)Show SMILES CCCCNC(=O)NS(=O)(=O)c1ccccc1-c1ccc(CN2c3ccccc3CCc3ccccc3C2=O)cc1 Show InChI InChI=1S/C33H33N3O4S/c1-2-3-22-34-33(38)35-41(39,40)31-15-9-7-12-28(31)26-18-16-24(17-19-26)23-36-30-14-8-5-11-27(30)21-20-25-10-4-6-13-29(25)32(36)37/h4-19H,2-3,20-23H2,1H3,(H2,34,35,38) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Affinity at human EP1 receptor. |
Bioorg Med Chem Lett 9: 2699-704 (1999)
BindingDB Entry DOI: 10.7270/Q2HX1BV9 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50117696
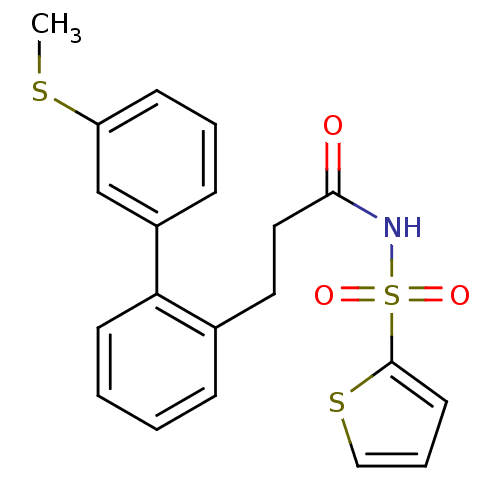
(CHEMBL315974 | Thiophene-2-sulfonic acid [3-(3'-me...)Show SMILES CSc1cccc(c1)-c1ccccc1CCC(=O)NS(=O)(=O)c1cccs1 Show InChI InChI=1S/C20H19NO3S3/c1-25-17-8-4-7-16(14-17)18-9-3-2-6-15(18)11-12-19(22)21-27(23,24)20-10-5-13-26-20/h2-10,13-14H,11-12H2,1H3,(H,21,22) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity at human Prostanoid EP3 receptor. |
Bioorg Med Chem Lett 12: 2583-6 (2002)
BindingDB Entry DOI: 10.7270/Q2QN663S |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50355514
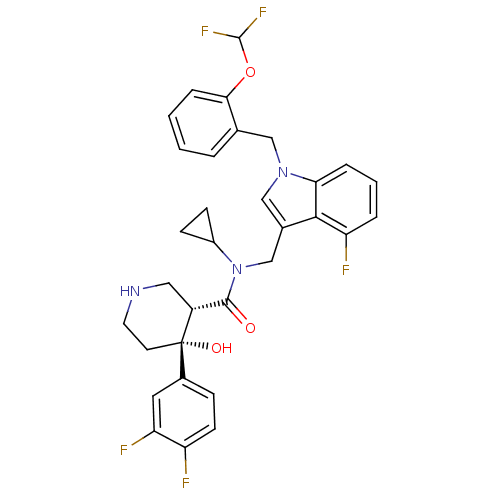
(CHEMBL1910316)Show SMILES O[C@@]1(CCNC[C@@H]1C(=O)N(Cc1cn(Cc2ccccc2OC(F)F)c2cccc(F)c12)C1CC1)c1ccc(F)c(F)c1 |r| Show InChI InChI=1S/C32H30F5N3O3/c33-24-11-8-21(14-26(24)35)32(42)12-13-38-15-23(32)30(41)40(22-9-10-22)18-20-17-39(27-6-3-5-25(34)29(20)27)16-19-4-1-2-7-28(19)43-31(36)37/h1-8,11,14,17,22-23,31,38,42H,9-10,12-13,15-16,18H2/t23-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 3976-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.014
BindingDB Entry DOI: 10.7270/Q21N81HN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50355517
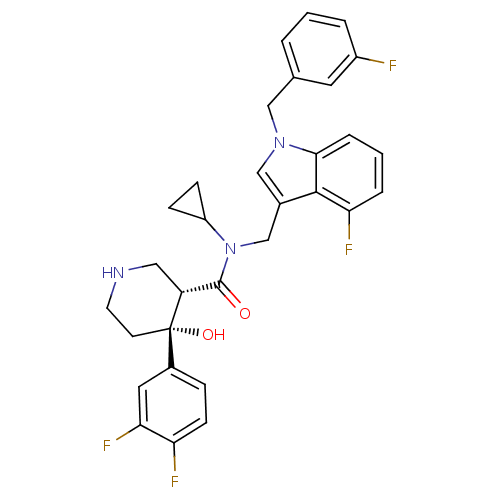
(CHEMBL1910319)Show SMILES O[C@@]1(CCNC[C@@H]1C(=O)N(Cc1cn(Cc2cccc(F)c2)c2cccc(F)c12)C1CC1)c1ccc(F)c(F)c1 |r| Show InChI InChI=1S/C31H29F4N3O2/c32-22-4-1-3-19(13-22)16-37-17-20(29-26(34)5-2-6-28(29)37)18-38(23-8-9-23)30(39)24-15-36-12-11-31(24,40)21-7-10-25(33)27(35)14-21/h1-7,10,13-14,17,23-24,36,40H,8-9,11-12,15-16,18H2/t24-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 3976-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.014
BindingDB Entry DOI: 10.7270/Q21N81HN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50355513
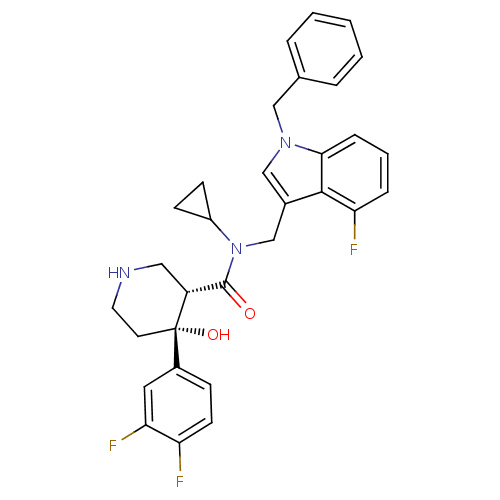
(CHEMBL1910315)Show SMILES O[C@@]1(CCNC[C@@H]1C(=O)N(Cc1cn(Cc2ccccc2)c2cccc(F)c12)C1CC1)c1ccc(F)c(F)c1 |r| Show InChI InChI=1S/C31H30F3N3O2/c32-25-12-9-22(15-27(25)34)31(39)13-14-35-16-24(31)30(38)37(23-10-11-23)19-21-18-36(17-20-5-2-1-3-6-20)28-8-4-7-26(33)29(21)28/h1-9,12,15,18,23-24,35,39H,10-11,13-14,16-17,19H2/t24-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 3976-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.014
BindingDB Entry DOI: 10.7270/Q21N81HN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50355518
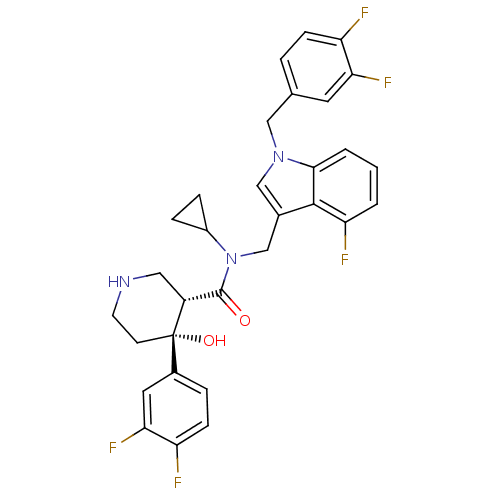
(CHEMBL1910320)Show SMILES O[C@@]1(CCNC[C@@H]1C(=O)N(Cc1cn(Cc2ccc(F)c(F)c2)c2cccc(F)c12)C1CC1)c1ccc(F)c(F)c1 |r| Show InChI InChI=1S/C31H28F5N3O2/c32-23-8-4-18(12-26(23)35)15-38-16-19(29-25(34)2-1-3-28(29)38)17-39(21-6-7-21)30(40)22-14-37-11-10-31(22,41)20-5-9-24(33)27(36)13-20/h1-5,8-9,12-13,16,21-22,37,41H,6-7,10-11,14-15,17H2/t22-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 3976-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.014
BindingDB Entry DOI: 10.7270/Q21N81HN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50355515
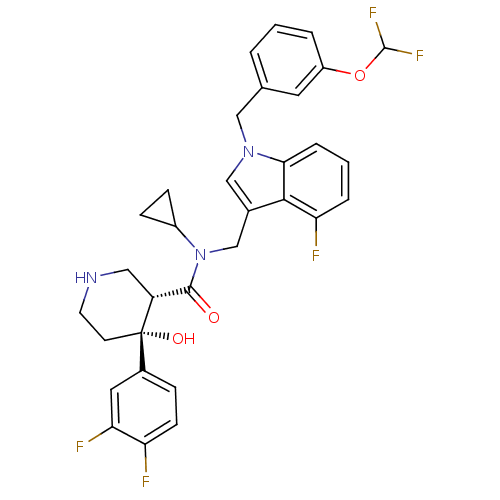
(CHEMBL1910317)Show SMILES O[C@@]1(CCNC[C@@H]1C(=O)N(Cc1cn(Cc2cccc(OC(F)F)c2)c2cccc(F)c12)C1CC1)c1ccc(F)c(F)c1 |r| Show InChI InChI=1S/C32H30F5N3O3/c33-25-10-7-21(14-27(25)35)32(42)11-12-38-15-24(32)30(41)40(22-8-9-22)18-20-17-39(28-6-2-5-26(34)29(20)28)16-19-3-1-4-23(13-19)43-31(36)37/h1-7,10,13-14,17,22,24,31,38,42H,8-9,11-12,15-16,18H2/t24-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 3976-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.014
BindingDB Entry DOI: 10.7270/Q21N81HN |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50117691
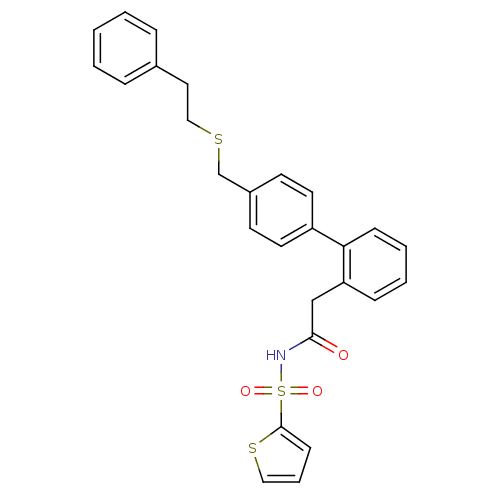
(CHEMBL86886 | Thiophene-2-sulfonic acid [2-(4'-phe...)Show SMILES O=C(Cc1ccccc1-c1ccc(CSCCc2ccccc2)cc1)NS(=O)(=O)c1cccs1 Show InChI InChI=1S/C27H25NO3S3/c29-26(28-34(30,31)27-11-6-17-33-27)19-24-9-4-5-10-25(24)23-14-12-22(13-15-23)20-32-18-16-21-7-2-1-3-8-21/h1-15,17H,16,18-20H2,(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity at human Prostanoid EP3 receptor. |
Bioorg Med Chem Lett 12: 2583-6 (2002)
BindingDB Entry DOI: 10.7270/Q2QN663S |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50117687

(CHEMBL314200 | Thiophene-2-sulfonic acid {2-methyl...)Show SMILES CC(Cc1ccccc1-c1ccc(OCCCOc2cccc(C)c2)cc1)C(=O)NS(=O)(=O)c1cccs1 Show InChI InChI=1S/C30H31NO5S2/c1-22-8-5-10-27(20-22)36-18-7-17-35-26-15-13-24(14-16-26)28-11-4-3-9-25(28)21-23(2)30(32)31-38(33,34)29-12-6-19-37-29/h3-6,8-16,19-20,23H,7,17-18,21H2,1-2H3,(H,31,32) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity at human Prostanoid EP3 receptor. |
Bioorg Med Chem Lett 12: 2583-6 (2002)
BindingDB Entry DOI: 10.7270/Q2QN663S |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50355519

(CHEMBL1910321)Show SMILES O[C@@]1(CCNC[C@@H]1C(=O)N(Cc1cn(Cc2cccnc2)c2cccc(F)c12)C1CC1)c1ccc(F)c(F)c1 |r| Show InChI InChI=1S/C30H29F3N4O2/c31-24-9-6-21(13-26(24)33)30(39)10-12-35-15-23(30)29(38)37(22-7-8-22)18-20-17-36(16-19-3-2-11-34-14-19)27-5-1-4-25(32)28(20)27/h1-6,9,11,13-14,17,22-23,35,39H,7-8,10,12,15-16,18H2/t23-,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 3976-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.014
BindingDB Entry DOI: 10.7270/Q21N81HN |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50081437

(CHEMBL327596 | sulfonylurea analogue)Show SMILES CN(C(=O)NCCc1ccccc1)S(=O)(=O)c1ccccc1-c1ccc(CN2c3ccccc3CCc3ccccc3C2=O)cc1 Show InChI InChI=1S/C38H35N3O4S/c1-40(38(43)39-26-25-28-11-3-2-4-12-28)46(44,45)36-18-10-8-15-33(36)31-21-19-29(20-22-31)27-41-35-17-9-6-14-32(35)24-23-30-13-5-7-16-34(30)37(41)42/h2-22H,23-27H2,1H3,(H,39,43) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Affinity at human EP1 receptor. |
Bioorg Med Chem Lett 9: 2699-704 (1999)
BindingDB Entry DOI: 10.7270/Q2HX1BV9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data