

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50085044 ((S)-2-(2-Benzoyl-phenylamino)-3-{4-[2-(5-methyl-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity to human Peroxisome proliferator activated receptor gamma using scintillation proximity assay | Bioorg Med Chem Lett 11: 3111-3 (2001) BindingDB Entry DOI: 10.7270/Q2B27TKM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50063920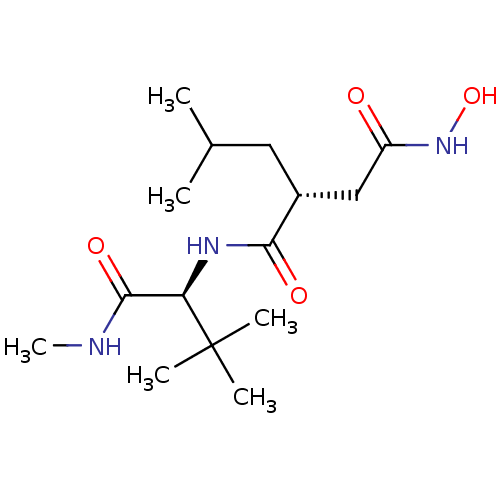 ((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of Matrix metalloproteinase-9 | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50063920 ((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of Matrix metalloproteinase-1 | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50085046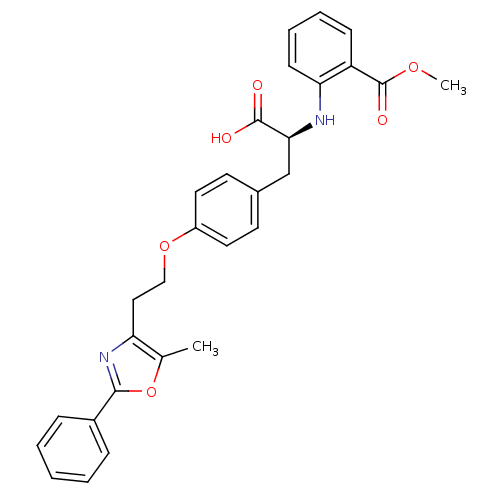 (2-((S)-1-carboxy-2-{4-[2-(5-methyl-2-phenyl-oxazol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity to human Peroxisome proliferator activated receptor gamma using scintillation proximity assay | Bioorg Med Chem Lett 11: 3111-3 (2001) BindingDB Entry DOI: 10.7270/Q2B27TKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50121622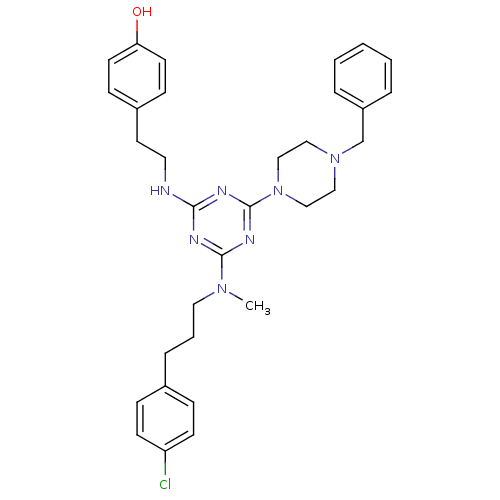 (4-[2-(4-(4-Benzyl-piperazin-1-yl)-6-{[3-(4-chloro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards human Estrogen receptor 2 using [3H]-17-beta-estradiol as radioligand | J Med Chem 45: 5492-505 (2002) BindingDB Entry DOI: 10.7270/Q2J965RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50103099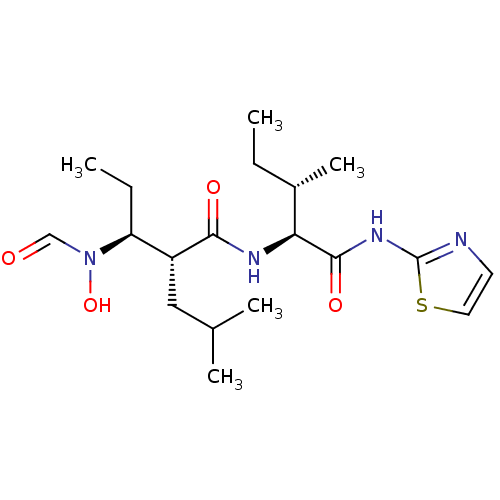 ((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibitionof Tumor necrosis factor alpha converting enzyme | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50121598 (4-{2-[4-{[3-(4-Chloro-phenyl)-propyl]-methyl-amino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards human Estrogen receptor 2 using [3H]-17-beta-estradiol as radioligand | J Med Chem 45: 5492-505 (2002) BindingDB Entry DOI: 10.7270/Q2J965RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50121626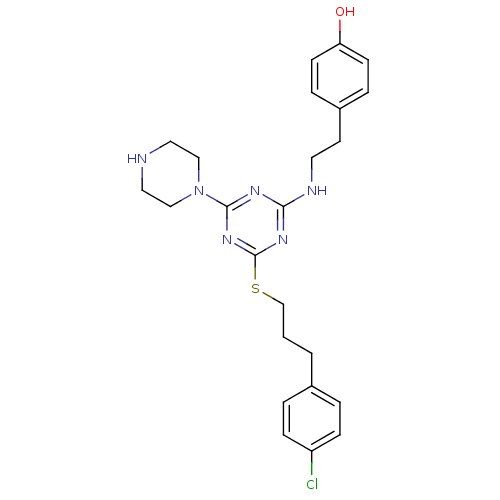 (4-(2-{4-[3-(4-Chloro-phenyl)-propylsulfanyl]-6-pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards human Estrogen receptor 2 using [3H]-17-beta-estradiol as radioligand | J Med Chem 45: 5492-505 (2002) BindingDB Entry DOI: 10.7270/Q2J965RW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50121590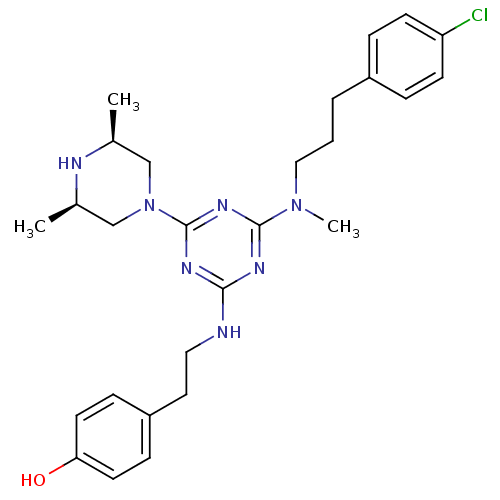 (4-{2-[4-{[3-(4-Chloro-phenyl)-propyl]-methyl-amino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards human Estrogen receptor 2 using [3H]-17-beta-estradiol as radioligand | J Med Chem 45: 5492-505 (2002) BindingDB Entry DOI: 10.7270/Q2J965RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50121608 (4-[2-(4-{[3-(4-Chloro-phenyl)-propyl]-methyl-amino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards human Estrogen receptor 2 using [3H]-17-beta-estradiol as radioligand | J Med Chem 45: 5492-505 (2002) BindingDB Entry DOI: 10.7270/Q2J965RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50121602 (4-[2-(4-{[3-(4-Fluoro-phenyl)-propyl]-methyl-amino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards human Estrogen receptor 2 using [3H]-17-beta-estradiol as radioligand | J Med Chem 45: 5492-505 (2002) BindingDB Entry DOI: 10.7270/Q2J965RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50121589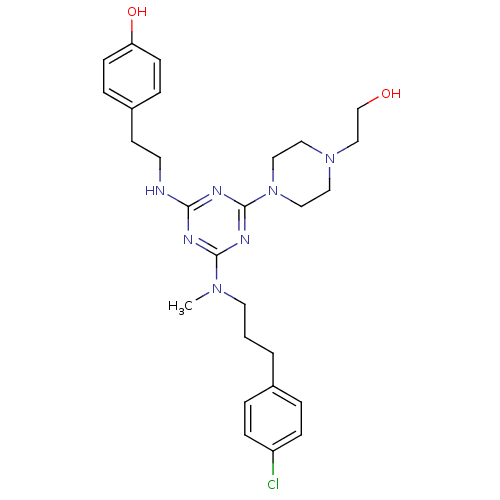 (4-(2-{4-{[3-(4-Chloro-phenyl)-propyl]-methyl-amino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards human Estrogen receptor 2 using [3H]-17-beta-estradiol as radioligand | J Med Chem 45: 5492-505 (2002) BindingDB Entry DOI: 10.7270/Q2J965RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50103097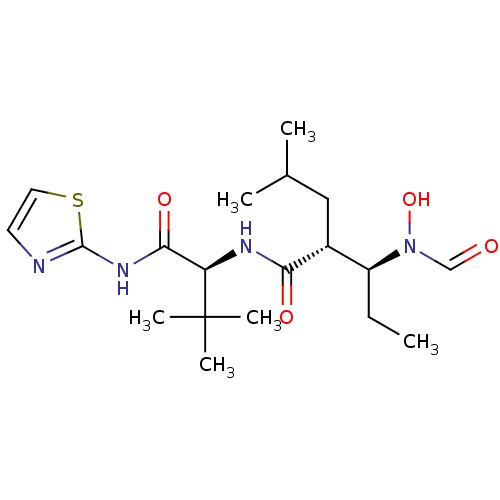 ((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of Matrix metalloproteinase-1 | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50103097 ((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibitionof Tumor necrosis factor alpha converting enzyme | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50103092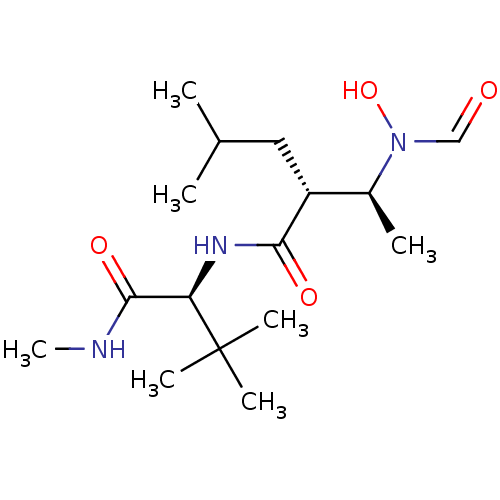 ((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of Matrix metalloproteinase-1 | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50103096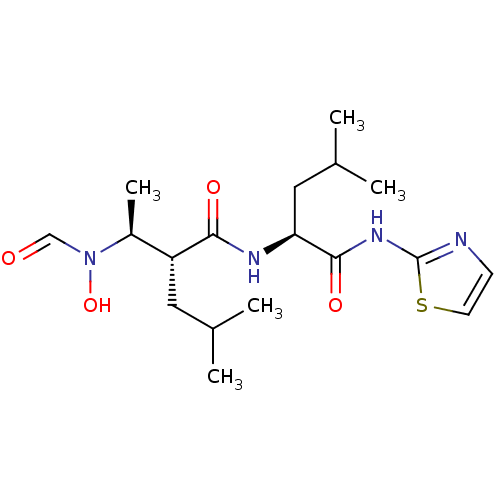 ((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of Matrix metalloproteinase-9 | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50103102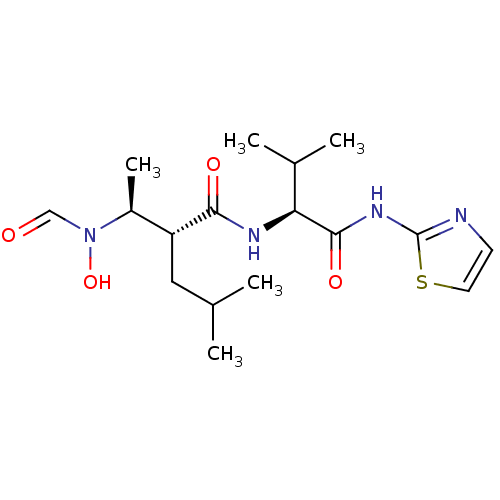 ((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of Matrix metalloproteinase-9 | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50121584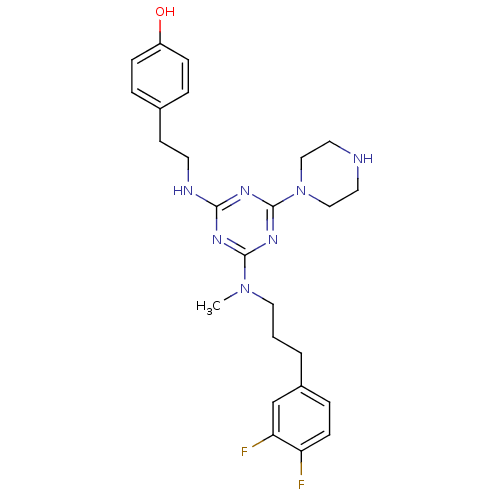 (4-[2-(4-{[3-(3,4-Difluoro-phenyl)-propyl]-methyl-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards human Estrogen receptor 2 using [3H]-17-beta-estradiol as radioligand | J Med Chem 45: 5492-505 (2002) BindingDB Entry DOI: 10.7270/Q2J965RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50103098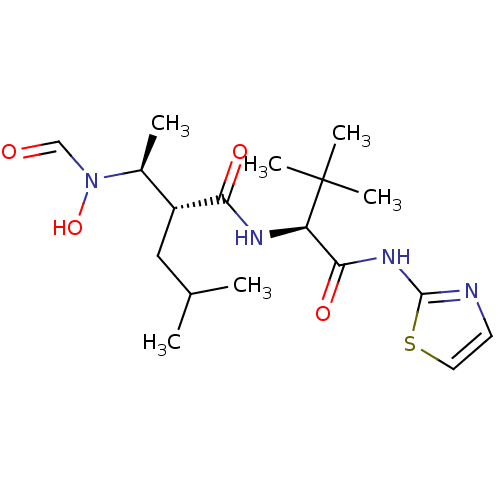 ((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibitionof Tumor necrosis factor alpha converting enzyme | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50103093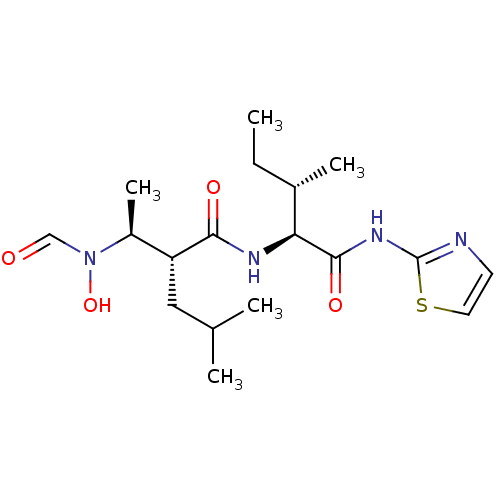 ((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibitionof Tumor necrosis factor alpha converting enzyme | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50103092 ((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of Matrix metalloproteinase-9 | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50103099 ((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of Matrix metalloproteinase-9 | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50121604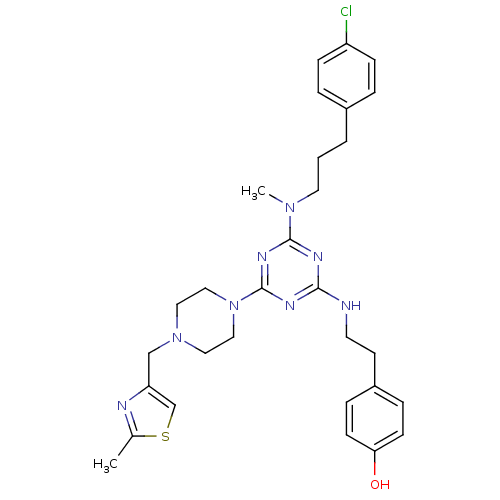 (4-(2-{4-{[3-(4-Chloro-phenyl)-propyl]-methyl-amino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards human Estrogen receptor 2 using [3H]-17-beta-estradiol as radioligand | J Med Chem 45: 5492-505 (2002) BindingDB Entry DOI: 10.7270/Q2J965RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50121601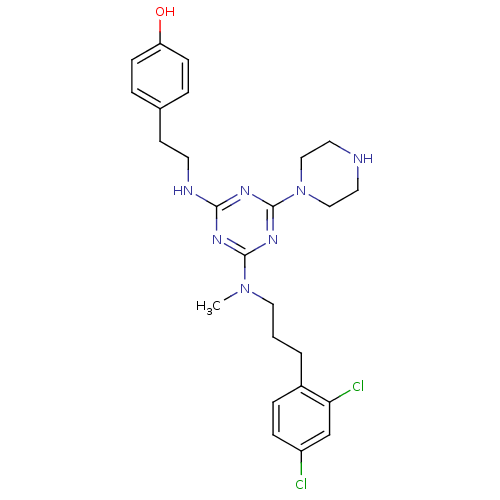 (4-[2-(4-{[3-(2,4-Dichloro-phenyl)-propyl]-methyl-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards human Estrogen receptor 2 using [3H]-17-beta-estradiol as radioligand | J Med Chem 45: 5492-505 (2002) BindingDB Entry DOI: 10.7270/Q2J965RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50103098 ((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of Matrix metalloproteinase-1 | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50103102 ((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibitionof Tumor necrosis factor alpha converting enzyme | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50103097 ((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of Matrix metalloproteinase-9 | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50103093 ((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of Matrix metalloproteinase-9 | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50103095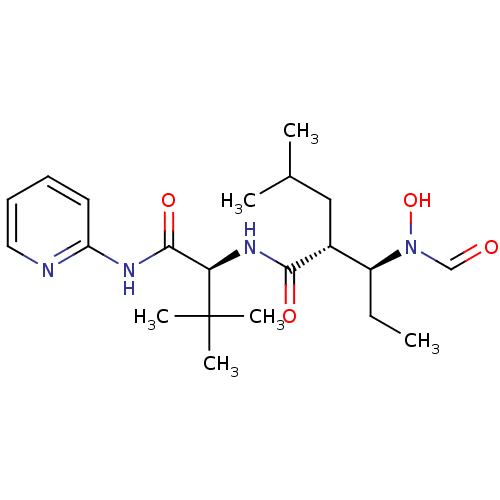 ((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibitionof Tumor necrosis factor alpha converting enzyme | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50121634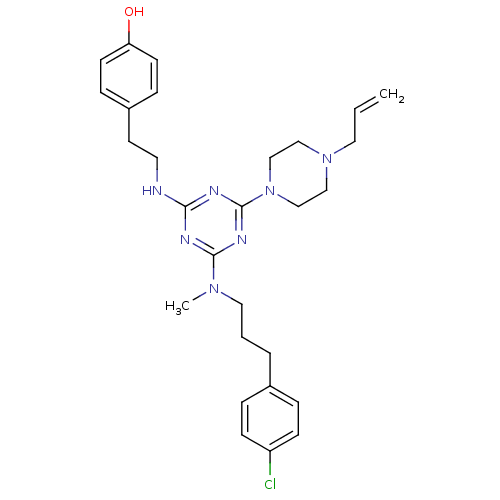 (4-[2-(4-(4-Allyl-piperazin-1-yl)-6-{[3-(4-chloro-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards human Estrogen receptor 2 using [3H]-17-beta-estradiol as radioligand | J Med Chem 45: 5492-505 (2002) BindingDB Entry DOI: 10.7270/Q2J965RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50121603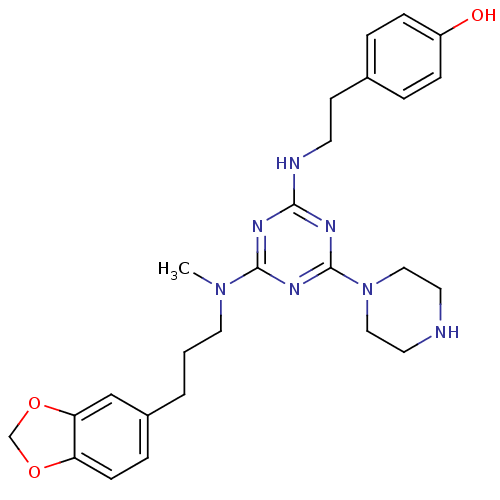 (4-(2-{4-[(3-Benzo[1,3]dioxol-5-yl-propyl)-methyl-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards human Estrogen receptor 2 using [3H]-17-beta-estradiol as radioligand | J Med Chem 45: 5492-505 (2002) BindingDB Entry DOI: 10.7270/Q2J965RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50103101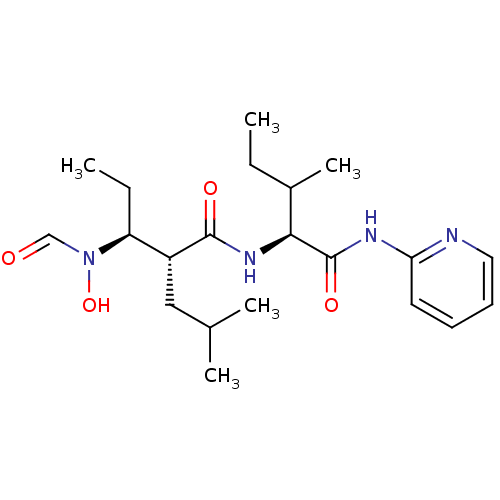 ((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibitionof Tumor necrosis factor alpha converting enzyme | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50103100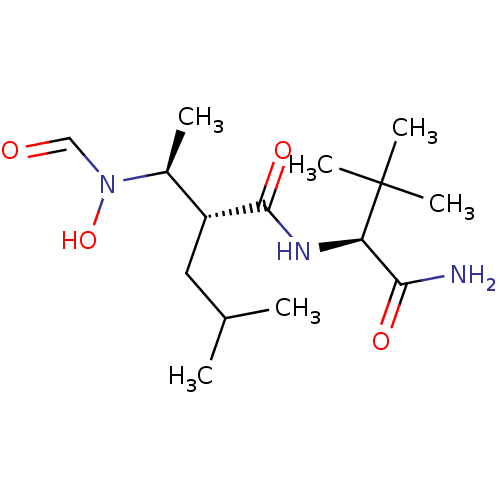 ((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of Matrix metalloproteinase-9 | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50103098 ((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of Matrix metalloproteinase-9 | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50121600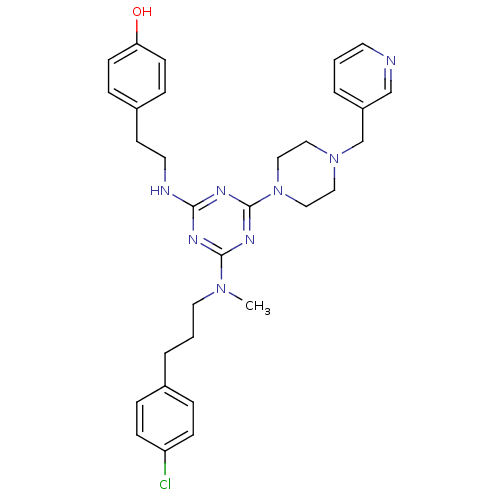 (4-{2-[4-{[3-(4-Chloro-phenyl)-propyl]-methyl-amino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards human Estrogen receptor 2 using [3H]-17-beta-estradiol as radioligand | J Med Chem 45: 5492-505 (2002) BindingDB Entry DOI: 10.7270/Q2J965RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50103102 ((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of Matrix metalloproteinase-1 | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50103101 ((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of matrix metalloprotease-3 (MMP3) | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50121636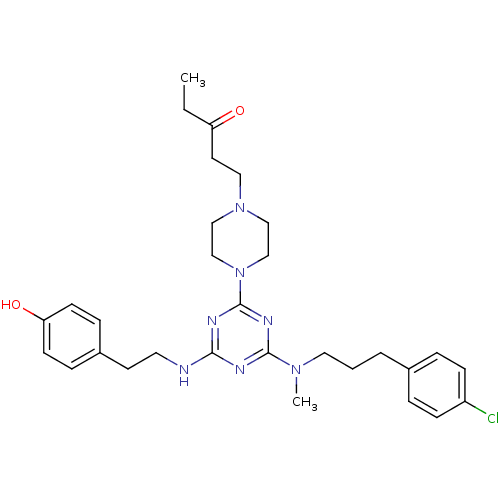 (1-(4-{4-{[3-(4-Chloro-phenyl)-propyl]-methyl-amino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards human Estrogen receptor 2 using [3H]-17-beta-estradiol as radioligand | J Med Chem 45: 5492-505 (2002) BindingDB Entry DOI: 10.7270/Q2J965RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50121625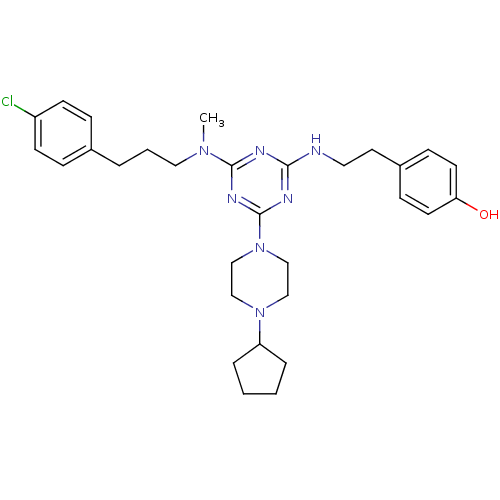 (4-{2-[4-{[3-(4-Chloro-phenyl)-propyl]-methyl-amino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards human Estrogen receptor 2 using [3H]-17-beta-estradiol as radioligand | J Med Chem 45: 5492-505 (2002) BindingDB Entry DOI: 10.7270/Q2J965RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50121633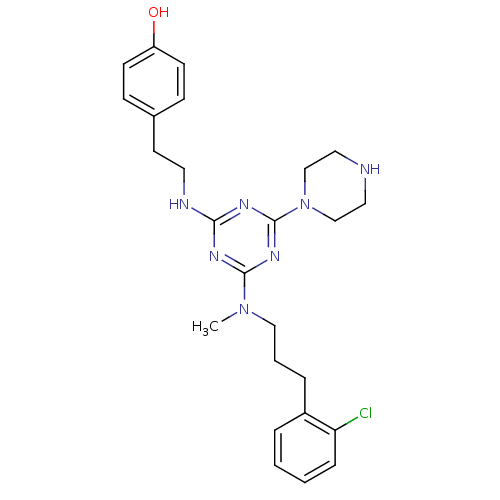 (4-[2-(4-{[3-(2-Chloro-phenyl)-propyl]-methyl-amino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards human Estrogen receptor 2 using [3H]-17-beta-estradiol as radioligand | J Med Chem 45: 5492-505 (2002) BindingDB Entry DOI: 10.7270/Q2J965RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50121587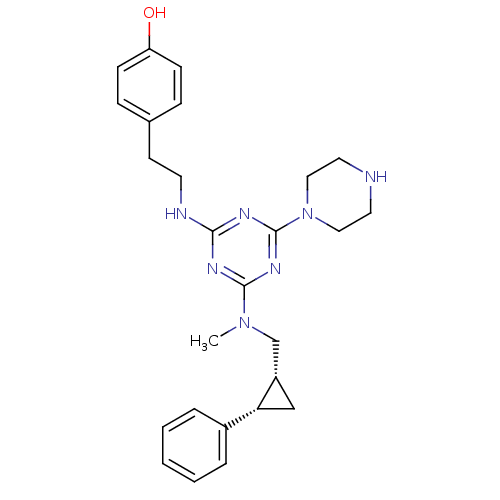 (CHEMBL153395 | cis-4-(2-{4-[Methyl-(2-phenyl-cyclo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards human Estrogen receptor 2 using [3H]-17-beta-estradiol as radioligand | J Med Chem 45: 5492-505 (2002) BindingDB Entry DOI: 10.7270/Q2J965RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50103093 ((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of Matrix metalloproteinase-1 | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50103099 ((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of Matrix metalloproteinase-1 | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50103099 ((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of matrix metalloprotease-3 (MMP3) | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50103095 ((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of Matrix metalloproteinase-1 | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50002865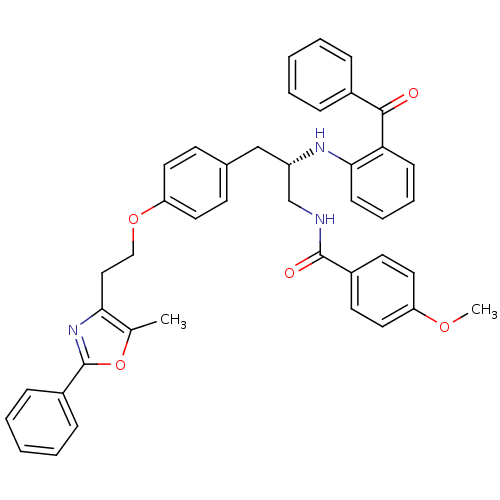 (CHEMBL230730) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]BRL49653 from PPARgamma by scintillation proximity assay | Bioorg Med Chem Lett 17: 3916-20 (2007) Checked by Author Article DOI: 10.1016/j.bmcl.2007.04.111 BindingDB Entry DOI: 10.7270/Q2R49S33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50121588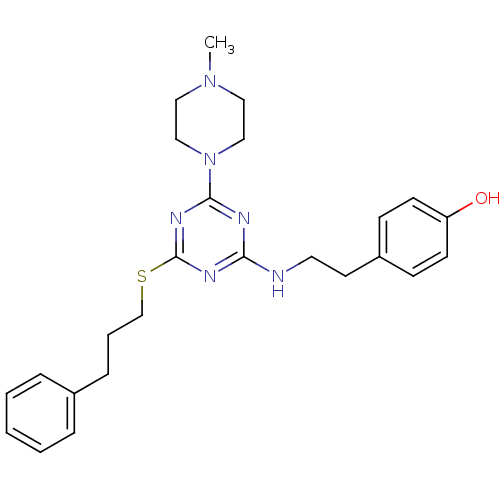 (4-{2-[4-(4-Methyl-piperazin-1-yl)-6-(3-phenyl-prop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards human Estrogen receptor 2 using [3H]-17-beta-estradiol as radioligand | J Med Chem 45: 5492-505 (2002) BindingDB Entry DOI: 10.7270/Q2J965RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50121593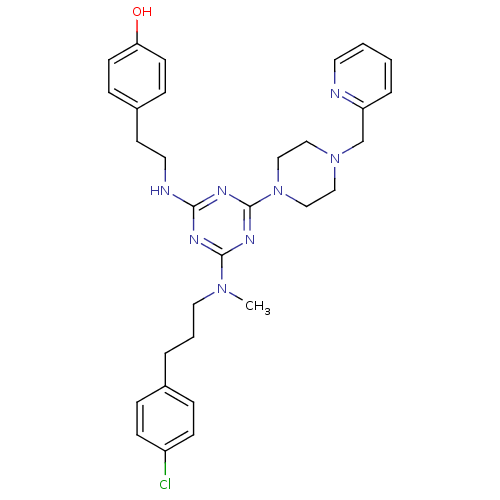 (4-{2-[4-{[3-(4-Chloro-phenyl)-propyl]-methyl-amino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards human Estrogen receptor 2 using [3H]-17-beta-estradiol as radioligand | J Med Chem 45: 5492-505 (2002) BindingDB Entry DOI: 10.7270/Q2J965RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM34017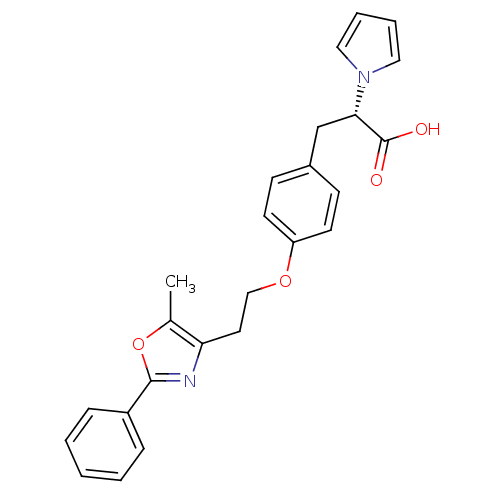 (CHEMBL104850 | phenylpropanoic acid derivative, 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity to human Peroxisome proliferator activated receptor gamma using scintillation proximity assay | Bioorg Med Chem Lett 11: 3111-3 (2001) BindingDB Entry DOI: 10.7270/Q2B27TKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM34017 (CHEMBL104850 | phenylpropanoic acid derivative, 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Maximal reporter activity against human Peroxisome proliferator activated receptor gamma Gal4 chimeric in transiently transfected CV-1 cells by funct... | Bioorg Med Chem Lett 11: 3111-3 (2001) BindingDB Entry DOI: 10.7270/Q2B27TKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 915 total ) | Next | Last >> |