

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metabotropic glutamate receptor 1 (RAT) | BDBM86211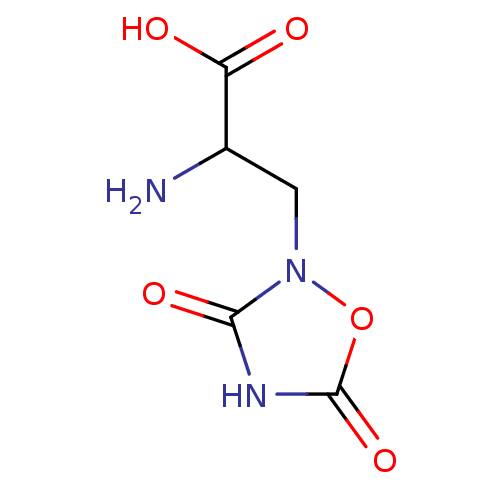 (CAS_52809-07-1 | NSC_40539 | Quisqualate) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse Curated by PDSP Ki Database | Mol Pharmacol 63: 1082-93 (2003) Article DOI: 10.1124/mol.63.5.1082 BindingDB Entry DOI: 10.7270/Q2BV7F62 | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50002371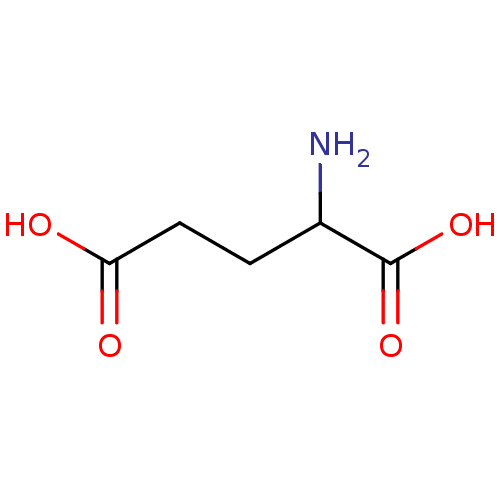 (2-aminopentanedioic acidglutamic acid | CHEMBL2763...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse Curated by PDSP Ki Database | Mol Pharmacol 63: 1082-93 (2003) Article DOI: 10.1124/mol.63.5.1082 BindingDB Entry DOI: 10.7270/Q2BV7F62 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50159110 (1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development, LLC Curated by PDSP Ki Database | Br J Pharmacol 143: 649-61 (2004) Article DOI: 10.1038/sj.bjp.0705964 BindingDB Entry DOI: 10.7270/Q2319TF2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50089896 (4-(Amino-carboxy-methyl)-3-methyl-benzoic acid((+)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse Curated by PDSP Ki Database | Mol Pharmacol 63: 1082-93 (2003) Article DOI: 10.1124/mol.63.5.1082 BindingDB Entry DOI: 10.7270/Q2BV7F62 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM50159110 (1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development, LLC Curated by PDSP Ki Database | Br J Pharmacol 143: 649-61 (2004) Article DOI: 10.1038/sj.bjp.0705964 BindingDB Entry DOI: 10.7270/Q2319TF2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50163606 (1-(3,4-Dihydro-2H-1-oxa-9-aza-anthracen-6-yl)-2-ph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse Curated by PDSP Ki Database | Mol Pharmacol 63: 1082-93 (2003) Article DOI: 10.1124/mol.63.5.1082 BindingDB Entry DOI: 10.7270/Q2BV7F62 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50231744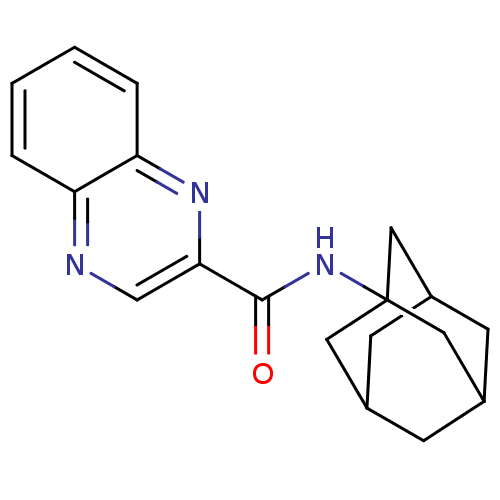 (CHEMBL399160 | NPS 2390 | quinoxaline-2-carboxylic...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse Curated by PDSP Ki Database | Mol Pharmacol 63: 1082-93 (2003) Article DOI: 10.1124/mol.63.5.1082 BindingDB Entry DOI: 10.7270/Q2BV7F62 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (Homo sapiens (Human)) | BDBM50146518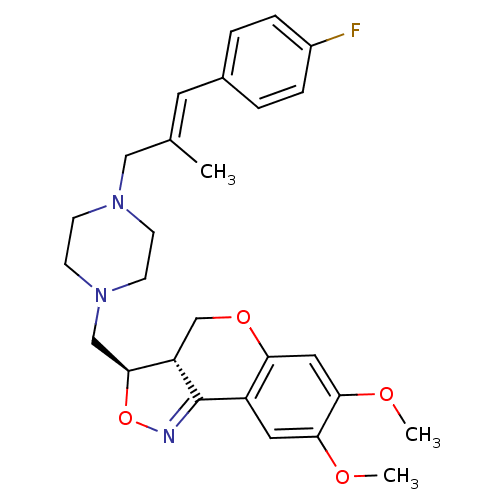 ((3R,3aS)-3-{4-[3-(4-Fluoro-phenyl)-2-methyl-allyl]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]rawolscine from human cloned adrenergic alpha-2B receptor transfected in CHO cells | Bioorg Med Chem 15: 3649-60 (2007) Article DOI: 10.1016/j.bmc.2007.03.053 BindingDB Entry DOI: 10.7270/Q2BZ66VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50030628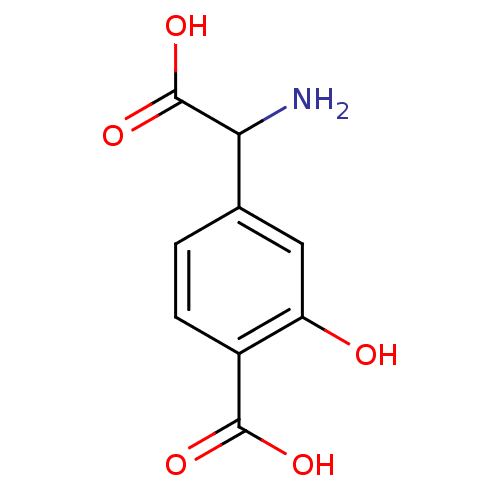 ((S)-4C3HPG | 4-(Amino-carboxy-methyl)-2-hydroxy-be...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse Curated by PDSP Ki Database | Mol Pharmacol 63: 1082-93 (2003) Article DOI: 10.1124/mol.63.5.1082 BindingDB Entry DOI: 10.7270/Q2BV7F62 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50146518 ((3R,3aS)-3-{4-[3-(4-Fluoro-phenyl)-2-methyl-allyl]...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from human cloned 5HTT receptor transfected in CHO cells | Bioorg Med Chem 15: 3649-60 (2007) Article DOI: 10.1016/j.bmc.2007.03.053 BindingDB Entry DOI: 10.7270/Q2BZ66VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50146518 ((3R,3aS)-3-{4-[3-(4-Fluoro-phenyl)-2-methyl-allyl]...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from human cloned 5HTT receptor transfected in CHO cells | Bioorg Med Chem 15: 3649-60 (2007) Article DOI: 10.1016/j.bmc.2007.03.053 BindingDB Entry DOI: 10.7270/Q2BZ66VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM86212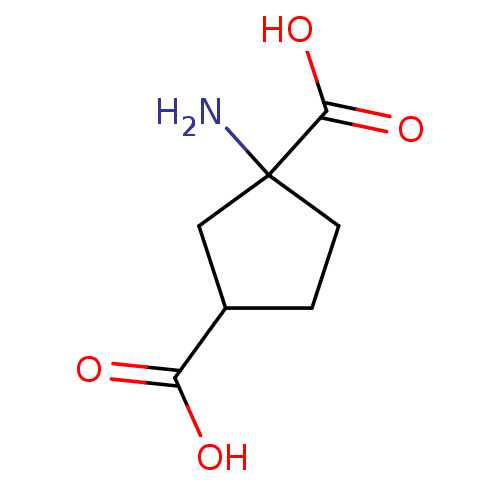 (1S, 3R-ACPD | CAS_104766 | NSC_104766) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse Curated by PDSP Ki Database | Mol Pharmacol 63: 1082-93 (2003) Article DOI: 10.1124/mol.63.5.1082 BindingDB Entry DOI: 10.7270/Q2BV7F62 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50209685 (CHEMBL226636 | cis-(+)-7,8-dimethoxy-3-[4-(3-(4-fl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from human cloned 5HTT receptor transfected in CHO cells | Bioorg Med Chem 15: 3649-60 (2007) Article DOI: 10.1016/j.bmc.2007.03.053 BindingDB Entry DOI: 10.7270/Q2BZ66VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50209685 (CHEMBL226636 | cis-(+)-7,8-dimethoxy-3-[4-(3-(4-fl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from human cloned 5HTT receptor transfected in CHO cells | Bioorg Med Chem 15: 3649-60 (2007) Article DOI: 10.1016/j.bmc.2007.03.053 BindingDB Entry DOI: 10.7270/Q2BZ66VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50209684 (CHEMBL390718 | cis-(+)-7,8-dimethoxy-3-[4-(2-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]rawolscine from human cloned adrenergic alpha2A receptor transfected in CHO cells | Bioorg Med Chem 15: 3649-60 (2007) Article DOI: 10.1016/j.bmc.2007.03.053 BindingDB Entry DOI: 10.7270/Q2BZ66VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50209684 (CHEMBL390718 | cis-(+)-7,8-dimethoxy-3-[4-(2-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]rawolscine from human cloned adrenergic alpha2A receptor transfected in CHO cells | Bioorg Med Chem 15: 3649-60 (2007) Article DOI: 10.1016/j.bmc.2007.03.053 BindingDB Entry DOI: 10.7270/Q2BZ66VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM22914 (CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development, LLC Curated by PDSP Ki Database | Br J Pharmacol 143: 649-61 (2004) Article DOI: 10.1038/sj.bjp.0705964 BindingDB Entry DOI: 10.7270/Q2319TF2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50030629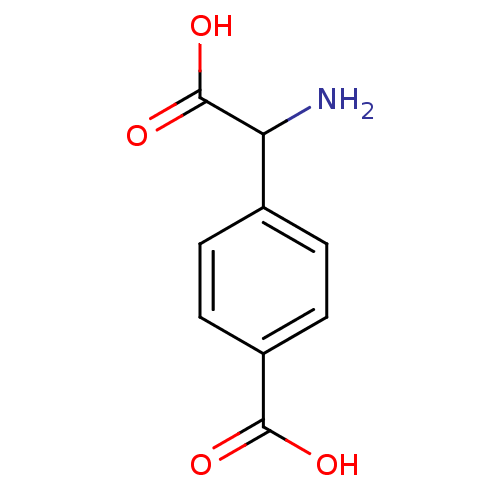 ((R,S)-4-Carboxy-3-hydroxyphenylglycine | (S)-4CPG ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse Curated by PDSP Ki Database | Mol Pharmacol 63: 1082-93 (2003) Article DOI: 10.1124/mol.63.5.1082 BindingDB Entry DOI: 10.7270/Q2BV7F62 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50209684 (CHEMBL390718 | cis-(+)-7,8-dimethoxy-3-[4-(2-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]rawolscine from human cloned adrenergic Alpha-2C receptor transfected in CHO cells | Bioorg Med Chem 15: 3649-60 (2007) Article DOI: 10.1016/j.bmc.2007.03.053 BindingDB Entry DOI: 10.7270/Q2BZ66VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50209684 (CHEMBL390718 | cis-(+)-7,8-dimethoxy-3-[4-(2-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]rawolscine from human cloned adrenergic Alpha-2C receptor transfected in CHO cells | Bioorg Med Chem 15: 3649-60 (2007) Article DOI: 10.1016/j.bmc.2007.03.053 BindingDB Entry DOI: 10.7270/Q2BZ66VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50209684 (CHEMBL390718 | cis-(+)-7,8-dimethoxy-3-[4-(2-methy...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from human cloned 5HTT receptor transfected in CHO cells | Bioorg Med Chem 15: 3649-60 (2007) Article DOI: 10.1016/j.bmc.2007.03.053 BindingDB Entry DOI: 10.7270/Q2BZ66VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50209684 (CHEMBL390718 | cis-(+)-7,8-dimethoxy-3-[4-(2-methy...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from human cloned 5HTT receptor transfected in CHO cells | Bioorg Med Chem 15: 3649-60 (2007) Article DOI: 10.1016/j.bmc.2007.03.053 BindingDB Entry DOI: 10.7270/Q2BZ66VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50146518 ((3R,3aS)-3-{4-[3-(4-Fluoro-phenyl)-2-methyl-allyl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]rawolscine from human cloned adrenergic Alpha-2C receptor transfected in CHO cells | Bioorg Med Chem 15: 3649-60 (2007) Article DOI: 10.1016/j.bmc.2007.03.053 BindingDB Entry DOI: 10.7270/Q2BZ66VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50146518 ((3R,3aS)-3-{4-[3-(4-Fluoro-phenyl)-2-methyl-allyl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]rawolscine from human cloned adrenergic Alpha-2C receptor transfected in CHO cells | Bioorg Med Chem 15: 3649-60 (2007) Article DOI: 10.1016/j.bmc.2007.03.053 BindingDB Entry DOI: 10.7270/Q2BZ66VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50212323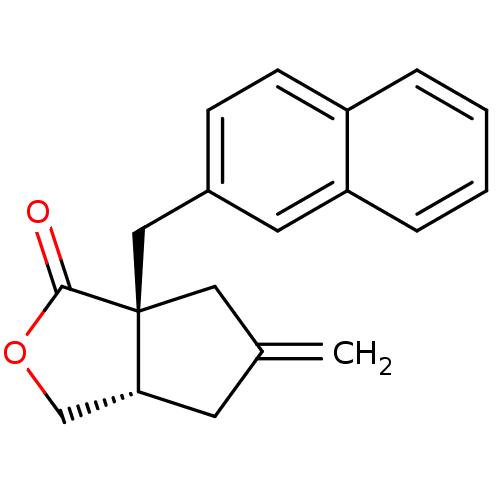 ((3aS,6aS)-5-methylene-6a-(naphthalen-2-ylmethyl)-h...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 11.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse Curated by PDSP Ki Database | Mol Pharmacol 63: 1082-93 (2003) Article DOI: 10.1124/mol.63.5.1082 BindingDB Entry DOI: 10.7270/Q2BV7F62 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (Homo sapiens (Human)) | BDBM50209684 (CHEMBL390718 | cis-(+)-7,8-dimethoxy-3-[4-(2-methy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]rawolscine from human cloned adrenergic alpha-2B receptor transfected in CHO cells | Bioorg Med Chem 15: 3649-60 (2007) Article DOI: 10.1016/j.bmc.2007.03.053 BindingDB Entry DOI: 10.7270/Q2BZ66VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50163631 (1-Methyl-4-piperidin-4-ylidene-1,4-dihydro-1,5,7a-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity for H1 histamine receptor expressed in CHO cells | J Med Chem 48: 2154-66 (2005) Article DOI: 10.1021/jm049495j BindingDB Entry DOI: 10.7270/Q2N0179T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50163629 (CHEMBL539843 | spiro[6,11-dihydro-5H-benzo[d]imida...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity for H1 histamine receptor expressed in CHO cells | J Med Chem 48: 2154-66 (2005) Article DOI: 10.1021/jm049495j BindingDB Entry DOI: 10.7270/Q2N0179T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50163630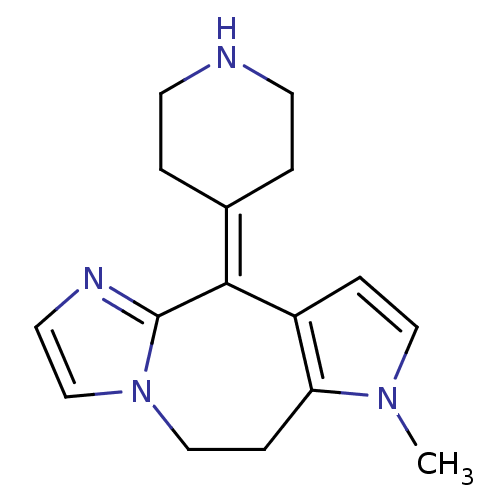 (1-Methyl-4-piperidin-4-ylidene-1,4,8,9-tetrahydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity for H1 histamine receptor expressed in CHO cells | J Med Chem 48: 2154-66 (2005) Article DOI: 10.1021/jm049495j BindingDB Entry DOI: 10.7270/Q2N0179T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50146518 ((3R,3aS)-3-{4-[3-(4-Fluoro-phenyl)-2-methyl-allyl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]rawolscine from human cloned adrenergic alpha2A receptor transfected in CHO cells | Bioorg Med Chem 15: 3649-60 (2007) Article DOI: 10.1016/j.bmc.2007.03.053 BindingDB Entry DOI: 10.7270/Q2BZ66VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50146518 ((3R,3aS)-3-{4-[3-(4-Fluoro-phenyl)-2-methyl-allyl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]rawolscine from human cloned adrenergic alpha2A receptor transfected in CHO cells | Bioorg Med Chem 15: 3649-60 (2007) Article DOI: 10.1016/j.bmc.2007.03.053 BindingDB Entry DOI: 10.7270/Q2BZ66VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM22876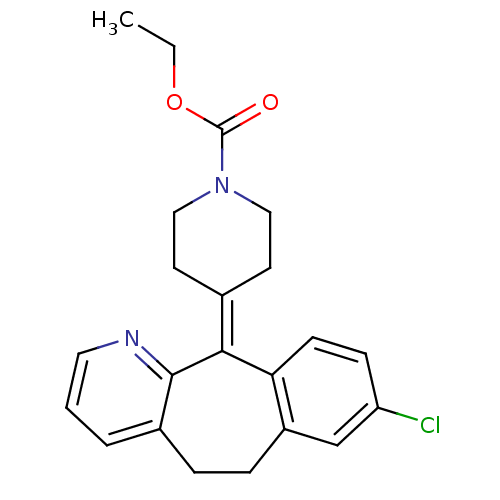 (CHEMBL998 | Claritin | Loratadine | Sch 29851 | US...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity for H1 histamine receptor expressed in CHO cells | J Med Chem 48: 2154-66 (2005) Article DOI: 10.1021/jm049495j BindingDB Entry DOI: 10.7270/Q2N0179T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22914 (CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 39.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development, LLC Curated by PDSP Ki Database | Br J Pharmacol 143: 649-61 (2004) Article DOI: 10.1038/sj.bjp.0705964 BindingDB Entry DOI: 10.7270/Q2319TF2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM22890 (2-(2-{4-[(4-chlorophenyl)(phenyl)methyl]piperazin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity for H1 histamine receptor expressed in CHO cells | J Med Chem 48: 2154-66 (2005) Article DOI: 10.1021/jm049495j BindingDB Entry DOI: 10.7270/Q2N0179T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transporter (Rattus norvegicus) | BDBM50146518 ((3R,3aS)-3-{4-[3-(4-Fluoro-phenyl)-2-methyl-allyl]...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]nisoxetine from rat NET | Bioorg Med Chem 15: 3649-60 (2007) Article DOI: 10.1016/j.bmc.2007.03.053 BindingDB Entry DOI: 10.7270/Q2BZ66VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50209685 (CHEMBL226636 | cis-(+)-7,8-dimethoxy-3-[4-(3-(4-fl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]rawolscine from human cloned adrenergic Alpha-2C receptor transfected in CHO cells | Bioorg Med Chem 15: 3649-60 (2007) Article DOI: 10.1016/j.bmc.2007.03.053 BindingDB Entry DOI: 10.7270/Q2BZ66VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50209685 (CHEMBL226636 | cis-(+)-7,8-dimethoxy-3-[4-(3-(4-fl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]rawolscine from human cloned adrenergic Alpha-2C receptor transfected in CHO cells | Bioorg Med Chem 15: 3649-60 (2007) Article DOI: 10.1016/j.bmc.2007.03.053 BindingDB Entry DOI: 10.7270/Q2BZ66VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50146518 ((3R,3aS)-3-{4-[3-(4-Fluoro-phenyl)-2-methyl-allyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]pyrilamine from human cloned histamine H1 receptor | Bioorg Med Chem 15: 3649-60 (2007) Article DOI: 10.1016/j.bmc.2007.03.053 BindingDB Entry DOI: 10.7270/Q2BZ66VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22914 (CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 79.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development, LLC Curated by PDSP Ki Database | Br J Pharmacol 143: 649-61 (2004) Article DOI: 10.1038/sj.bjp.0705964 BindingDB Entry DOI: 10.7270/Q2319TF2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50030630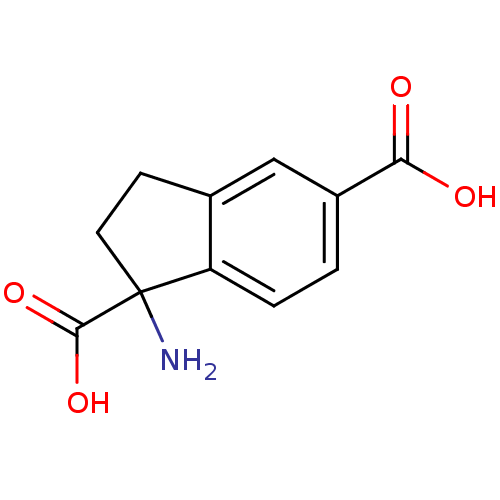 ((RS)-1-aminoindan-1,5-dicarboxylic acid | 1-Amino-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 98.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse Curated by PDSP Ki Database | Mol Pharmacol 63: 1082-93 (2003) Article DOI: 10.1124/mol.63.5.1082 BindingDB Entry DOI: 10.7270/Q2BV7F62 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50209685 (CHEMBL226636 | cis-(+)-7,8-dimethoxy-3-[4-(3-(4-fl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]rawolscine from human cloned adrenergic alpha2A receptor transfected in CHO cells | Bioorg Med Chem 15: 3649-60 (2007) Article DOI: 10.1016/j.bmc.2007.03.053 BindingDB Entry DOI: 10.7270/Q2BZ66VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50209685 (CHEMBL226636 | cis-(+)-7,8-dimethoxy-3-[4-(3-(4-fl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]rawolscine from human cloned adrenergic alpha2A receptor transfected in CHO cells | Bioorg Med Chem 15: 3649-60 (2007) Article DOI: 10.1016/j.bmc.2007.03.053 BindingDB Entry DOI: 10.7270/Q2BZ66VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50146518 ((3R,3aS)-3-{4-[3-(4-Fluoro-phenyl)-2-methyl-allyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from human cloned alpha-1A receptor | Bioorg Med Chem 15: 3649-60 (2007) Article DOI: 10.1016/j.bmc.2007.03.053 BindingDB Entry DOI: 10.7270/Q2BZ66VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (Homo sapiens (Human)) | BDBM50209685 (CHEMBL226636 | cis-(+)-7,8-dimethoxy-3-[4-(3-(4-fl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]rawolscine from human cloned adrenergic alpha-2B receptor transfected in CHO cells | Bioorg Med Chem 15: 3649-60 (2007) Article DOI: 10.1016/j.bmc.2007.03.053 BindingDB Entry DOI: 10.7270/Q2BZ66VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50030627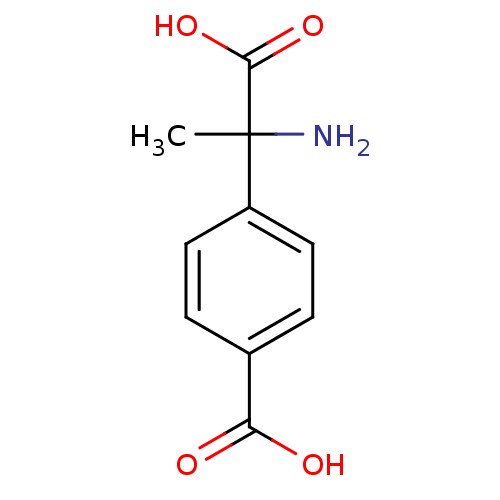 ((+-)-MCPG | (R,S)-alpha-Methyl-4-carboxyphenylglyc...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 165 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse Curated by PDSP Ki Database | Mol Pharmacol 63: 1082-93 (2003) Article DOI: 10.1124/mol.63.5.1082 BindingDB Entry DOI: 10.7270/Q2BV7F62 | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50146518 ((3R,3aS)-3-{4-[3-(4-Fluoro-phenyl)-2-methyl-allyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human cloned NK1 receptor | Bioorg Med Chem 15: 3649-60 (2007) Article DOI: 10.1016/j.bmc.2007.03.053 BindingDB Entry DOI: 10.7270/Q2BZ66VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50146518 ((3R,3aS)-3-{4-[3-(4-Fluoro-phenyl)-2-methyl-allyl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 375 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [125I]iodosulpiride from human cloned dopamine D3 receptor | Bioorg Med Chem 15: 3649-60 (2007) Article DOI: 10.1016/j.bmc.2007.03.053 BindingDB Entry DOI: 10.7270/Q2BZ66VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50146518 ((3R,3aS)-3-{4-[3-(4-Fluoro-phenyl)-2-methyl-allyl]...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 583 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]WIN-35428 from rat DAT | Bioorg Med Chem 15: 3649-60 (2007) Article DOI: 10.1016/j.bmc.2007.03.053 BindingDB Entry DOI: 10.7270/Q2BZ66VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50212323 ((3aS,6aS)-5-methylene-6a-(naphthalen-2-ylmethyl)-h...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse Curated by PDSP Ki Database | Mol Pharmacol 63: 1082-93 (2003) Article DOI: 10.1124/mol.63.5.1082 BindingDB Entry DOI: 10.7270/Q2BV7F62 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM86213 (CAS_5126051 | CHEMBL327783 | CPCCOEt | NSC_5126051) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse Curated by PDSP Ki Database | Mol Pharmacol 63: 1082-93 (2003) Article DOI: 10.1124/mol.63.5.1082 BindingDB Entry DOI: 10.7270/Q2BV7F62 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 400 total ) | Next | Last >> |