

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50530705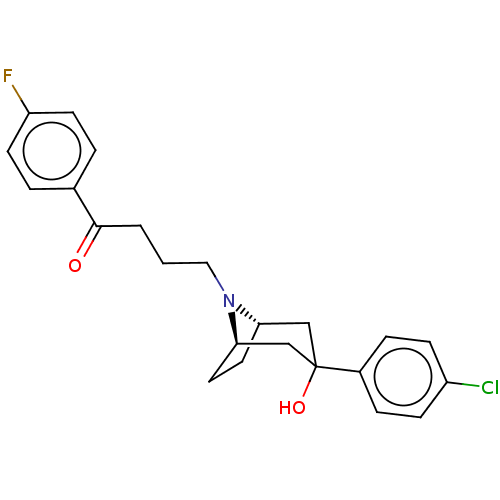 (CHEMBL4451384) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0525 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Displacement of PPHT-red from SNAP-tagged human D2LR expressed in CHOK1 cell membranes by TR-FRET assay | J Med Chem 62: 9488-9520 (2019) Article DOI: 10.1021/acs.jmedchem.9b00864 BindingDB Entry DOI: 10.7270/Q2NP27WQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50530705 (CHEMBL4451384) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0525 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Displacement of PPHT-red from SNAP-tagged human D2LR expressed in CHOK1 cell membranes by TR-FRET assay | J Med Chem 62: 9488-9520 (2019) Article DOI: 10.1021/acs.jmedchem.9b00864 BindingDB Entry DOI: 10.7270/Q2NP27WQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50264895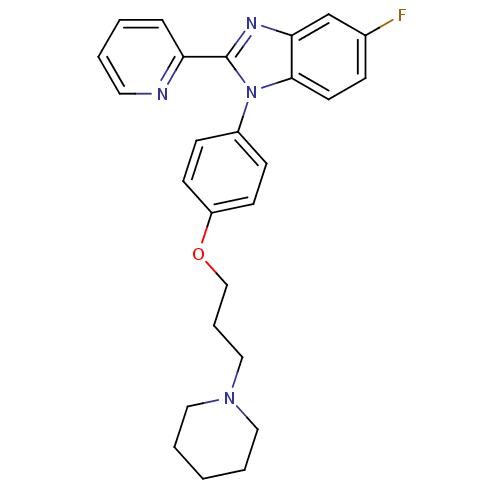 (5-fluoro-1-(4-(3-(piperidin-1-yl)propoxy)phenyl)-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in HEK293 cells assessed as reversal of N-alpha-methylhistamine-induced inhibition of fo... | Bioorg Med Chem Lett 18: 5032-6 (2008) Article DOI: 10.1016/j.bmcl.2008.08.008 BindingDB Entry DOI: 10.7270/Q2CZ370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50067497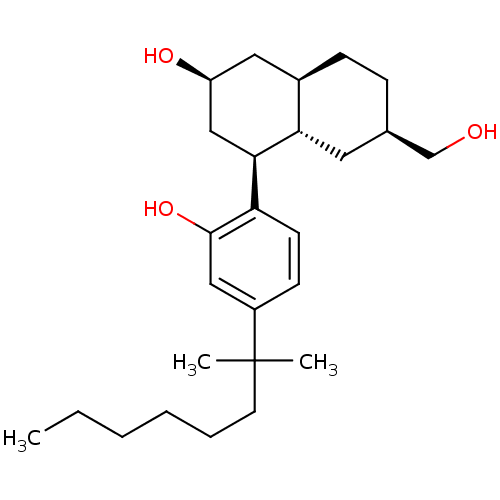 ((2S,4S,4aS,6R,8aR)-4-[4-(1,1-Dimethyl-heptyl)-2-hy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Louis University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 binding to Cannabinoid receptor 1 in rat brain membranes | J Med Chem 41: 4207-15 (1998) Article DOI: 10.1021/jm970239z BindingDB Entry DOI: 10.7270/Q2C24VK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50530680 (CHEMBL4570273) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Displacement of PPHT-red from SNAP-tagged human D2LR expressed in CHOK1 cell membranes by TR-FRET assay | J Med Chem 62: 9488-9520 (2019) Article DOI: 10.1021/acs.jmedchem.9b00864 BindingDB Entry DOI: 10.7270/Q2NP27WQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50530680 (CHEMBL4570273) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Displacement of PPHT-red from SNAP-tagged human D2LR expressed in CHOK1 cell membranes by TR-FRET assay | J Med Chem 62: 9488-9520 (2019) Article DOI: 10.1021/acs.jmedchem.9b00864 BindingDB Entry DOI: 10.7270/Q2NP27WQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50064340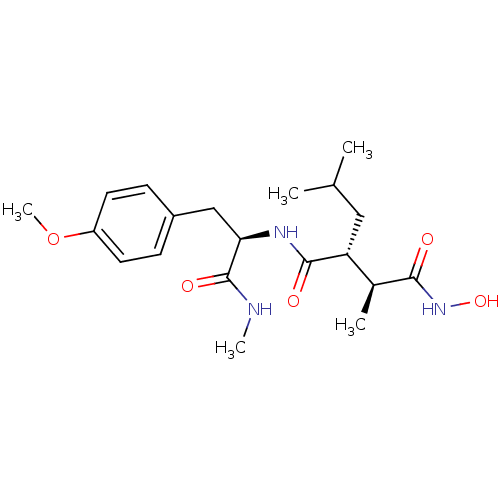 ((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-[(R)-2-(4-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of MMP-9 (Matrix metalloproteinase-9) | J Med Chem 41: 1745-8 (1998) Article DOI: 10.1021/jm970849z BindingDB Entry DOI: 10.7270/Q2GB235N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor corepressor 1 (Homo sapiens (Human)) | BDBM50354086 (FK-228 | Istodax | ROMIDEPSIN) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human HDAC3/NcoR1 enzyme using flurogenic Ac-LeuGlyLys (Ac)-AMC as substrate after 15 to 30 mins | J Med Chem 56: 6512-20 (2013) Article DOI: 10.1021/jm4008449 BindingDB Entry DOI: 10.7270/Q27S7Q6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50062051 (1-Dimethylcarbamoyl-6-(4-fluoro-phenyl)-3-[4-(2-me...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency against Platelet activating factor (PAF) receptor using [3H]-C18-PAF as radioligand on rabbit platelet membranes | J Med Chem 41: 74-95 (1998) Article DOI: 10.1021/jm970389+ BindingDB Entry DOI: 10.7270/Q2MC8Z4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50325449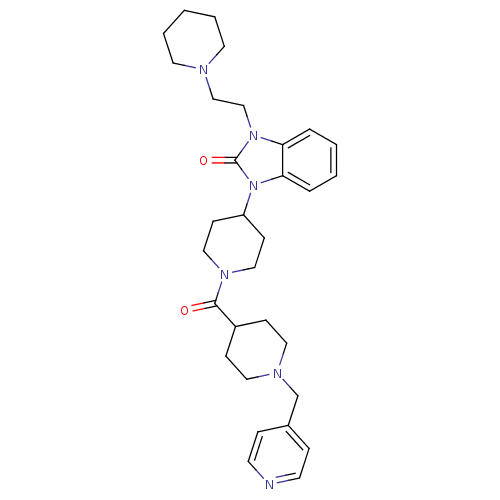 (1-(2-(piperidin-1-yl)ethyl)-3-(1-(1-(pyridin-4-ylm...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]Nalpha-methylhistamine from histamine H3 receptor in guinea pig brain | Bioorg Med Chem Lett 20: 5004-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.052 BindingDB Entry DOI: 10.7270/Q2N016QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50328970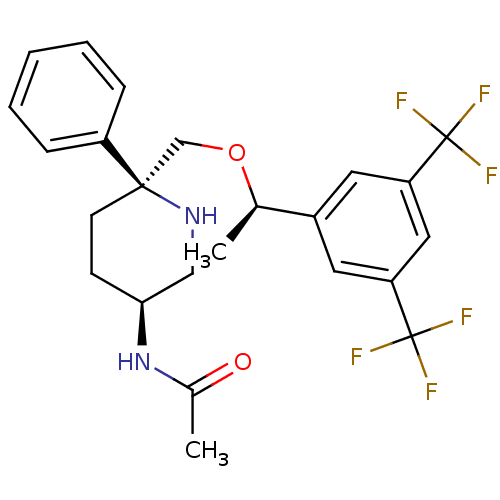 (CHEMBL1270066 | N-((3S,6S)-6-(((R)-1-(3,5-bis(trif...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SAr-Met from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6313-5 (2010) Article DOI: 10.1016/j.bmcl.2010.08.059 BindingDB Entry DOI: 10.7270/Q23X86VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50530700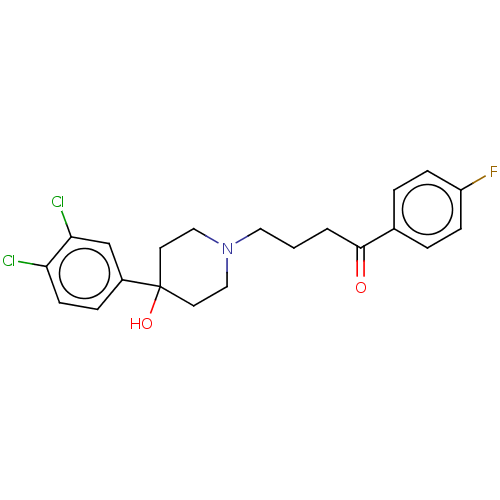 (CHEMBL4544491) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Displacement of PPHT-red from SNAP-tagged human D2LR expressed in CHOK1 cell membranes by TR-FRET assay | J Med Chem 62: 9488-9520 (2019) Article DOI: 10.1021/acs.jmedchem.9b00864 BindingDB Entry DOI: 10.7270/Q2NP27WQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50530700 (CHEMBL4544491) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Displacement of PPHT-red from SNAP-tagged human D2LR expressed in CHOK1 cell membranes by TR-FRET assay | J Med Chem 62: 9488-9520 (2019) Article DOI: 10.1021/acs.jmedchem.9b00864 BindingDB Entry DOI: 10.7270/Q2NP27WQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50530676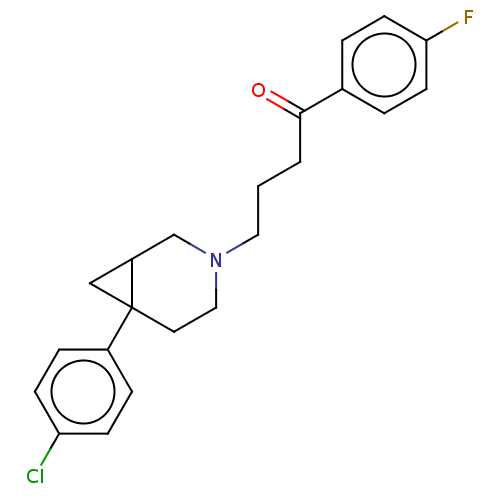 (CHEMBL4528835) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Displacement of PPHT-red from SNAP-tagged human D2LR expressed in CHOK1 cell membranes by TR-FRET assay | J Med Chem 62: 9488-9520 (2019) Article DOI: 10.1021/acs.jmedchem.9b00864 BindingDB Entry DOI: 10.7270/Q2NP27WQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50530676 (CHEMBL4528835) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Displacement of PPHT-red from SNAP-tagged human D2LR expressed in CHOK1 cell membranes by TR-FRET assay | J Med Chem 62: 9488-9520 (2019) Article DOI: 10.1021/acs.jmedchem.9b00864 BindingDB Entry DOI: 10.7270/Q2NP27WQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50328981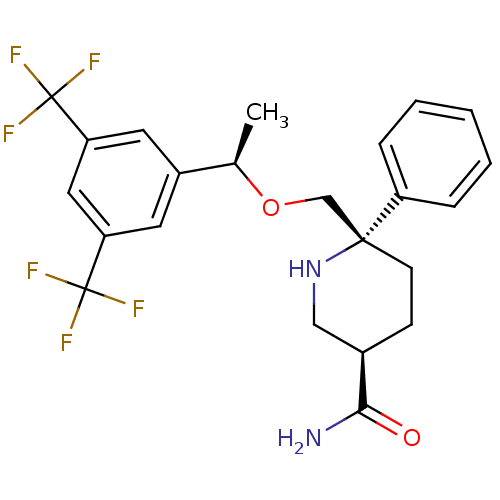 ((3R,6S)-6-(((R)-1-(3,5-bis(trifluoromethyl)phenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SAr-Met from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6313-5 (2010) Article DOI: 10.1016/j.bmcl.2010.08.059 BindingDB Entry DOI: 10.7270/Q23X86VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50062066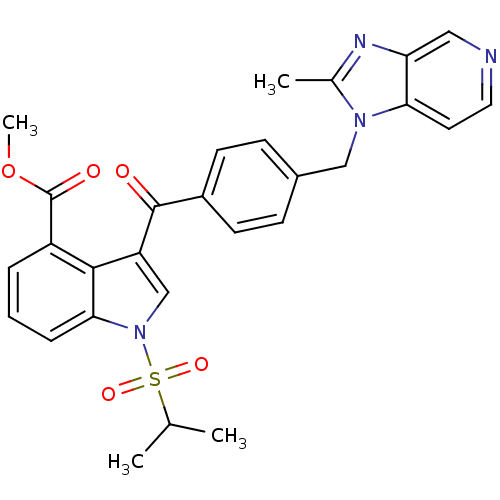 (3-[4-(2-Methyl-imidazo[4,5-c]pyridin-1-ylmethyl)-b...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency against Platelet activating factor (PAF) receptor using [3H]-C18-PAF as radioligand on rabbit platelet membranes | J Med Chem 41: 74-95 (1998) Article DOI: 10.1021/jm970389+ BindingDB Entry DOI: 10.7270/Q2MC8Z4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50139771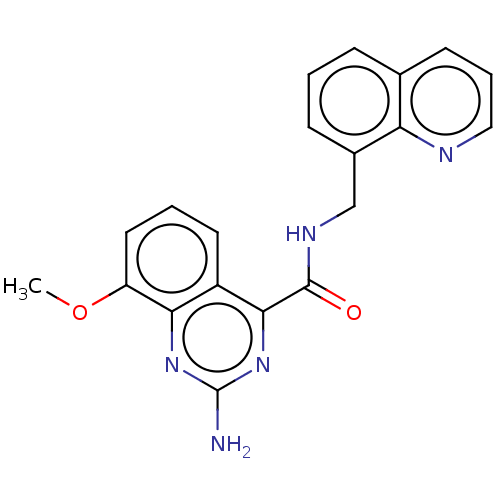 (CHEMBL3765580 | US10138212, Example 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCH-58261 from human adenosine A2A receptor expressed in HEK cell membranes after 60 mins by microplate scintillation counting an... | Bioorg Med Chem Lett 26: 1348-54 (2016) Article DOI: 10.1016/j.bmcl.2015.11.048 BindingDB Entry DOI: 10.7270/Q2S46TT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50139773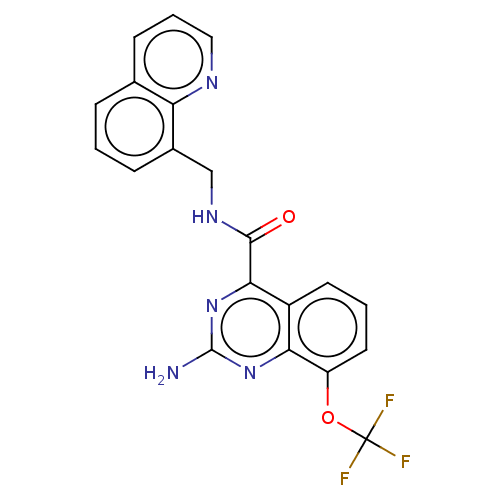 (CHEMBL3765379 | US10138212, Example 101) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCH-58261 from human adenosine A2A receptor expressed in HEK cell membranes after 60 mins by microplate scintillation counting an... | Bioorg Med Chem Lett 26: 1348-54 (2016) Article DOI: 10.1016/j.bmcl.2015.11.048 BindingDB Entry DOI: 10.7270/Q2S46TT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50175828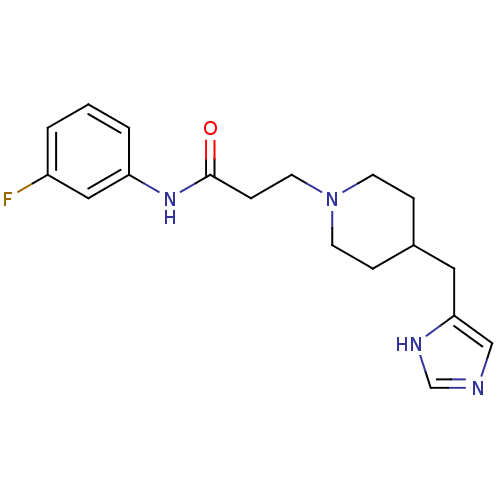 (3-(4-((1H-imidazol-4-yl)methyl)piperidin-1-yl)-N-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from histamine H3 receptor in guinea pig brain | Bioorg Med Chem Lett 16: 395-9 (2005) Article DOI: 10.1016/j.bmcl.2005.09.076 BindingDB Entry DOI: 10.7270/Q29S1QK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50175843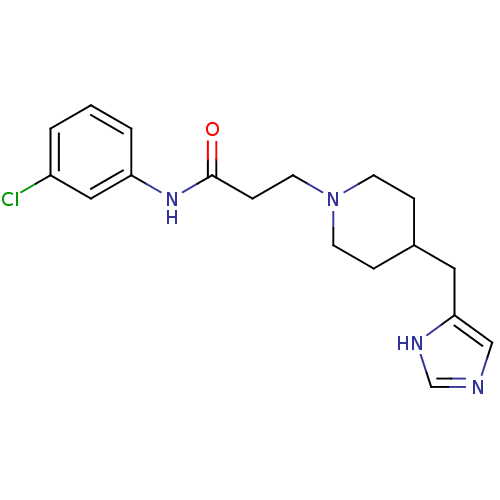 (3-(4-((1H-imidazol-4-yl)methyl)piperidin-1-yl)-N-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from histamine H3 receptor in guinea pig brain | Bioorg Med Chem Lett 16: 395-9 (2005) Article DOI: 10.1016/j.bmcl.2005.09.076 BindingDB Entry DOI: 10.7270/Q29S1QK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50325450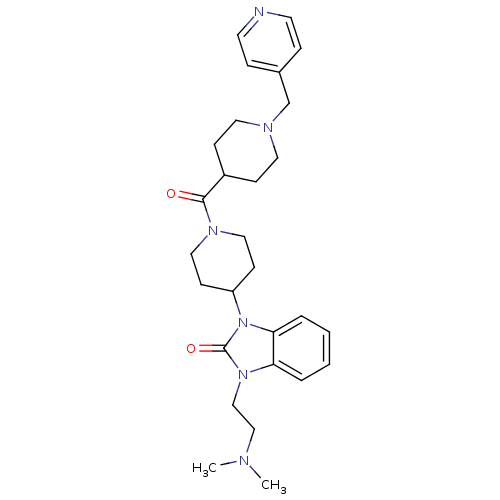 (1-(2-(dimethylamino)ethyl)-3-(1-(1-(pyridin-4-ylme...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]Nalpha-methylhistamine from histamine H3 receptor in guinea pig brain | Bioorg Med Chem Lett 20: 5004-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.052 BindingDB Entry DOI: 10.7270/Q2N016QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50001019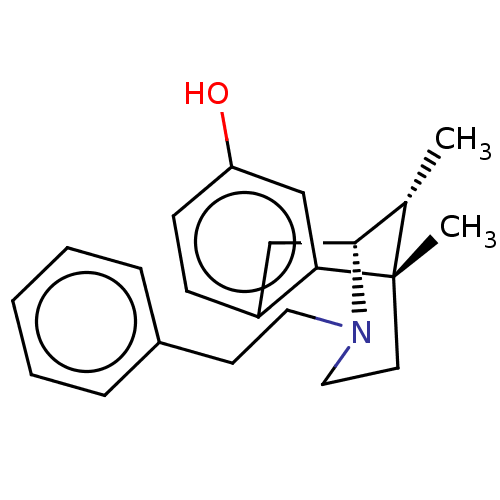 (6,11-Dimethyl-3-phenethyl-1,2,3,4,5,6-hexahydro-2,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocin from sigma1 receptor in guinea pig brain P2 membranes after 120 mins | Eur J Med Chem 125: 603-610 (2017) Article DOI: 10.1016/j.ejmech.2016.09.077 BindingDB Entry DOI: 10.7270/Q2J38VTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50325465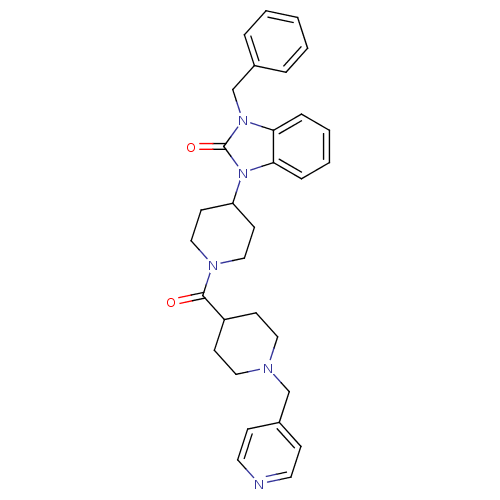 (1-benzyl-3-(1-(1-(pyridin-4-ylmethyl)piperidine-4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]Nalpha-methylhistamine from histamine H3 receptor in guinea pig brain | Bioorg Med Chem Lett 20: 5004-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.052 BindingDB Entry DOI: 10.7270/Q2N016QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50325466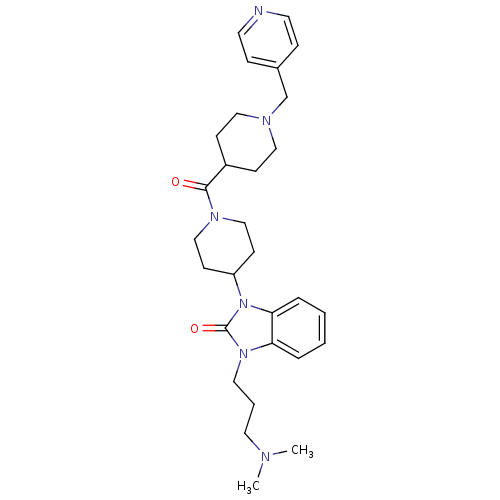 (1-(3-(dimethylamino)propyl)-3-(1-(1-(pyridin-4-ylm...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]Nalpha-methylhistamine from histamine H3 receptor in guinea pig brain | Bioorg Med Chem Lett 20: 5004-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.052 BindingDB Entry DOI: 10.7270/Q2N016QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50229129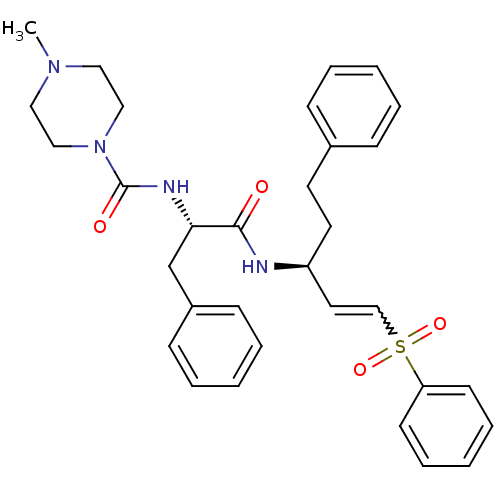 (4-Methyl-piperazine-1-carboxylic acid [(S)-1-((E)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Time dependent inhibition of Trypanosoma cruzi cruzain using Cbz-Phe-Arg-AMC as substrate assessed as inhibition constant for EI complex measured for... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00628 BindingDB Entry DOI: 10.7270/Q2445RC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50328980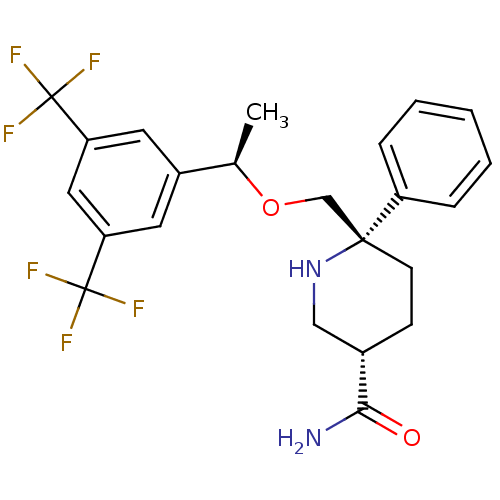 ((3S,6S)-6-(((R)-1-(3,5-bis(trifluoromethyl)phenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SAr-Met from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6313-5 (2010) Article DOI: 10.1016/j.bmcl.2010.08.059 BindingDB Entry DOI: 10.7270/Q23X86VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50067499 ((6aR,10aR)-3(1,1-dimethylheptyl)-9-hydroxymethyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Louis University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 binding to Cannabinoid receptor 1 in rat brain membranes | J Med Chem 41: 4207-15 (1998) Article DOI: 10.1021/jm970239z BindingDB Entry DOI: 10.7270/Q2C24VK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM210759 (US9290454, 4.4) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Curated by ChEMBL | Assay Description Antagonist activity at CRTh2 (unknown origin) assessed as inhibition of CD11b activation | Bioorg Med Chem Lett 27: 5344-5348 (2017) Article DOI: 10.1016/j.bmcl.2017.07.064 BindingDB Entry DOI: 10.7270/Q2HX1G7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50328973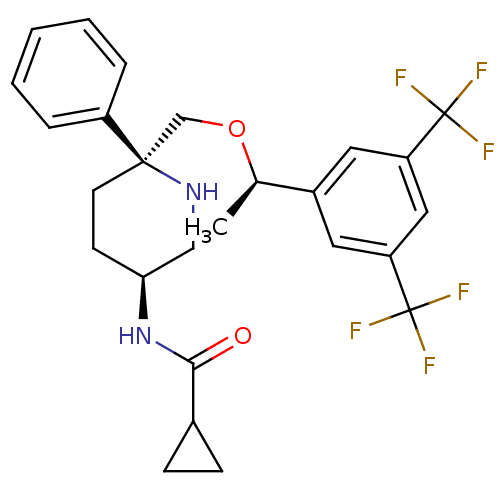 (CHEMBL1270175 | N-((3S,6S)-6-(((R)-1-(3,5-bis(trif...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SAr-Met from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6313-5 (2010) Article DOI: 10.1016/j.bmcl.2010.08.059 BindingDB Entry DOI: 10.7270/Q23X86VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50325460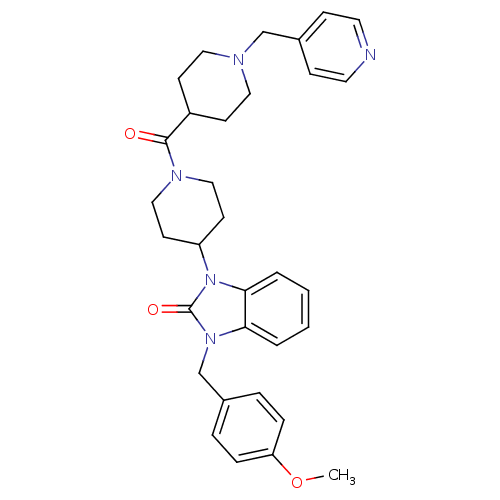 (1-(4-methoxybenzyl)-3-(1-(1-(pyridin-4-ylmethyl)pi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]Nalpha-methylhistamine from histamine H3 receptor in guinea pig brain | Bioorg Med Chem Lett 20: 5004-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.052 BindingDB Entry DOI: 10.7270/Q2N016QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50328974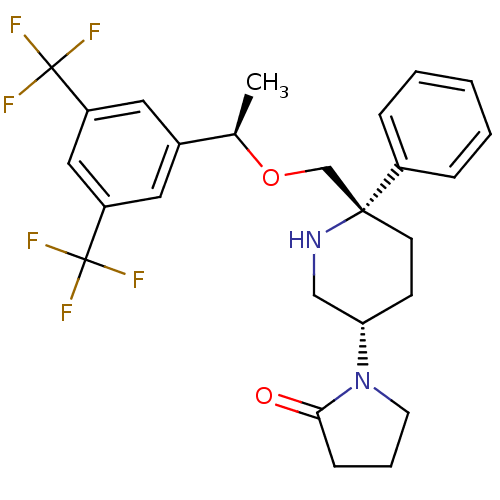 (1-((3S,6S)-6-(((R)-1-(3,5-bis(trifluoromethyl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SAr-Met from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6313-5 (2010) Article DOI: 10.1016/j.bmcl.2010.08.059 BindingDB Entry DOI: 10.7270/Q23X86VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50328982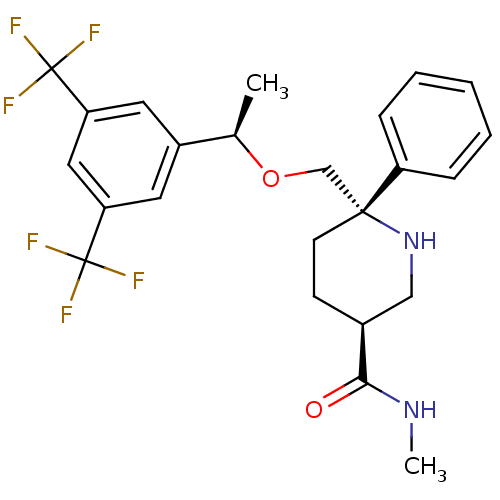 ((3S,6S)-6-(((R)-1-(3,5-bis(trifluoromethyl)phenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SAr-Met from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6313-5 (2010) Article DOI: 10.1016/j.bmcl.2010.08.059 BindingDB Entry DOI: 10.7270/Q23X86VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50017698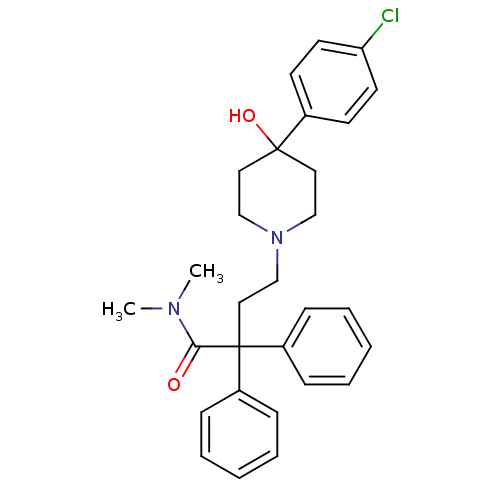 (4-(4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl)-N,N...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.268 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in HEK293 cell membrane incubated for 60 mins by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00611 BindingDB Entry DOI: 10.7270/Q2N301V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50328979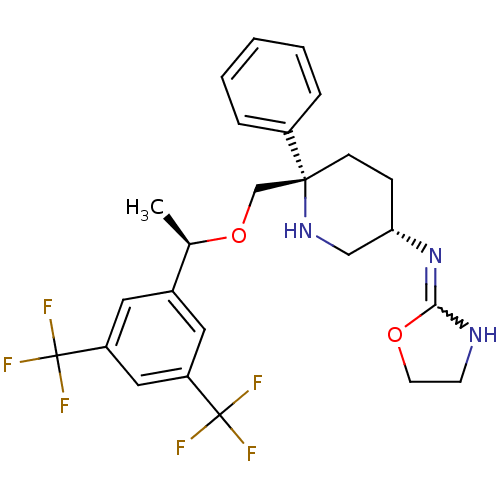 (CHEMBL1270465 | N-((3S,6S)-6-(((R)-1-(3,5-bis(trif...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SAr-Met from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6313-5 (2010) Article DOI: 10.1016/j.bmcl.2010.08.059 BindingDB Entry DOI: 10.7270/Q23X86VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50328972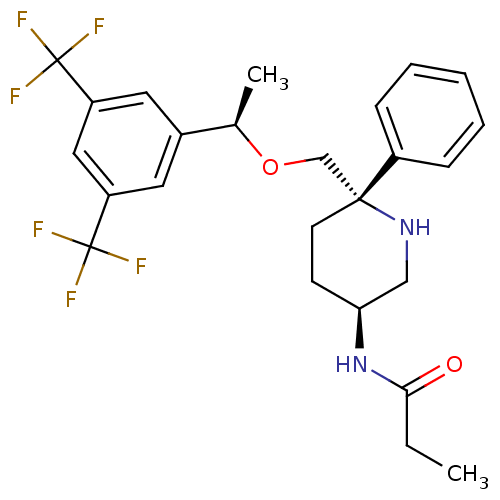 (CHEMBL1270174 | N-((3S,6S)-6-(((R)-1-(3,5-bis(trif...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SAr-Met from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6313-5 (2010) Article DOI: 10.1016/j.bmcl.2010.08.059 BindingDB Entry DOI: 10.7270/Q23X86VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM92649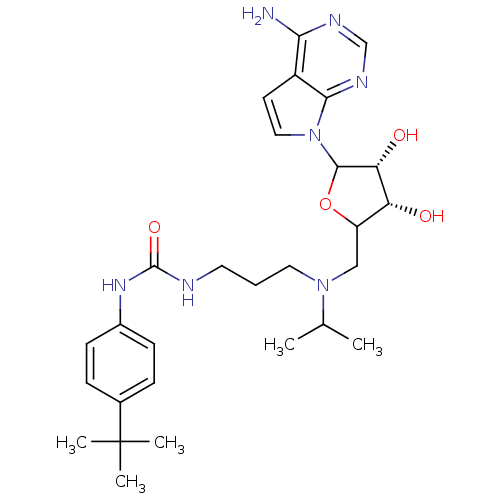 (EPZ004777) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. | Assay Description Assay of DOT1L enzymatic activity were performed under balanced conditions using a radiometric assay. | Chem Biol Drug Des 80: 971-80 (2012) Article DOI: 10.1111/cbdd.12050 BindingDB Entry DOI: 10.7270/Q2Z89B12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM35226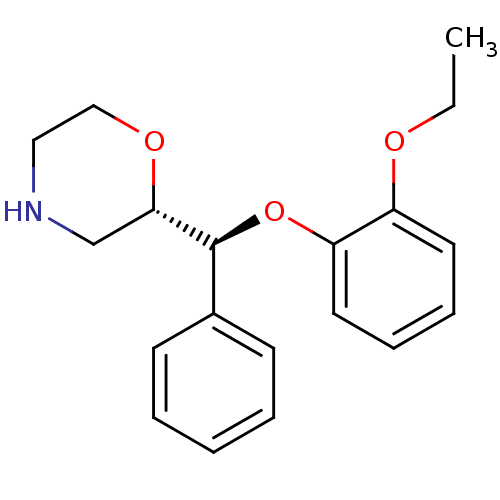 ((S,S)-reboxetine | Reboxetine | Vestra) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.300 | -56.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Wyeth Research | Assay Description Compounds were evaluated the inhibition of [3H] nisoxetine binding to MDCK-Net6 cells, stably transfected with the human norepinephrine transporter (... | Bioorg Med Chem 17: 7802-15 (2009) Article DOI: 10.1016/j.bmc.2009.09.023 BindingDB Entry DOI: 10.7270/Q26Q1VK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50328988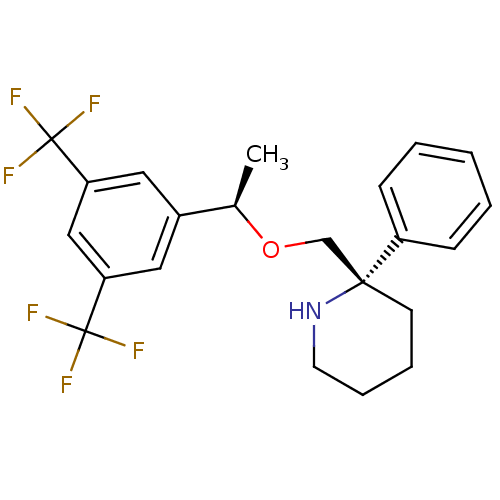 ((S)-2-(((R)-1-(3,5-bis(trifluoromethyl)phenyl)etho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SAr-Met from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6313-5 (2010) Article DOI: 10.1016/j.bmcl.2010.08.059 BindingDB Entry DOI: 10.7270/Q23X86VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50325461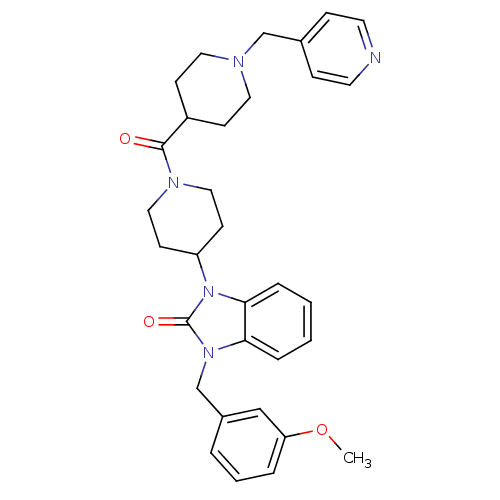 (1-(3-methoxybenzyl)-3-(1-(1-(pyridin-4-ylmethyl)pi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]Nalpha-methylhistamine from histamine H3 receptor in guinea pig brain | Bioorg Med Chem Lett 20: 5004-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.052 BindingDB Entry DOI: 10.7270/Q2N016QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50169599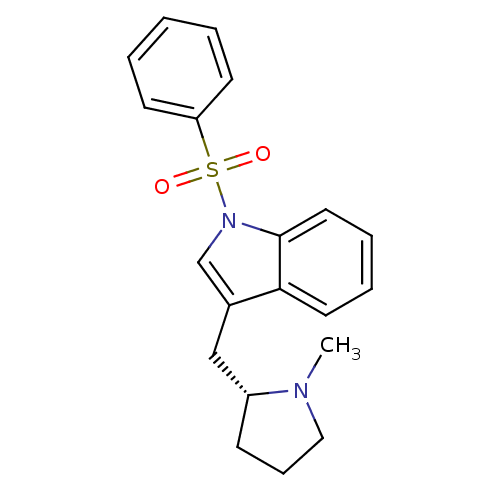 (1-Benzenesulfonyl-3-((R)-1-methyl-pyrrolidin-2-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells | J Med Chem 51: 603-11 (2008) Article DOI: 10.1021/jm070910s BindingDB Entry DOI: 10.7270/Q2CJ8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50169599 (1-Benzenesulfonyl-3-((R)-1-methyl-pyrrolidin-2-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity against h5-HT6 receptor transiently expressed in HEK293 cells | Bioorg Med Chem Lett 15: 3510-3 (2005) Article DOI: 10.1016/j.bmcl.2005.05.092 BindingDB Entry DOI: 10.7270/Q20Z7411 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50139748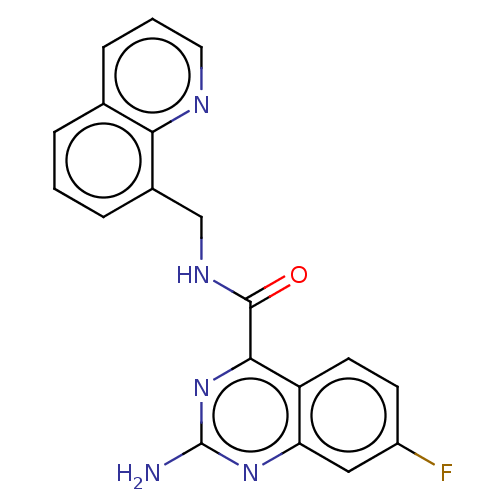 (CHEMBL3763717) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCH-58261 from human adenosine A2A receptor expressed in HEK cell membranes after 60 mins by microplate scintillation counting an... | Bioorg Med Chem Lett 26: 1348-54 (2016) Article DOI: 10.1016/j.bmcl.2015.11.048 BindingDB Entry DOI: 10.7270/Q2S46TT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50086063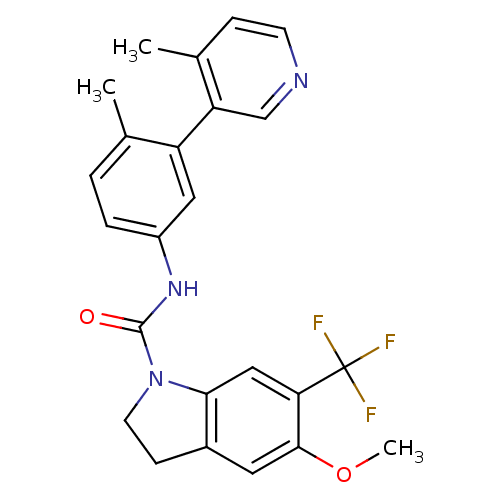 (5-Methoxy-6-trifluoromethyl-2,3-dihydro-indole-1-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity towards human cloned 5-hydroxytryptamine receptor 2C in HEK293 cells, using [3H]mesulergine as radioligand. | J Med Chem 43: 1123-34 (2000) Checked by Author BindingDB Entry DOI: 10.7270/Q23X85V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50530683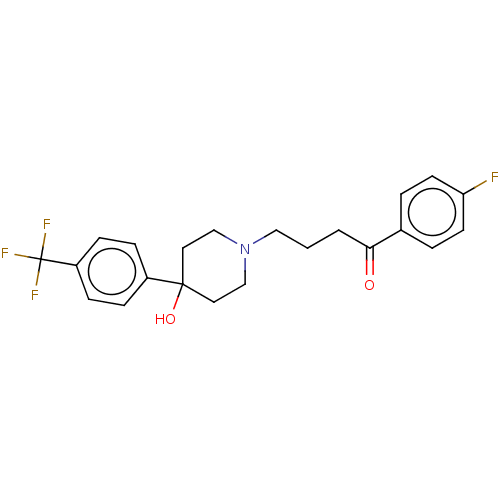 (CHEMBL4436610) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.324 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Displacement of PPHT-red from SNAP-tagged human D2LR expressed in CHOK1 cell membranes by TR-FRET assay | J Med Chem 62: 9488-9520 (2019) Article DOI: 10.1021/acs.jmedchem.9b00864 BindingDB Entry DOI: 10.7270/Q2NP27WQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50530683 (CHEMBL4436610) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.324 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Displacement of PPHT-red from SNAP-tagged human D2LR expressed in CHOK1 cell membranes by TR-FRET assay | J Med Chem 62: 9488-9520 (2019) Article DOI: 10.1021/acs.jmedchem.9b00864 BindingDB Entry DOI: 10.7270/Q2NP27WQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50328978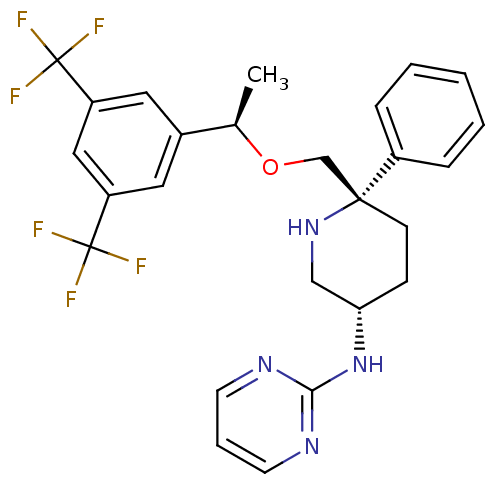 (CHEMBL1270464 | N-((3S,6S)-6-(((R)-1-(3,5-bis(trif...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SAr-Met from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6313-5 (2010) Article DOI: 10.1016/j.bmcl.2010.08.059 BindingDB Entry DOI: 10.7270/Q23X86VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50328986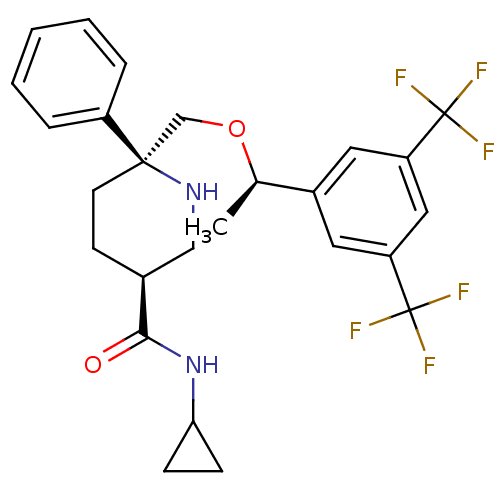 ((3S,6S)-6-(((R)-1-(3,5-bis(trifluoromethyl)phenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SAr-Met from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6313-5 (2010) Article DOI: 10.1016/j.bmcl.2010.08.059 BindingDB Entry DOI: 10.7270/Q23X86VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50328971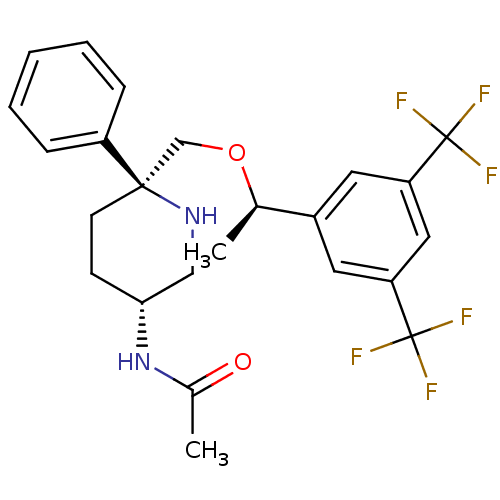 (CHEMBL1270067 | N-((3R,6S)-6-(((R)-1-(3,5-bis(trif...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SAr-Met from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6313-5 (2010) Article DOI: 10.1016/j.bmcl.2010.08.059 BindingDB Entry DOI: 10.7270/Q23X86VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50328984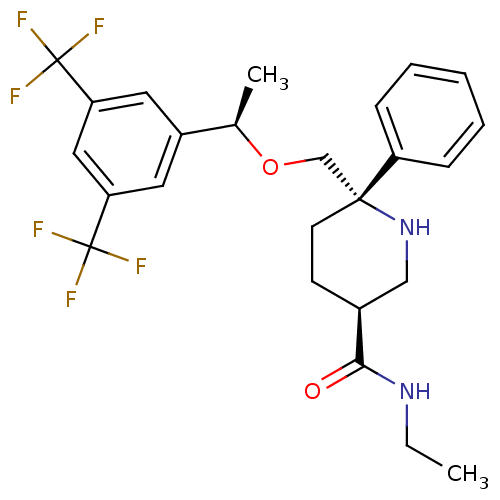 ((3S,6S)-6-(((R)-1-(3,5-bis(trifluoromethyl)phenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SAr-Met from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6313-5 (2010) Article DOI: 10.1016/j.bmcl.2010.08.059 BindingDB Entry DOI: 10.7270/Q23X86VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 28354 total ) | Next | Last >> |