

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50280333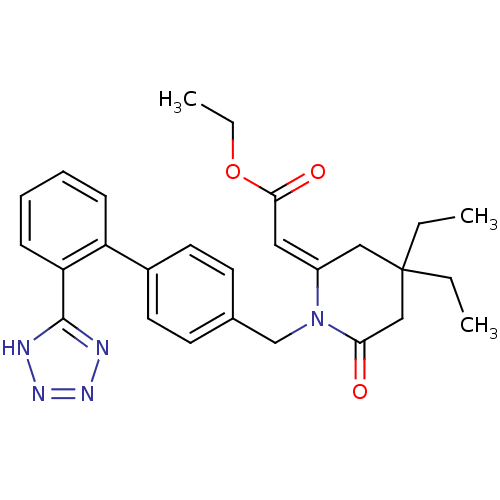 (CHEMBL49207 | [4,4-Diethyl-6-oxo-1-[2'-(1H-tetrazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability of the compound to displace [125I]- labelled [Sar1,Ileu8] from angiotensin II receptor of bovine adrenal cortex membranes | Bioorg Med Chem Lett 2: 1775-1779 (1992) Article DOI: 10.1016/S0960-894X(00)80474-6 BindingDB Entry DOI: 10.7270/Q2NP24B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50280320 (CHEMBL51084 | [4-Oxo-3-[2'-(1H-tetrazol-5-yl)-biph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability of the compound to displace [125I]- labelled [Sar1,Ileu8] from angiotensin II receptor of bovine adrenal cortex membranes | Bioorg Med Chem Lett 2: 1775-1779 (1992) Article DOI: 10.1016/S0960-894X(00)80474-6 BindingDB Entry DOI: 10.7270/Q2NP24B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50281520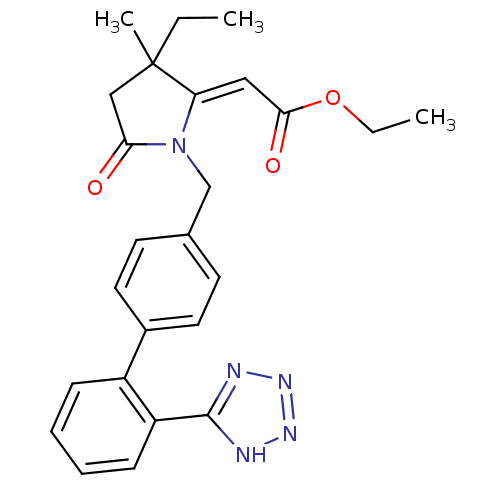 (CHEMBL123348 | [3-Ethyl-3-methyl-5-oxo-1-[2'-(1H-t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration that gives 50% inhibition for binding of 125-I-[Sar1, IIe8] angiotensin to Angiotensin II receptor, type 1 in bovine adrenal cortex mem... | Bioorg Med Chem Lett 3: 369-374 (1993) Article DOI: 10.1016/S0960-894X(01)80914-8 BindingDB Entry DOI: 10.7270/Q20G3K2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50281522 (CHEMBL121871 | [3-Oxo-2-[2'-(1H-tetrazol-5-yl)-bip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration that gives 50% inhibition for binding of 125-I-[Sar1, IIe8] angiotensin to Angiotensin II receptor, type 1 in bovine adrenal cortex mem... | Bioorg Med Chem Lett 3: 369-374 (1993) Article DOI: 10.1016/S0960-894X(01)80914-8 BindingDB Entry DOI: 10.7270/Q20G3K2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50280325 (CHEMBL48459 | [4-Ethyl-6-oxo-4-propyl-1-[2'-(1H-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability of the compound to displace [125I]- labelled [Sar1,Ileu8] from angiotensin II receptor of bovine adrenal cortex membranes | Bioorg Med Chem Lett 2: 1775-1779 (1992) Article DOI: 10.1016/S0960-894X(00)80474-6 BindingDB Entry DOI: 10.7270/Q2NP24B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50281524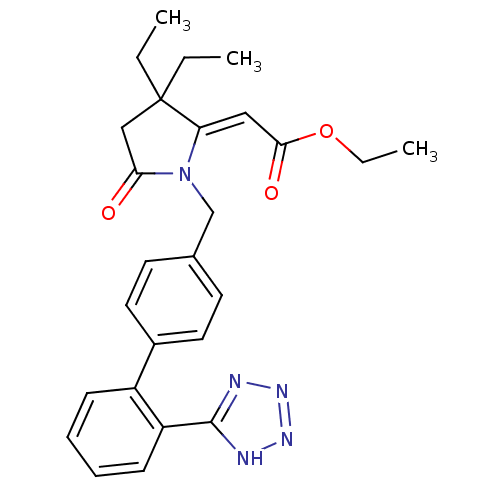 (CHEMBL123713 | [3,3-Diethyl-5-oxo-1-[2'-(1H-tetraz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration that gives 50% inhibition for binding of 125-I-[Sar1, IIe8] angiotensin to Angiotensin II receptor, type 1 in bovine adrenal cortex mem... | Bioorg Med Chem Lett 3: 369-374 (1993) Article DOI: 10.1016/S0960-894X(01)80914-8 BindingDB Entry DOI: 10.7270/Q20G3K2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50280329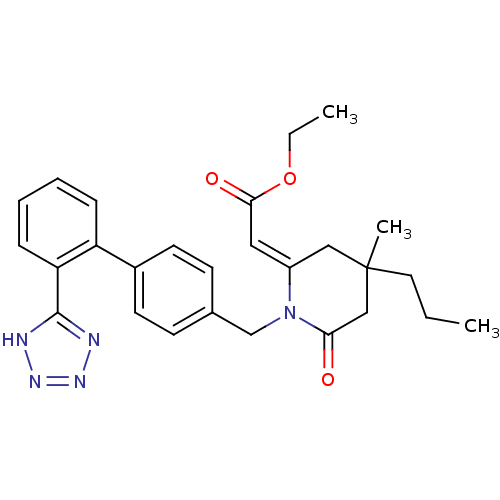 (CHEMBL50906 | [4-Methyl-6-oxo-4-propyl-1-[2'-(1H-t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability of the compound to displace [125I]- labelled [Sar1,Ileu8] from angiotensin II receptor of bovine adrenal cortex membranes | Bioorg Med Chem Lett 2: 1775-1779 (1992) Article DOI: 10.1016/S0960-894X(00)80474-6 BindingDB Entry DOI: 10.7270/Q2NP24B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50280321 (CHEMBL296731 | [4-Isopropyl-4-methyl-6-oxo-1-[2'-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability of the compound to displace [125I]- labelled [Sar1,Ileu8] from angiotensin II receptor of bovine adrenal cortex membranes | Bioorg Med Chem Lett 2: 1775-1779 (1992) Article DOI: 10.1016/S0960-894X(00)80474-6 BindingDB Entry DOI: 10.7270/Q2NP24B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50281519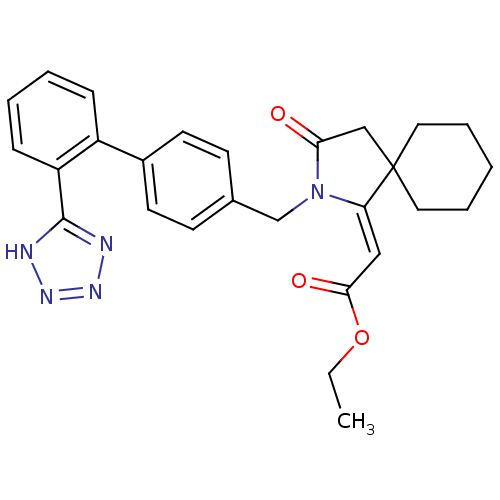 (CHEMBL124449 | [3-Oxo-2-[2'-(1H-tetrazol-5-yl)-bip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration that gives 50% inhibition for binding of 125-I-[Sar1, IIe8] angiotensin to Angiotensin II receptor, type 1 in bovine adrenal cortex mem... | Bioorg Med Chem Lett 3: 369-374 (1993) Article DOI: 10.1016/S0960-894X(01)80914-8 BindingDB Entry DOI: 10.7270/Q20G3K2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50280323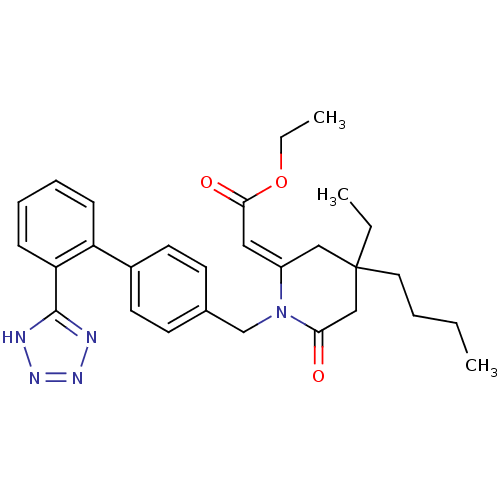 (CHEMBL51622 | [4-Butyl-4-ethyl-6-oxo-1-[2'-(1H-tet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability of the compound to displace [125I]- labelled [Sar1,Ileu8] from angiotensin II receptor of bovine adrenal cortex membranes | Bioorg Med Chem Lett 2: 1775-1779 (1992) Article DOI: 10.1016/S0960-894X(00)80474-6 BindingDB Entry DOI: 10.7270/Q2NP24B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50280322 (CHEMBL413098 | [4-Methyl-6-oxo-1-[2'-(1H-tetrazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability of the compound to displace [125I]- labelled [Sar1,Ileu8] from angiotensin II receptor of bovine adrenal cortex membranes | Bioorg Med Chem Lett 2: 1775-1779 (1992) Article DOI: 10.1016/S0960-894X(00)80474-6 BindingDB Entry DOI: 10.7270/Q2NP24B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50280328 (CHEMBL418269 | [4,4-Diethyl-6-oxo-1-[2'-(1H-tetraz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability of the compound to displace [125I]- labelled [Sar1,Ileu8] from angiotensin II receptor of bovine adrenal cortex membranes | Bioorg Med Chem Lett 2: 1775-1779 (1992) Article DOI: 10.1016/S0960-894X(00)80474-6 BindingDB Entry DOI: 10.7270/Q2NP24B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50281523 (CHEMBL421094 | [3-Oxo-2-[2'-(1H-tetrazol-5-yl)-bip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration that gives 50% inhibition for binding of 125-I-[Sar1, IIe8] angiotensin to Angiotensin II receptor, type 1 in bovine adrenal cortex mem... | Bioorg Med Chem Lett 3: 369-374 (1993) Article DOI: 10.1016/S0960-894X(01)80914-8 BindingDB Entry DOI: 10.7270/Q20G3K2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049205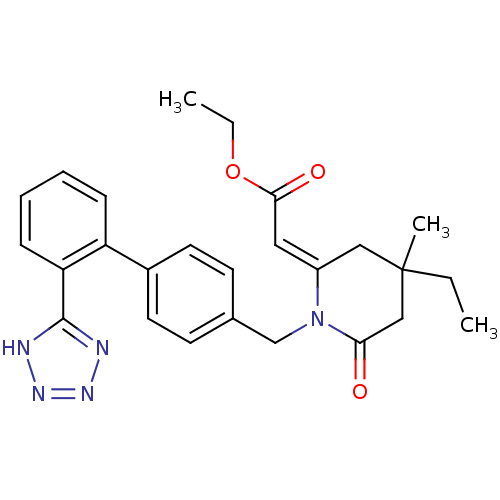 (CHEMBL48602 | RWJ-46458 | [4-Ethyl-4-methyl-6-oxo-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability of the compound to displace [125I]- labelled [Sar1,Ileu8] from angiotensin II receptor of bovine adrenal cortex membranes | Bioorg Med Chem Lett 2: 1775-1779 (1992) Article DOI: 10.1016/S0960-894X(00)80474-6 BindingDB Entry DOI: 10.7270/Q2NP24B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50280330 (CHEMBL51001 | [4-Ethyl-4-methyl-6-oxo-1-[2'-(1H-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability of the compound to displace [125I]- labelled [Sar1,Ileu8] from angiotensin II receptor of bovine adrenal cortex membranes | Bioorg Med Chem Lett 2: 1775-1779 (1992) Article DOI: 10.1016/S0960-894X(00)80474-6 BindingDB Entry DOI: 10.7270/Q2NP24B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50280327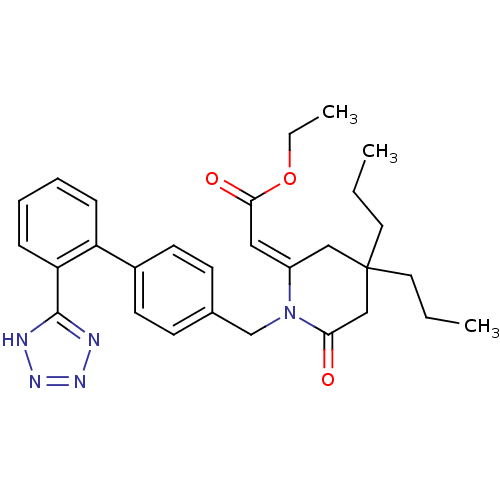 (CHEMBL295369 | [6-Oxo-4,4-dipropyl-1-[2'-(1H-tetra...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability of the compound to displace [125I]- labelled [Sar1,Ileu8] from angiotensin II receptor of bovine adrenal cortex membranes | Bioorg Med Chem Lett 2: 1775-1779 (1992) Article DOI: 10.1016/S0960-894X(00)80474-6 BindingDB Entry DOI: 10.7270/Q2NP24B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50009714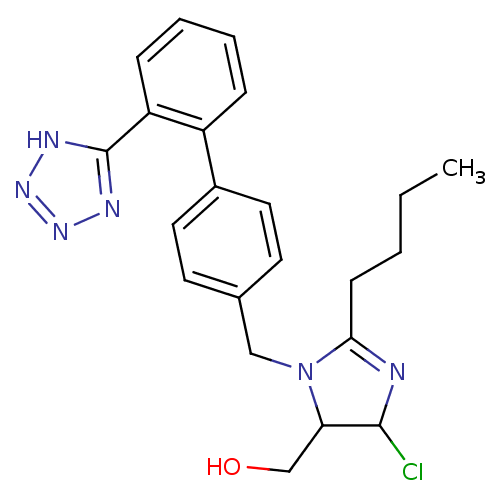 (CHEMBL191 | {2-Butyl-5-chloro-3-[2'-(2H-tetrazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability of the compound to displace [125I]- labelled [Sar1,Ileu8] from angiotensin II receptor of bovine adrenal cortex membranes | Bioorg Med Chem Lett 2: 1775-1779 (1992) Article DOI: 10.1016/S0960-894X(00)80474-6 BindingDB Entry DOI: 10.7270/Q2NP24B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50009714 (CHEMBL191 | {2-Butyl-5-chloro-3-[2'-(2H-tetrazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 425 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration that gives 50% inhibition for binding of 125-I-[Sar1, IIe8] angiotensin to Angiotensin II receptor, type 1 in bovine adrenal cortex mem... | Bioorg Med Chem Lett 3: 369-374 (1993) Article DOI: 10.1016/S0960-894X(01)80914-8 BindingDB Entry DOI: 10.7270/Q20G3K2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50280319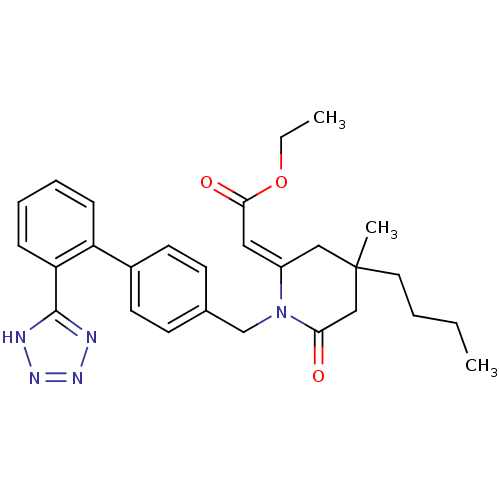 (CHEMBL299151 | [4-Butyl-4-methyl-6-oxo-1-[2'-(1H-t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability of the compound to displace [125I]- labelled [Sar1,Ileu8] from angiotensin II receptor of bovine adrenal cortex membranes | Bioorg Med Chem Lett 2: 1775-1779 (1992) Article DOI: 10.1016/S0960-894X(00)80474-6 BindingDB Entry DOI: 10.7270/Q2NP24B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50280331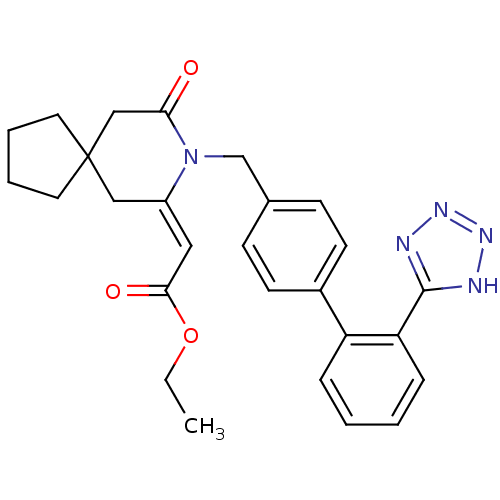 (CHEMBL49192 | [9-Oxo-8-[2'-(1H-tetrazol-5-yl)-biph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability of the compound to displace [125I]- labelled [Sar1,Ileu8] from angiotensin II receptor of bovine adrenal cortex membranes | Bioorg Med Chem Lett 2: 1775-1779 (1992) Article DOI: 10.1016/S0960-894X(00)80474-6 BindingDB Entry DOI: 10.7270/Q2NP24B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50280326 (CHEMBL51078 | [4-Methyl-6-oxo-4-pentyl-1-[2'-(1H-t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability of the compound to displace [125I]- labelled [Sar1,Ileu8] from angiotensin II receptor of bovine adrenal cortex membranes | Bioorg Med Chem Lett 2: 1775-1779 (1992) Article DOI: 10.1016/S0960-894X(00)80474-6 BindingDB Entry DOI: 10.7270/Q2NP24B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50280332 (4,4-Diethyl-6-[2-oxo-prop-(E)-ylidene]-1-[2'-(1H-t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability of the compound to displace [125I]- labelled [Sar1,Ileu8] from angiotensin II receptor of bovine adrenal cortex membranes | Bioorg Med Chem Lett 2: 1775-1779 (1992) Article DOI: 10.1016/S0960-894X(00)80474-6 BindingDB Entry DOI: 10.7270/Q2NP24B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50281521 (CHEMBL330866 | [3-Methyl-5-oxo-1-[2'-(1H-tetrazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration that gives 50% inhibition for binding of 125-I-[Sar1, IIe8] angiotensin to Angiotensin II receptor, type 1 in bovine adrenal cortex mem... | Bioorg Med Chem Lett 3: 369-374 (1993) Article DOI: 10.1016/S0960-894X(01)80914-8 BindingDB Entry DOI: 10.7270/Q20G3K2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50280324 (CHEMBL49262 | [4,4-Dimethyl-6-oxo-1-[2'-(1H-tetraz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability of the compound to displace [125I]- labelled [Sar1,Ileu8] from angiotensin II receptor of bovine adrenal cortex membranes | Bioorg Med Chem Lett 2: 1775-1779 (1992) Article DOI: 10.1016/S0960-894X(00)80474-6 BindingDB Entry DOI: 10.7270/Q2NP24B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50403241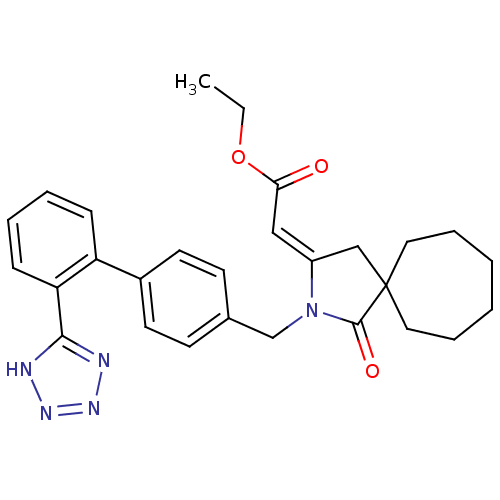 (CHEMBL422912) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity against Angiotensin II receptor in rabbit aorta rings | Bioorg Med Chem Lett 4: 87-92 (1994) Article DOI: 10.1016/S0960-894X(01)81127-6 BindingDB Entry DOI: 10.7270/Q2BC40QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50280325 (CHEMBL48459 | [4-Ethyl-6-oxo-4-propyl-1-[2'-(1H-te...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity against Angiotensin II receptor in rabbit aorta rings | Bioorg Med Chem Lett 4: 87-92 (1994) Article DOI: 10.1016/S0960-894X(01)81127-6 BindingDB Entry DOI: 10.7270/Q2BC40QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50280333 (CHEMBL49207 | [4,4-Diethyl-6-oxo-1-[2'-(1H-tetrazo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | 7.94 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity against Angiotensin II receptor in rabbit aorta rings | Bioorg Med Chem Lett 4: 87-92 (1994) Article DOI: 10.1016/S0960-894X(01)81127-6 BindingDB Entry DOI: 10.7270/Q2BC40QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50403240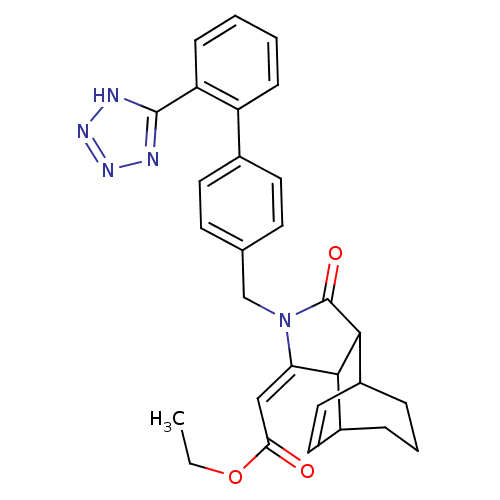 (CHEMBL122855) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | 19.9 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity against Angiotensin II receptor in rabbit aorta rings | Bioorg Med Chem Lett 4: 87-92 (1994) Article DOI: 10.1016/S0960-894X(01)81127-6 BindingDB Entry DOI: 10.7270/Q2BC40QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM82258 (CAS_114798-26-4 | Losartan | NSC_3961) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | n/a | 1.58 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity against Angiotensin II receptor in rabbit aorta rings | Bioorg Med Chem Lett 4: 87-92 (1994) Article DOI: 10.1016/S0960-894X(01)81127-6 BindingDB Entry DOI: 10.7270/Q2BC40QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50403239 (CHEMBL425139) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity against Angiotensin II receptor in rabbit aorta rings | Bioorg Med Chem Lett 4: 87-92 (1994) Article DOI: 10.1016/S0960-894X(01)81127-6 BindingDB Entry DOI: 10.7270/Q2BC40QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50280323 (CHEMBL51622 | [4-Butyl-4-ethyl-6-oxo-1-[2'-(1H-tet...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity against Angiotensin II receptor in rabbit aorta rings | Bioorg Med Chem Lett 4: 87-92 (1994) Article DOI: 10.1016/S0960-894X(01)81127-6 BindingDB Entry DOI: 10.7270/Q2BC40QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50280319 (CHEMBL299151 | [4-Butyl-4-methyl-6-oxo-1-[2'-(1H-t...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | 31.6 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity against Angiotensin II receptor in rabbit aorta rings | Bioorg Med Chem Lett 4: 87-92 (1994) Article DOI: 10.1016/S0960-894X(01)81127-6 BindingDB Entry DOI: 10.7270/Q2BC40QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50049205 (CHEMBL48602 | RWJ-46458 | [4-Ethyl-4-methyl-6-oxo-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity against Angiotensin II receptor in rabbit aorta rings | Bioorg Med Chem Lett 4: 87-92 (1994) Article DOI: 10.1016/S0960-894X(01)81127-6 BindingDB Entry DOI: 10.7270/Q2BC40QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50403238 (CHEMBL170196) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | 3.16 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity against Angiotensin II receptor in rabbit aorta rings | Bioorg Med Chem Lett 4: 87-92 (1994) Article DOI: 10.1016/S0960-894X(01)81127-6 BindingDB Entry DOI: 10.7270/Q2BC40QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50403237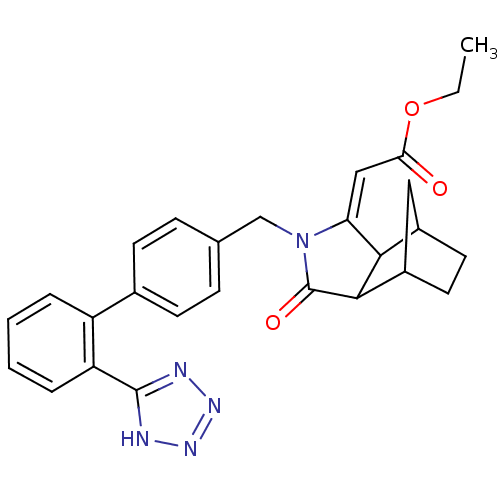 (CHEMBL122891) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity against Angiotensin II receptor in rabbit aorta rings | Bioorg Med Chem Lett 4: 87-92 (1994) Article DOI: 10.1016/S0960-894X(01)81127-6 BindingDB Entry DOI: 10.7270/Q2BC40QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50403236 (CHEMBL123208) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | 6.31 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity against Angiotensin II receptor in rabbit aorta rings | Bioorg Med Chem Lett 4: 87-92 (1994) Article DOI: 10.1016/S0960-894X(01)81127-6 BindingDB Entry DOI: 10.7270/Q2BC40QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50280320 (CHEMBL51084 | [4-Oxo-3-[2'-(1H-tetrazol-5-yl)-biph...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity against Angiotensin II receptor in rabbit aorta rings | Bioorg Med Chem Lett 4: 87-92 (1994) Article DOI: 10.1016/S0960-894X(01)81127-6 BindingDB Entry DOI: 10.7270/Q2BC40QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50280331 (CHEMBL49192 | [9-Oxo-8-[2'-(1H-tetrazol-5-yl)-biph...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity against Angiotensin II receptor in rabbit aorta rings | Bioorg Med Chem Lett 4: 87-92 (1994) Article DOI: 10.1016/S0960-894X(01)81127-6 BindingDB Entry DOI: 10.7270/Q2BC40QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50280324 (CHEMBL49262 | [4,4-Dimethyl-6-oxo-1-[2'-(1H-tetraz...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | 39.8 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity against Angiotensin II receptor in rabbit aorta rings | Bioorg Med Chem Lett 4: 87-92 (1994) Article DOI: 10.1016/S0960-894X(01)81127-6 BindingDB Entry DOI: 10.7270/Q2BC40QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50280333 (CHEMBL49207 | [4,4-Diethyl-6-oxo-1-[2'-(1H-tetrazo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity against Angiotensin II receptor in rabbit aorta rings | Bioorg Med Chem Lett 4: 87-92 (1994) Article DOI: 10.1016/S0960-894X(01)81127-6 BindingDB Entry DOI: 10.7270/Q2BC40QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50280321 (CHEMBL296731 | [4-Isopropyl-4-methyl-6-oxo-1-[2'-(...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | 15.8 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity against Angiotensin II receptor in rabbit aorta rings | Bioorg Med Chem Lett 4: 87-92 (1994) Article DOI: 10.1016/S0960-894X(01)81127-6 BindingDB Entry DOI: 10.7270/Q2BC40QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50049205 (CHEMBL48602 | RWJ-46458 | [4-Ethyl-4-methyl-6-oxo-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity against Angiotensin II receptor in rabbit aorta rings | Bioorg Med Chem Lett 4: 87-92 (1994) Article DOI: 10.1016/S0960-894X(01)81127-6 BindingDB Entry DOI: 10.7270/Q2BC40QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50403236 (CHEMBL123208) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity against Angiotensin II receptor in rabbit aorta rings | Bioorg Med Chem Lett 4: 87-92 (1994) Article DOI: 10.1016/S0960-894X(01)81127-6 BindingDB Entry DOI: 10.7270/Q2BC40QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||