

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1,4-dihydroxy-2-naphthoyl-CoA synthase (Mycobacterium tuberculosis) | BDBM50382338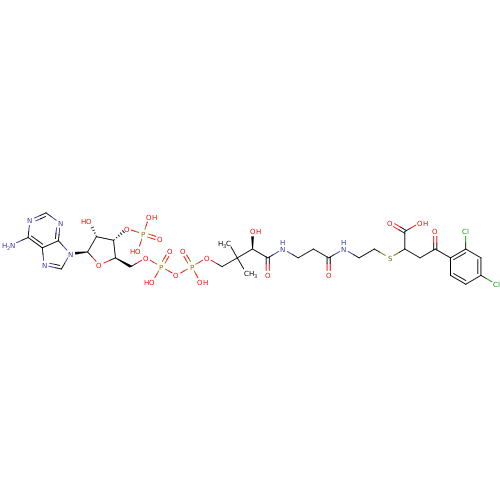 (CHEMBL2024344) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis MenB expressed in Escherichia coli BL21 (DE3) by Lineweaver-Burk plot analysis | ACS Med Chem Lett 2: 818-823 (2011) Article DOI: 10.1021/ml200141e BindingDB Entry DOI: 10.7270/Q2B56KRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,4-dihydroxy-2-naphthoyl-CoA synthase (Mycobacterium tuberculosis) | BDBM50382339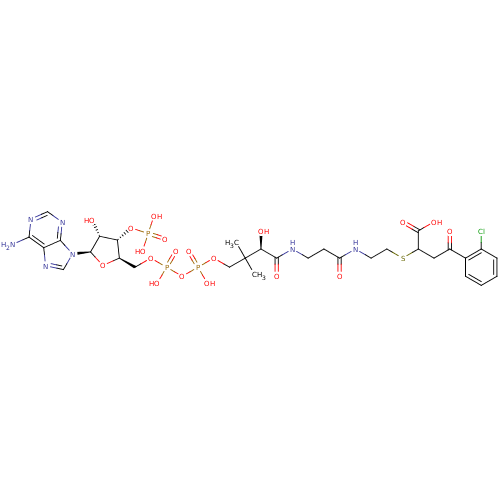 (CHEMBL2024338) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis MenB expressed in Escherichia coli BL21 (DE3) by Lineweaver-Burk plot analysis | ACS Med Chem Lett 2: 818-823 (2011) Article DOI: 10.1021/ml200141e BindingDB Entry DOI: 10.7270/Q2B56KRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] (Yersinia pestis (Enterobacteria)) | BDBM50373349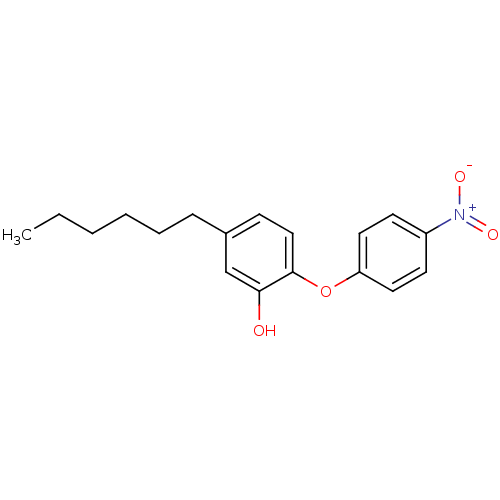 (CHEMBL264417 | PT12) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 100 | -40.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Würzburg | Assay Description Steady-state kinetics were performed on a Cary 100 Bio (Varian) spectrometer at 25 °C using 30 mM PIPES, 150 mM NaCl, and 1.0 mM EDTA (pH 8.0) as... | Biochemistry 55: 2992-3006 (2016) Article DOI: 10.1021/acs.biochem.5b01301 BindingDB Entry DOI: 10.7270/Q25D8QM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] (Yersinia pestis (Enterobacteria)) | BDBM190659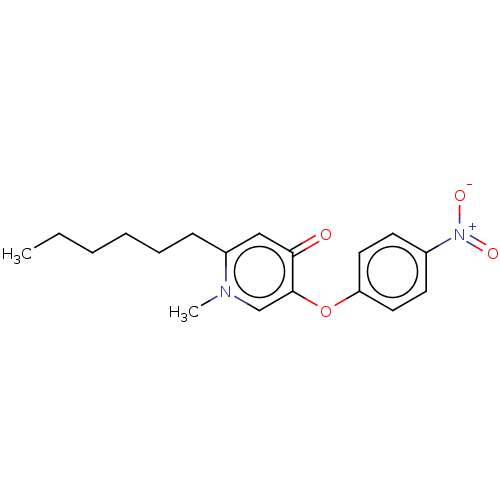 (PT156) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 200 | -38.2 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Würzburg | Assay Description Steady-state kinetics were performed on a Cary 100 Bio (Varian) spectrometer at 25 °C using 30 mM PIPES, 150 mM NaCl, and 1.0 mM EDTA (pH 8.0) as... | Biochemistry 55: 2992-3006 (2016) Article DOI: 10.1021/acs.biochem.5b01301 BindingDB Entry DOI: 10.7270/Q25D8QM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,4-dihydroxy-2-naphthoyl-CoA synthase (Mycobacterium tuberculosis) | BDBM50382338 (CHEMBL2024344) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 286 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Non-competitive inhibition of Mycobacterium tuberculosis MenB expressed in Escherichia coli BL21 (DE3) by Lineweaver-Burk plot analysis | ACS Med Chem Lett 2: 818-823 (2011) Article DOI: 10.1021/ml200141e BindingDB Entry DOI: 10.7270/Q2B56KRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,4-dihydroxy-2-naphthoyl-CoA synthase (Mycobacterium tuberculosis) | BDBM50382337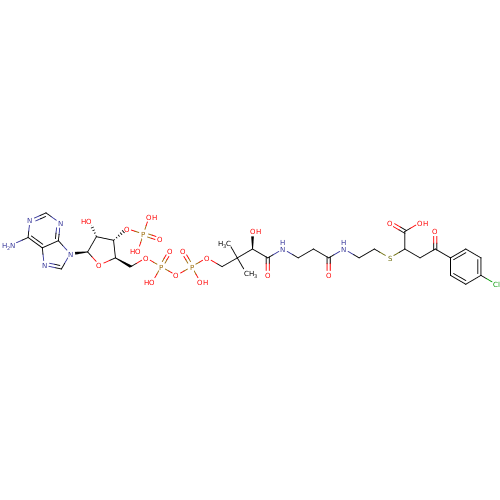 (CHEMBL2024335) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis MenB expressed in Escherichia coli BL21 (DE3) by Lineweaver-Burk plot analysis | ACS Med Chem Lett 2: 818-823 (2011) Article DOI: 10.1021/ml200141e BindingDB Entry DOI: 10.7270/Q2B56KRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 (Mycobacterium tuberculosis) | BDBM93049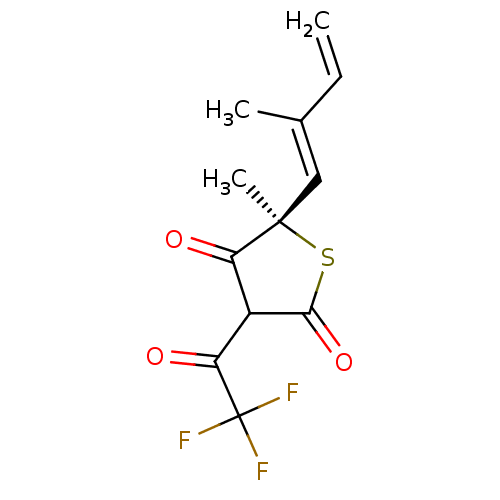 (TLM analog, 6) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Similars | Article PubMed | 460 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
Institute for Chemical Biology & Drug Discovery | Assay Description Binding of TLM and the TLM analogs to KasA was quantified by monitoring changes in the intrinsic tryptophan fluroescence of the enzyme using 280-nm e... | J Biol Chem 288: 6045-52 (2013) Article DOI: 10.1074/jbc.M112.414516 BindingDB Entry DOI: 10.7270/Q2KP80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] [T276S] (Yersinia pestis (Enterobacteria)) | BDBM190659 (PT156) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 500 | -36.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Würzburg | Assay Description Steady-state kinetics were performed on a Cary 100 Bio (Varian) spectrometer at 25 °C using 30 mM PIPES, 150 mM NaCl, and 1.0 mM EDTA (pH 8.0) as... | Biochemistry 55: 2992-3006 (2016) Article DOI: 10.1021/acs.biochem.5b01301 BindingDB Entry DOI: 10.7270/Q25D8QM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,4-dihydroxy-2-naphthoyl-CoA synthase (Mycobacterium tuberculosis) | BDBM50382339 (CHEMBL2024338) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 792 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Non-competitive inhibition of Mycobacterium tuberculosis MenB expressed in Escherichia coli BL21 (DE3) by Lineweaver-Burk plot analysis | ACS Med Chem Lett 2: 818-823 (2011) Article DOI: 10.1021/ml200141e BindingDB Entry DOI: 10.7270/Q2B56KRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 (Mycobacterium tuberculosis) | BDBM93048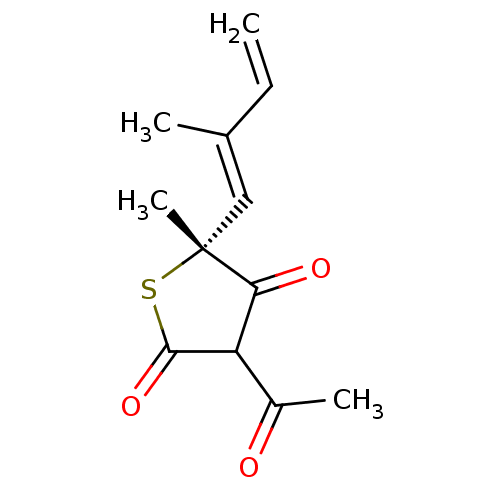 (TLM analog, 5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Similars | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
Institute for Chemical Biology & Drug Discovery | Assay Description Binding of TLM and the TLM analogs to KasA was quantified by monitoring changes in the intrinsic tryptophan fluroescence of the enzyme using 280-nm e... | J Biol Chem 288: 6045-52 (2013) Article DOI: 10.1074/jbc.M112.414516 BindingDB Entry DOI: 10.7270/Q2KP80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,4-dihydroxy-2-naphthoyl-CoA synthase (Mycobacterium tuberculosis) | BDBM50382337 (CHEMBL2024335) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Non-competitive inhibition of Mycobacterium tuberculosis MenB expressed in Escherichia coli BL21 (DE3) by Lineweaver-Burk plot analysis | ACS Med Chem Lett 2: 818-823 (2011) Article DOI: 10.1021/ml200141e BindingDB Entry DOI: 10.7270/Q2B56KRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 (Mycobacterium tuberculosis) | BDBM50241313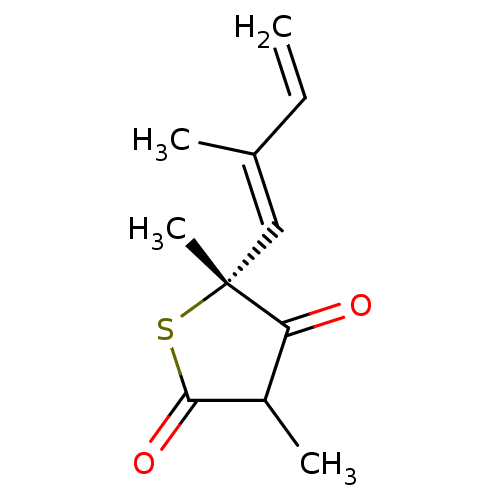 ((5R)-4-hydroxy-3,5-dimethyl-5-[(1E)-2-methylbuta-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
Institute for Chemical Biology & Drug Discovery | Assay Description Binding of TLM and the TLM analogs to KasA was quantified by monitoring changes in the intrinsic tryptophan fluroescence of the enzyme using 280-nm e... | J Biol Chem 288: 6045-52 (2013) Article DOI: 10.1074/jbc.M112.414516 BindingDB Entry DOI: 10.7270/Q2KP80S4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] [T276S] (Yersinia pestis (Enterobacteria)) | BDBM190656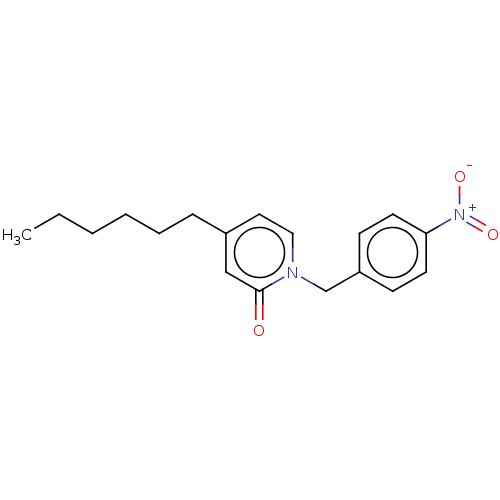 (PT424) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | -31.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Würzburg | Assay Description Steady-state kinetics were performed on a Cary 100 Bio (Varian) spectrometer at 25 °C using 30 mM PIPES, 150 mM NaCl, and 1.0 mM EDTA (pH 8.0) as... | Biochemistry 55: 2992-3006 (2016) Article DOI: 10.1021/acs.biochem.5b01301 BindingDB Entry DOI: 10.7270/Q25D8QM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] [T276S] (Yersinia pestis (Enterobacteria)) | BDBM50373349 (CHEMBL264417 | PT12) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 4.00E+3 | -30.8 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Würzburg | Assay Description Steady-state kinetics were performed on a Cary 100 Bio (Varian) spectrometer at 25 °C using 30 mM PIPES, 150 mM NaCl, and 1.0 mM EDTA (pH 8.0) as... | Biochemistry 55: 2992-3006 (2016) Article DOI: 10.1021/acs.biochem.5b01301 BindingDB Entry DOI: 10.7270/Q25D8QM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 (Mycobacterium tuberculosis) | BDBM93046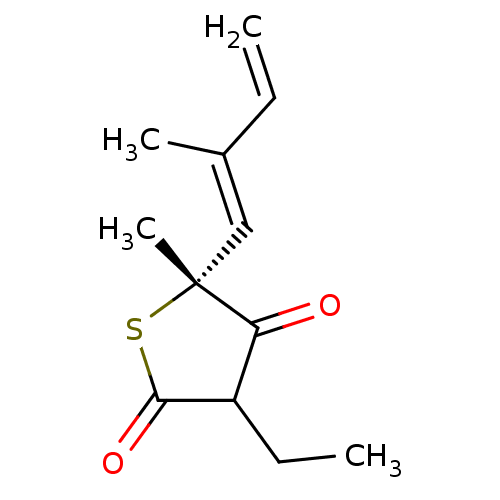 (TLM analog, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Similars | Article PubMed | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
Institute for Chemical Biology & Drug Discovery | Assay Description Binding of TLM and the TLM analogs to KasA was quantified by monitoring changes in the intrinsic tryptophan fluroescence of the enzyme using 280-nm e... | J Biol Chem 288: 6045-52 (2013) Article DOI: 10.1074/jbc.M112.414516 BindingDB Entry DOI: 10.7270/Q2KP80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] (Yersinia pestis (Enterobacteria)) | BDBM190656 (PT424) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.00E+3 | -29.1 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Würzburg | Assay Description Steady-state kinetics were performed on a Cary 100 Bio (Varian) spectrometer at 25 °C using 30 mM PIPES, 150 mM NaCl, and 1.0 mM EDTA (pH 8.0) as... | Biochemistry 55: 2992-3006 (2016) Article DOI: 10.1021/acs.biochem.5b01301 BindingDB Entry DOI: 10.7270/Q25D8QM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] [T276S] (Yersinia pestis (Enterobacteria)) | BDBM50373349 (CHEMBL264417 | PT12) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 8.00E+3 | -29.1 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Würzburg | Assay Description Steady-state kinetics were performed on a Cary 100 Bio (Varian) spectrometer at 25 °C using 30 mM PIPES, 150 mM NaCl, and 1.0 mM EDTA (pH 8.0) as... | Biochemistry 55: 2992-3006 (2016) Article DOI: 10.1021/acs.biochem.5b01301 BindingDB Entry DOI: 10.7270/Q25D8QM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 (Mycobacterium tuberculosis) | BDBM93054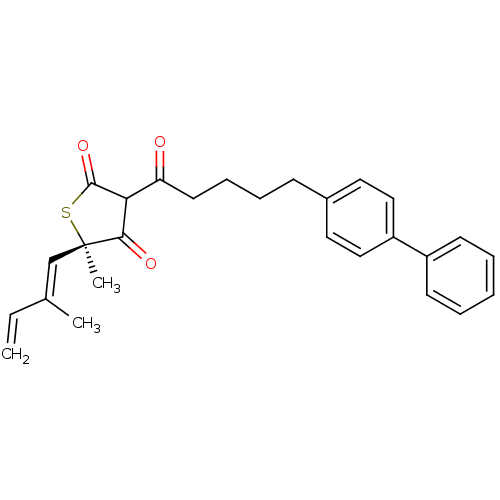 (TLM analog, 11) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
Institute for Chemical Biology & Drug Discovery | Assay Description Binding of TLM and the TLM analogs to KasA was quantified by monitoring changes in the intrinsic tryptophan fluroescence of the enzyme using 280-nm e... | J Biol Chem 288: 6045-52 (2013) Article DOI: 10.1074/jbc.M112.414516 BindingDB Entry DOI: 10.7270/Q2KP80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 (Mycobacterium tuberculosis) | BDBM93048 (TLM analog, 5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Similars | Article PubMed | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
Institute for Chemical Biology & Drug Discovery | Assay Description Binding of TLM and the TLM analogs to KasA was quantified by monitoring changes in the intrinsic tryptophan fluroescence of the enzyme using 280-nm e... | J Biol Chem 288: 6045-52 (2013) Article DOI: 10.1074/jbc.M112.414516 BindingDB Entry DOI: 10.7270/Q2KP80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 (Mycobacterium tuberculosis) | BDBM93049 (TLM analog, 6) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Similars | Article PubMed | 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
Institute for Chemical Biology & Drug Discovery | Assay Description Binding of TLM and the TLM analogs to KasA was quantified by monitoring changes in the intrinsic tryptophan fluroescence of the enzyme using 280-nm e... | J Biol Chem 288: 6045-52 (2013) Article DOI: 10.1074/jbc.M112.414516 BindingDB Entry DOI: 10.7270/Q2KP80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 (Mycobacterium tuberculosis) | BDBM93059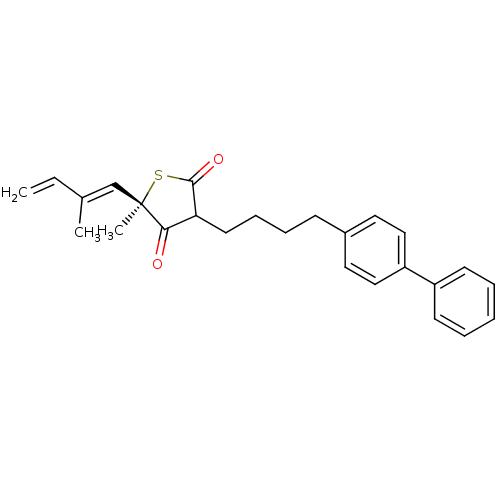 (TLM analog, 16) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
Institute for Chemical Biology & Drug Discovery | Assay Description Binding of TLM and the TLM analogs to KasA was quantified by monitoring changes in the intrinsic tryptophan fluroescence of the enzyme using 280-nm e... | J Biol Chem 288: 6045-52 (2013) Article DOI: 10.1074/jbc.M112.414516 BindingDB Entry DOI: 10.7270/Q2KP80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 (Mycobacterium tuberculosis) | BDBM93047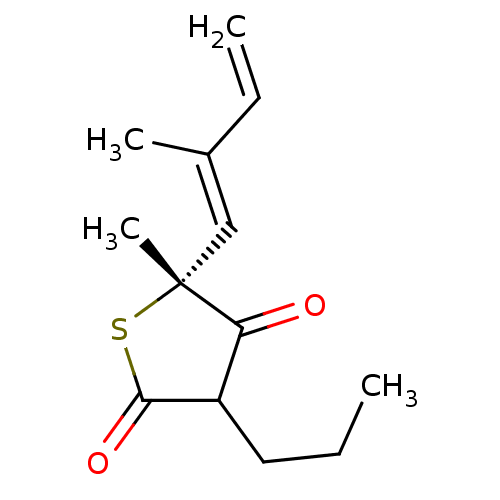 (TLM analog, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Similars | Article PubMed | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
Institute for Chemical Biology & Drug Discovery | Assay Description Binding of TLM and the TLM analogs to KasA was quantified by monitoring changes in the intrinsic tryptophan fluroescence of the enzyme using 280-nm e... | J Biol Chem 288: 6045-52 (2013) Article DOI: 10.1074/jbc.M112.414516 BindingDB Entry DOI: 10.7270/Q2KP80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] (Yersinia pestis (Enterobacteria)) | BDBM190656 (PT424) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.60E+4 | -27.4 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Würzburg | Assay Description Steady-state kinetics were performed on a Cary 100 Bio (Varian) spectrometer at 25 °C using 30 mM PIPES, 150 mM NaCl, and 1.0 mM EDTA (pH 8.0) as... | Biochemistry 55: 2992-3006 (2016) Article DOI: 10.1021/acs.biochem.5b01301 BindingDB Entry DOI: 10.7270/Q25D8QM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 (Mycobacterium tuberculosis) | BDBM93051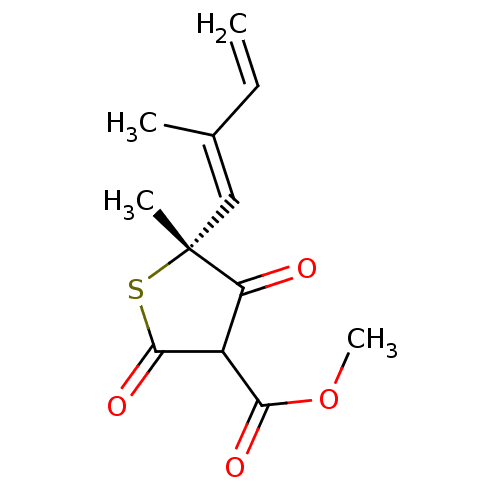 (TLM analog, 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
Institute for Chemical Biology & Drug Discovery | Assay Description Binding of TLM and the TLM analogs to KasA was quantified by monitoring changes in the intrinsic tryptophan fluroescence of the enzyme using 280-nm e... | J Biol Chem 288: 6045-52 (2013) Article DOI: 10.1074/jbc.M112.414516 BindingDB Entry DOI: 10.7270/Q2KP80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 (Mycobacterium tuberculosis) | BDBM93060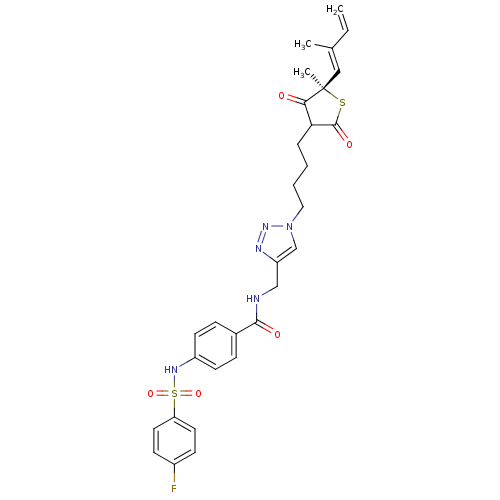 (TLM analog, 17) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
Institute for Chemical Biology & Drug Discovery | Assay Description Binding of TLM and the TLM analogs to KasA was quantified by monitoring changes in the intrinsic tryptophan fluroescence of the enzyme using 280-nm e... | J Biol Chem 288: 6045-52 (2013) Article DOI: 10.1074/jbc.M112.414516 BindingDB Entry DOI: 10.7270/Q2KP80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 (Mycobacterium tuberculosis) | BDBM93045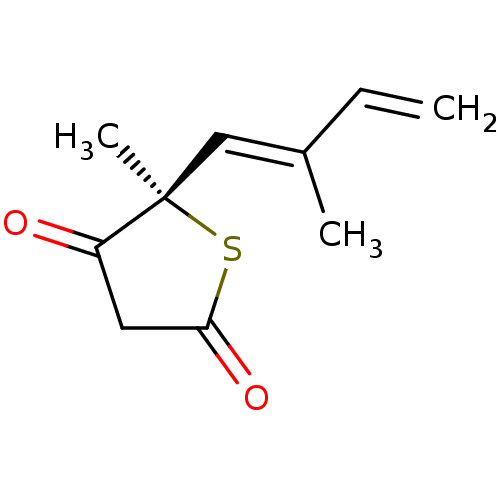 (TLM analog, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
Institute for Chemical Biology & Drug Discovery | Assay Description Binding of TLM and the TLM analogs to KasA was quantified by monitoring changes in the intrinsic tryptophan fluroescence of the enzyme using 280-nm e... | J Biol Chem 288: 6045-52 (2013) Article DOI: 10.1074/jbc.M112.414516 BindingDB Entry DOI: 10.7270/Q2KP80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 (Mycobacterium tuberculosis) | BDBM93050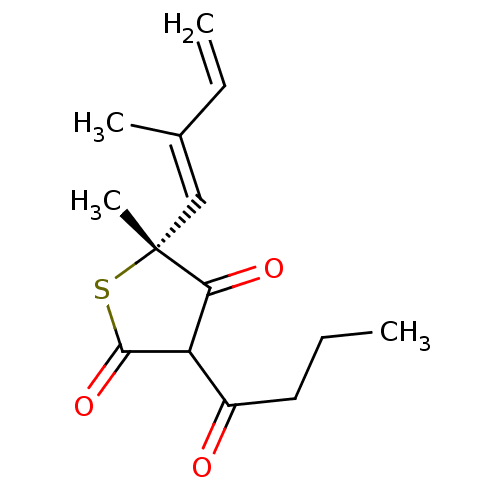 (TLM analog, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
Institute for Chemical Biology & Drug Discovery | Assay Description Binding of TLM and the TLM analogs to KasA was quantified by monitoring changes in the intrinsic tryptophan fluroescence of the enzyme using 280-nm e... | J Biol Chem 288: 6045-52 (2013) Article DOI: 10.1074/jbc.M112.414516 BindingDB Entry DOI: 10.7270/Q2KP80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 (Mycobacterium tuberculosis) | BDBM93057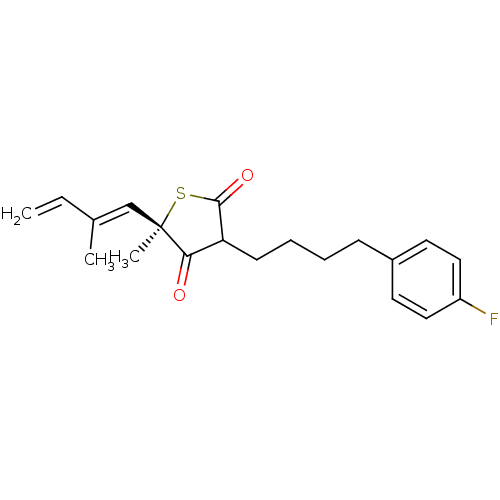 (TLM analog, 14) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
Institute for Chemical Biology & Drug Discovery | Assay Description Binding of TLM and the TLM analogs to KasA was quantified by monitoring changes in the intrinsic tryptophan fluroescence of the enzyme using 280-nm e... | J Biol Chem 288: 6045-52 (2013) Article DOI: 10.1074/jbc.M112.414516 BindingDB Entry DOI: 10.7270/Q2KP80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 (Mycobacterium tuberculosis) | BDBM93058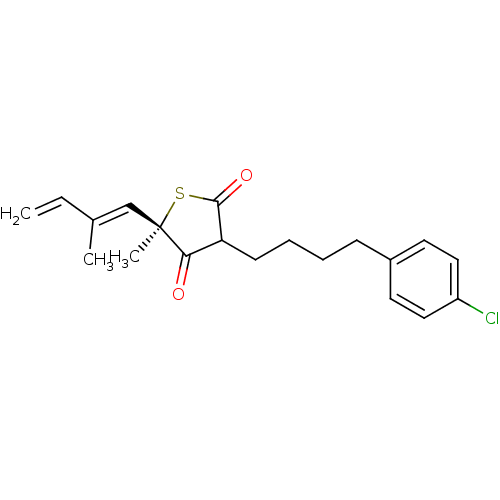 (TLM analog, 15) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
Institute for Chemical Biology & Drug Discovery | Assay Description Binding of TLM and the TLM analogs to KasA was quantified by monitoring changes in the intrinsic tryptophan fluroescence of the enzyme using 280-nm e... | J Biol Chem 288: 6045-52 (2013) Article DOI: 10.1074/jbc.M112.414516 BindingDB Entry DOI: 10.7270/Q2KP80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 (Mycobacterium tuberculosis) | BDBM93056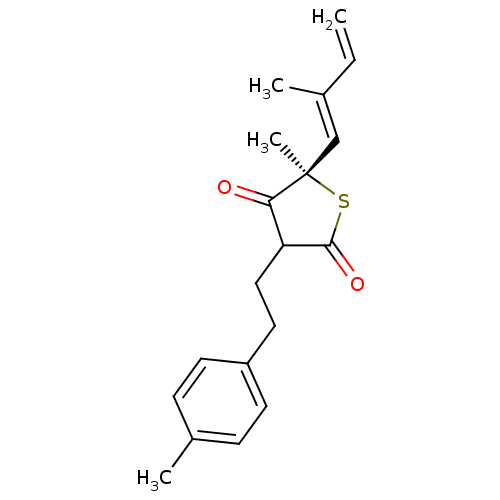 (TLM analog, 13) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
Institute for Chemical Biology & Drug Discovery | Assay Description Binding of TLM and the TLM analogs to KasA was quantified by monitoring changes in the intrinsic tryptophan fluroescence of the enzyme using 280-nm e... | J Biol Chem 288: 6045-52 (2013) Article DOI: 10.1074/jbc.M112.414516 BindingDB Entry DOI: 10.7270/Q2KP80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 (Mycobacterium tuberculosis) | BDBM93053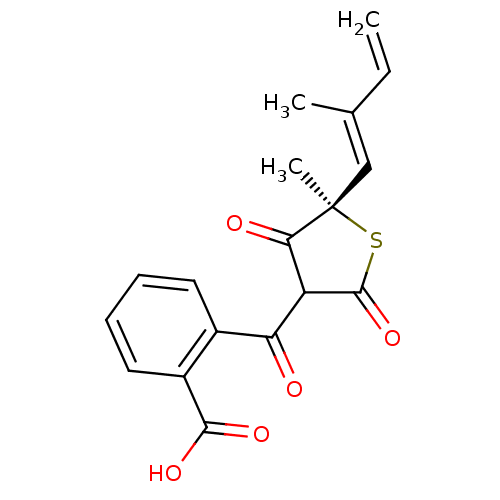 (TLM analog, 10) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
Institute for Chemical Biology & Drug Discovery | Assay Description Binding of TLM and the TLM analogs to KasA was quantified by monitoring changes in the intrinsic tryptophan fluroescence of the enzyme using 280-nm e... | J Biol Chem 288: 6045-52 (2013) Article DOI: 10.1074/jbc.M112.414516 BindingDB Entry DOI: 10.7270/Q2KP80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 (Mycobacterium tuberculosis) | BDBM93052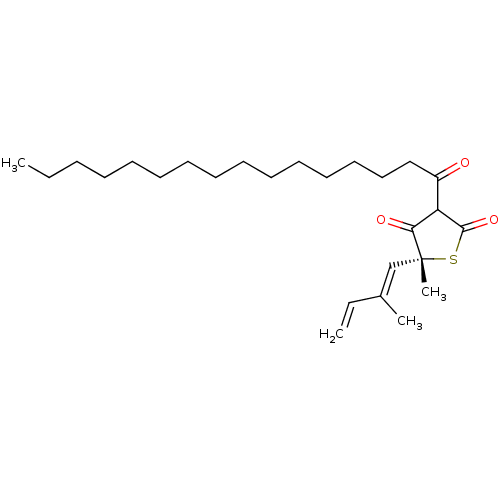 (TLM analog, 9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
Institute for Chemical Biology & Drug Discovery | Assay Description Binding of TLM and the TLM analogs to KasA was quantified by monitoring changes in the intrinsic tryptophan fluroescence of the enzyme using 280-nm e... | J Biol Chem 288: 6045-52 (2013) Article DOI: 10.1074/jbc.M112.414516 BindingDB Entry DOI: 10.7270/Q2KP80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 (Mycobacterium tuberculosis) | BDBM50241313 ((5R)-4-hydroxy-3,5-dimethyl-5-[(1E)-2-methylbuta-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.75E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
Institute for Chemical Biology & Drug Discovery | Assay Description Binding of TLM and the TLM analogs to KasA was quantified by monitoring changes in the intrinsic tryptophan fluroescence of the enzyme using 280-nm e... | J Biol Chem 288: 6045-52 (2013) Article DOI: 10.1074/jbc.M112.414516 BindingDB Entry DOI: 10.7270/Q2KP80S4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 (Mycobacterium tuberculosis) | BDBM93047 (TLM analog, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Similars | Article PubMed | 3.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
Institute for Chemical Biology & Drug Discovery | Assay Description Binding of TLM and the TLM analogs to KasA was quantified by monitoring changes in the intrinsic tryptophan fluroescence of the enzyme using 280-nm e... | J Biol Chem 288: 6045-52 (2013) Article DOI: 10.1074/jbc.M112.414516 BindingDB Entry DOI: 10.7270/Q2KP80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 (Mycobacterium tuberculosis) | BDBM93046 (TLM analog, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Similars | Article PubMed | 3.57E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
Institute for Chemical Biology & Drug Discovery | Assay Description Binding of TLM and the TLM analogs to KasA was quantified by monitoring changes in the intrinsic tryptophan fluroescence of the enzyme using 280-nm e... | J Biol Chem 288: 6045-52 (2013) Article DOI: 10.1074/jbc.M112.414516 BindingDB Entry DOI: 10.7270/Q2KP80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 (Mycobacterium tuberculosis) | BDBM93055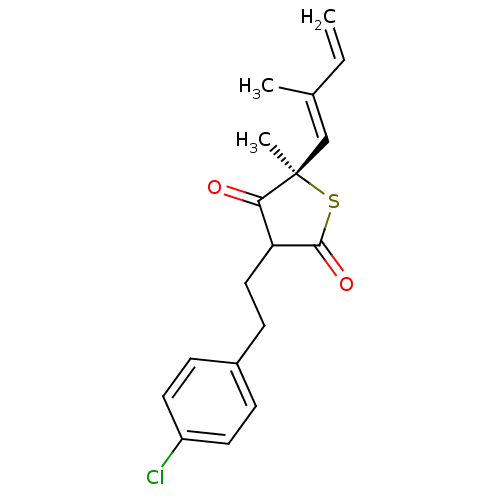 (TLM analog, 12) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
Institute for Chemical Biology & Drug Discovery | Assay Description Binding of TLM and the TLM analogs to KasA was quantified by monitoring changes in the intrinsic tryptophan fluroescence of the enzyme using 280-nm e... | J Biol Chem 288: 6045-52 (2013) Article DOI: 10.1074/jbc.M112.414516 BindingDB Entry DOI: 10.7270/Q2KP80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM518186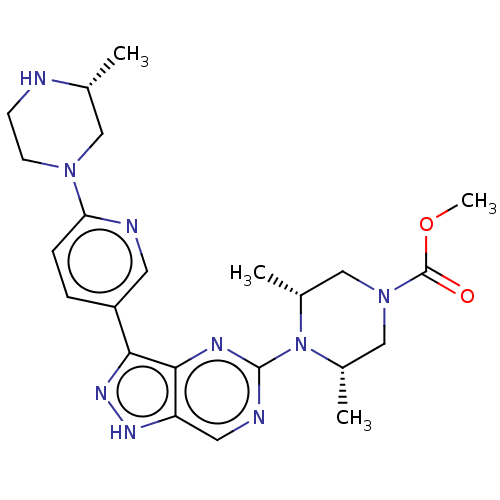 (US11111247, Example 17) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00206 BindingDB Entry DOI: 10.7270/Q2R78K72 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM518192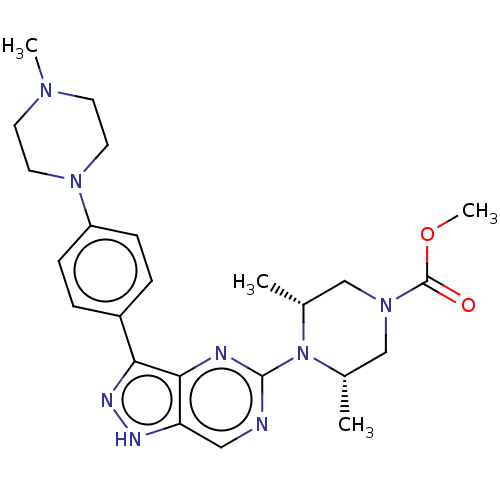 (US11111247, Example 22) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00206 BindingDB Entry DOI: 10.7270/Q2R78K72 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM102619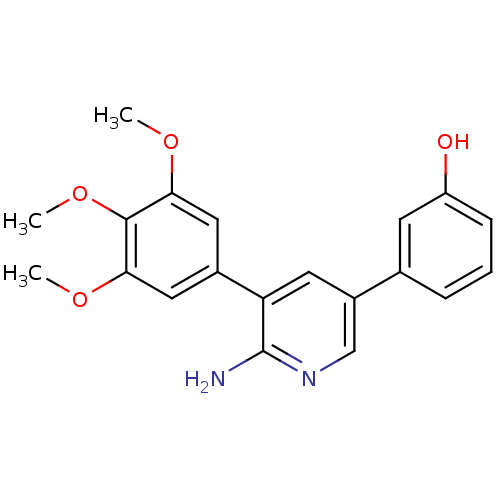 (K02288a | US10688093, Compound 382_0087_0284 | US1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128452 BindingDB Entry DOI: 10.7270/Q2JS9VGJ | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM518173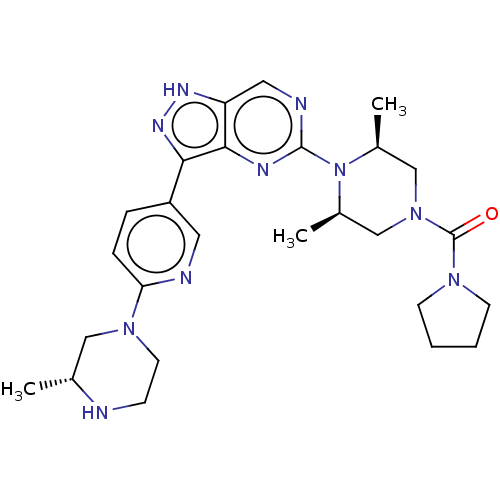 (US11111247, Example 9) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00206 BindingDB Entry DOI: 10.7270/Q2R78K72 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM518194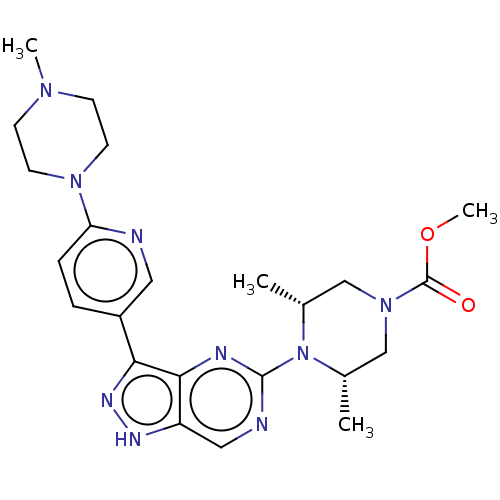 (US11111247, Example 24) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00206 BindingDB Entry DOI: 10.7270/Q2R78K72 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM518193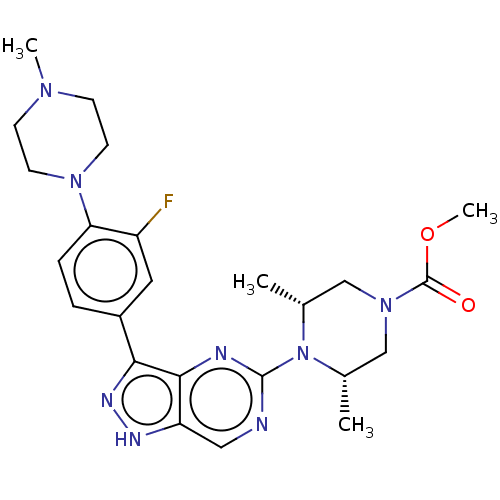 (US11111247, Example 23) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00206 BindingDB Entry DOI: 10.7270/Q2R78K72 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase receptor R3 (Homo sapiens (Human)) | BDBM102619 (K02288a | US10688093, Compound 382_0087_0284 | US1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128452 BindingDB Entry DOI: 10.7270/Q2JS9VGJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | CHEMBL5286909 | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL UniChem | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | CHEMBL5286909 | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL UniChem | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM518135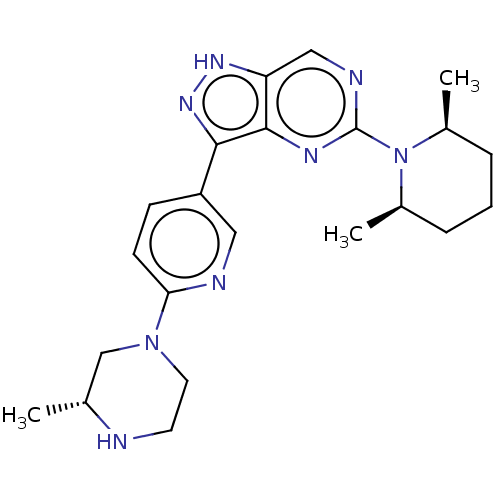 (US11111247, Example 1) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00206 BindingDB Entry DOI: 10.7270/Q2R78K72 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50596223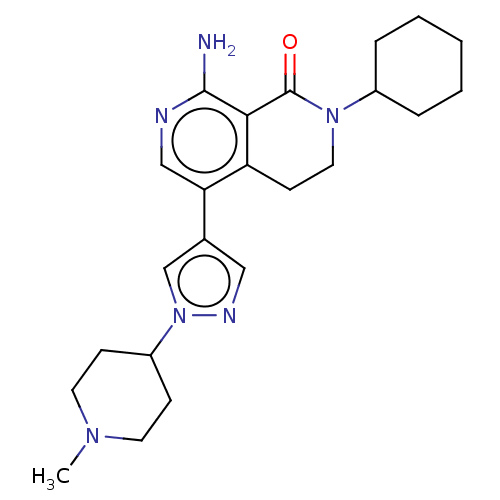 (CHEMBL5200241) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128452 BindingDB Entry DOI: 10.7270/Q2JS9VGJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM518220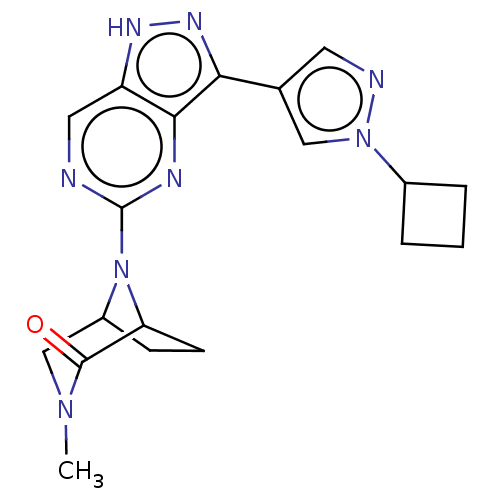 (US11111247, Example 63 | US11111247, Example 64) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50596221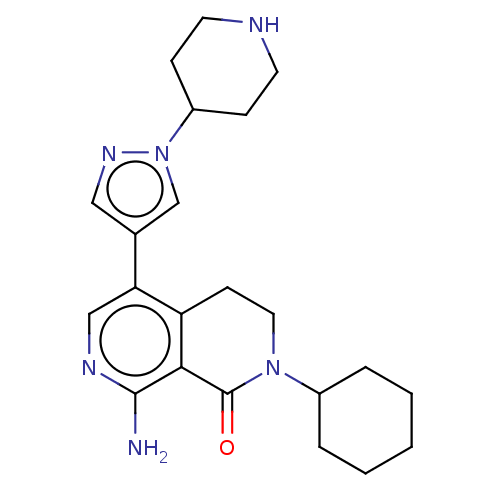 (CHEMBL5186575) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128452 BindingDB Entry DOI: 10.7270/Q2JS9VGJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50596220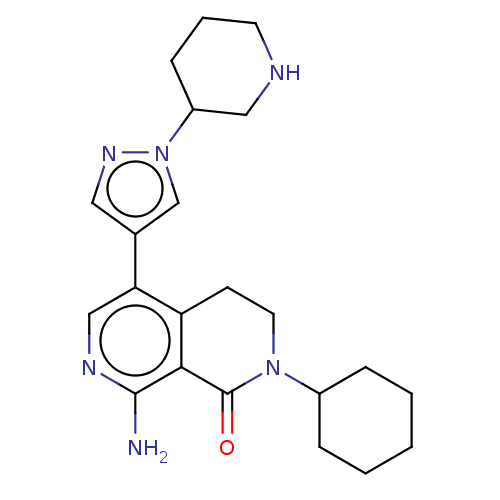 (CHEMBL5205781) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128452 BindingDB Entry DOI: 10.7270/Q2JS9VGJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 328 total ) | Next | Last >> |