 Found 630 hits with Last Name = 'largent-milnes' and Initial = 'tm'
Found 630 hits with Last Name = 'largent-milnes' and Initial = 'tm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Substance-P receptor
(Homo sapiens (Human)) | BDBM50439327
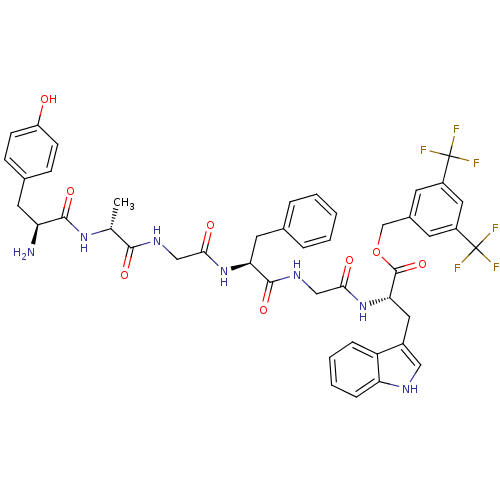
(CHEMBL2419544)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C45H45F6N7O8/c1-25(56-41(63)34(52)17-27-11-13-32(59)14-12-27)40(62)54-22-38(60)57-36(18-26-7-3-2-4-8-26)42(64)55-23-39(61)58-37(19-29-21-53-35-10-6-5-9-33(29)35)43(65)66-24-28-15-30(44(46,47)48)20-31(16-28)45(49,50)51/h2-16,20-21,25,34,36-37,53,59H,17-19,22-24,52H2,1H3,(H,54,62)(H,55,64)(H,56,63)(H,57,60)(H,58,61)/t25-,34+,36+,37+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 23: 4975-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.065
BindingDB Entry DOI: 10.7270/Q2222W64 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50341318
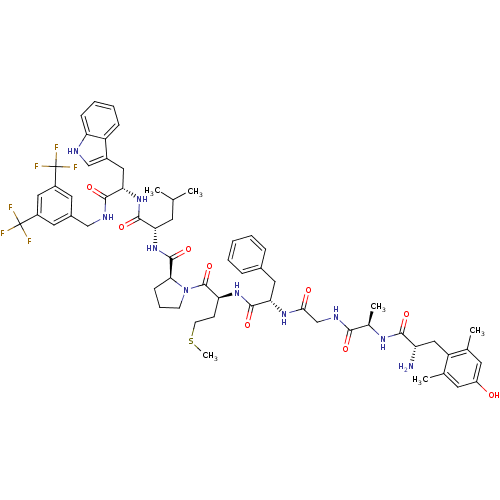
((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-15-(4-hyd...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C61H74F6N10O9S/c1-33(2)21-48(56(83)75-50(27-39-31-69-46-16-11-10-15-43(39)46)55(82)70-30-38-24-40(60(62,63)64)28-41(25-38)61(65,66)67)76-58(85)51-17-12-19-77(51)59(86)47(18-20-87-6)74-57(84)49(26-37-13-8-7-9-14-37)73-52(79)32-71-53(80)36(5)72-54(81)45(68)29-44-34(3)22-42(78)23-35(44)4/h7-11,13-16,22-25,28,31,33,36,45,47-51,69,78H,12,17-21,26-27,29-30,32,68H2,1-6H3,(H,70,82)(H,71,80)(H,72,81)(H,73,79)(H,74,84)(H,75,83)(H,76,85)/t36-,45+,47+,48+,49+,50+,51+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells |
J Med Chem 54: 2029-38 (2011)
Article DOI: 10.1021/jm101023r
BindingDB Entry DOI: 10.7270/Q2862GRK |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50341318

((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-15-(4-hyd...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C61H74F6N10O9S/c1-33(2)21-48(56(83)75-50(27-39-31-69-46-16-11-10-15-43(39)46)55(82)70-30-38-24-40(60(62,63)64)28-41(25-38)61(65,66)67)76-58(85)51-17-12-19-77(51)59(86)47(18-20-87-6)74-57(84)49(26-37-13-8-7-9-14-37)73-52(79)32-71-53(80)36(5)72-54(81)45(68)29-44-34(3)22-42(78)23-35(44)4/h7-11,13-16,22-25,28,31,33,36,45,47-51,69,78H,12,17-21,26-27,29-30,32,68H2,1-6H3,(H,70,82)(H,71,80)(H,72,81)(H,73,79)(H,74,84)(H,75,83)(H,76,85)/t36-,45+,47+,48+,49+,50+,51+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells |
J Med Chem 54: 2029-38 (2011)
Article DOI: 10.1021/jm101023r
BindingDB Entry DOI: 10.7270/Q2862GRK |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50439329
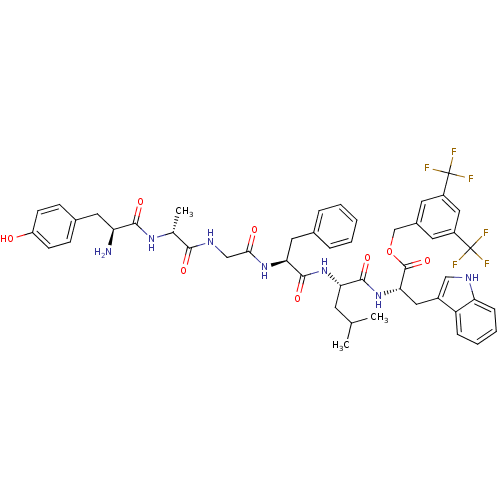
(CHEMBL2419542)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C49H53F6N7O8/c1-27(2)17-39(45(67)62-41(22-32-24-57-38-12-8-7-11-36(32)38)47(69)70-26-31-18-33(48(50,51)52)23-34(19-31)49(53,54)55)61-46(68)40(21-29-9-5-4-6-10-29)60-42(64)25-58-43(65)28(3)59-44(66)37(56)20-30-13-15-35(63)16-14-30/h4-16,18-19,23-24,27-28,37,39-41,57,63H,17,20-22,25-26,56H2,1-3H3,(H,58,65)(H,59,66)(H,60,64)(H,61,68)(H,62,67)/t28-,37+,39+,40+,41+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 23: 4975-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.065
BindingDB Entry DOI: 10.7270/Q2222W64 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50439326
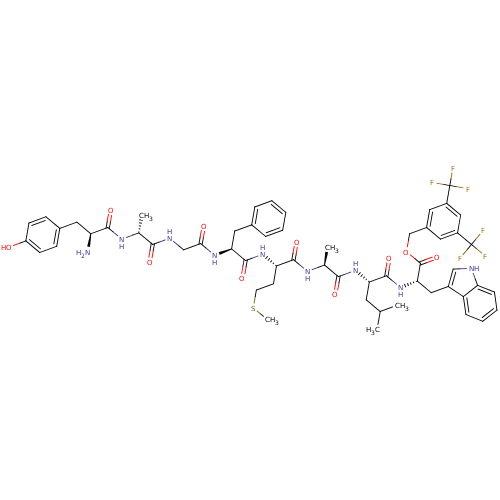
(CHEMBL2419537)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C57H67F6N9O10S/c1-31(2)21-45(53(79)72-47(26-37-28-65-43-14-10-9-13-41(37)43)55(81)82-30-36-22-38(56(58,59)60)27-39(23-36)57(61,62)63)71-50(76)33(4)68-52(78)44(19-20-83-5)70-54(80)46(25-34-11-7-6-8-12-34)69-48(74)29-66-49(75)32(3)67-51(77)42(64)24-35-15-17-40(73)18-16-35/h6-18,22-23,27-28,31-33,42,44-47,65,73H,19-21,24-26,29-30,64H2,1-5H3,(H,66,75)(H,67,77)(H,68,78)(H,69,74)(H,70,80)(H,71,76)(H,72,79)/t32-,33+,42+,44+,45+,46+,47+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 23: 4975-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.065
BindingDB Entry DOI: 10.7270/Q2222W64 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50439325
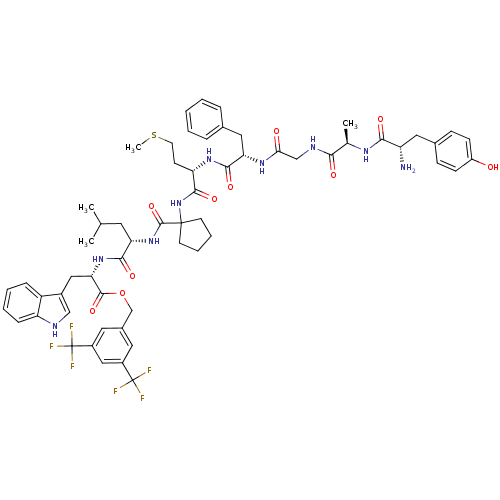
(CHEMBL2419538)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NC1(CCCC1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C60H71F6N9O10S/c1-34(2)24-47(53(80)73-49(29-39-31-68-45-15-9-8-14-43(39)45)56(83)85-33-38-25-40(59(61,62)63)30-41(26-38)60(64,65)66)74-57(84)58(21-10-11-22-58)75-55(82)46(20-23-86-4)72-54(81)48(28-36-12-6-5-7-13-36)71-50(77)32-69-51(78)35(3)70-52(79)44(67)27-37-16-18-42(76)19-17-37/h5-9,12-19,25-26,30-31,34-35,44,46-49,68,76H,10-11,20-24,27-29,32-33,67H2,1-4H3,(H,69,78)(H,70,79)(H,71,77)(H,72,81)(H,73,80)(H,74,84)(H,75,82)/t35-,44+,46+,47+,48+,49+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 23: 4975-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.065
BindingDB Entry DOI: 10.7270/Q2222W64 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50558721

(CHEMBL4784791)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human KOR expressed in mouse HN9.10 cell membranes incubated for 2 hrs by liquid scintillation counting based radiol... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZG6WWT |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50341314
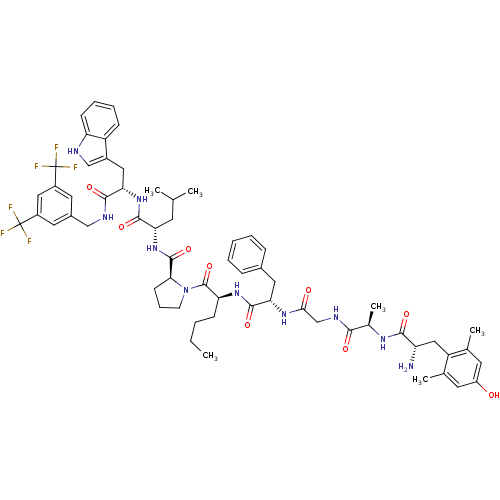
((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-2-butyl-1...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C62H76F6N10O9/c1-7-8-18-48(75-58(85)50(27-38-15-10-9-11-16-38)74-53(80)33-72-54(81)37(6)73-55(82)46(69)30-45-35(4)23-43(79)24-36(45)5)60(87)78-21-14-20-52(78)59(86)77-49(22-34(2)3)57(84)76-51(28-40-32-70-47-19-13-12-17-44(40)47)56(83)71-31-39-25-41(61(63,64)65)29-42(26-39)62(66,67)68/h9-13,15-17,19,23-26,29,32,34,37,46,48-52,70,79H,7-8,14,18,20-22,27-28,30-31,33,69H2,1-6H3,(H,71,83)(H,72,81)(H,73,82)(H,74,80)(H,75,85)(H,76,84)(H,77,86)/t37-,46+,48+,49+,50+,51+,52+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells |
J Med Chem 54: 2029-38 (2011)
Article DOI: 10.1021/jm101023r
BindingDB Entry DOI: 10.7270/Q2862GRK |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50341314

((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-2-butyl-1...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C62H76F6N10O9/c1-7-8-18-48(75-58(85)50(27-38-15-10-9-11-16-38)74-53(80)33-72-54(81)37(6)73-55(82)46(69)30-45-35(4)23-43(79)24-36(45)5)60(87)78-21-14-20-52(78)59(86)77-49(22-34(2)3)57(84)76-51(28-40-32-70-47-19-13-12-17-44(40)47)56(83)71-31-39-25-41(61(63,64)65)29-42(26-39)62(66,67)68/h9-13,15-17,19,23-26,29,32,34,37,46,48-52,70,79H,7-8,14,18,20-22,27-28,30-31,33,69H2,1-6H3,(H,71,83)(H,72,81)(H,73,82)(H,74,80)(H,75,85)(H,76,84)(H,77,86)/t37-,46+,48+,49+,50+,51+,52+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells |
J Med Chem 54: 2029-38 (2011)
Article DOI: 10.1021/jm101023r
BindingDB Entry DOI: 10.7270/Q2862GRK |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM21021
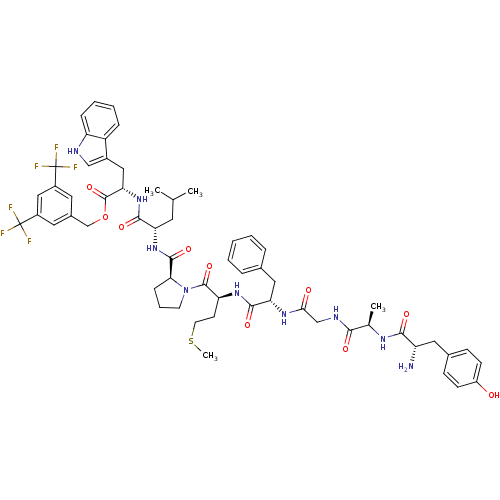
(Bifunctional Peptide Ligand, 5 (TY005) | CHEMBL389...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C59H69F6N9O10S/c1-33(2)23-46(53(79)73-48(28-38-30-67-44-14-9-8-13-42(38)44)57(83)84-32-37-24-39(58(60,61)62)29-40(25-37)59(63,64)65)72-55(81)49-15-10-21-74(49)56(82)45(20-22-85-4)71-54(80)47(27-35-11-6-5-7-12-35)70-50(76)31-68-51(77)34(3)69-52(78)43(66)26-36-16-18-41(75)19-17-36/h5-9,11-14,16-19,24-25,29-30,33-34,43,45-49,67,75H,10,15,20-23,26-28,31-32,66H2,1-4H3,(H,68,77)(H,69,78)(H,70,76)(H,71,80)(H,72,81)(H,73,79)/t34-,43+,45+,46+,47+,48+,49+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 23: 4975-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.065
BindingDB Entry DOI: 10.7270/Q2222W64 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50439329

(CHEMBL2419542)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C49H53F6N7O8/c1-27(2)17-39(45(67)62-41(22-32-24-57-38-12-8-7-11-36(32)38)47(69)70-26-31-18-33(48(50,51)52)23-34(19-31)49(53,54)55)61-46(68)40(21-29-9-5-4-6-10-29)60-42(64)25-58-43(65)28(3)59-44(66)37(56)20-30-13-15-35(63)16-14-30/h4-16,18-19,23-24,27-28,37,39-41,57,63H,17,20-22,25-26,56H2,1-3H3,(H,58,65)(H,59,66)(H,60,64)(H,61,68)(H,62,67)/t28-,37+,39+,40+,41+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from rat NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 23: 4975-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.065
BindingDB Entry DOI: 10.7270/Q2222W64 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM214798
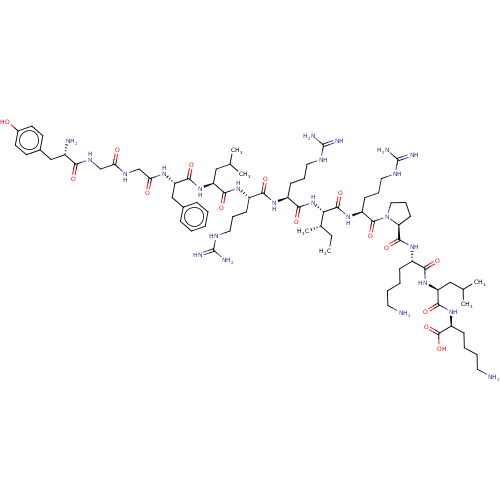
(Dynorphin A (1-17) | YGGFLRRIRPKLK)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(O)=O |r| Show InChI InChI=1S/C75H126N24O15/c1-7-45(6)61(70(111)94-53(25-17-35-87-75(83)84)71(112)99-36-18-26-58(99)69(110)93-50(21-11-13-31-76)64(105)96-56(38-44(4)5)67(108)95-54(72(113)114)22-12-14-32-77)98-65(106)52(24-16-34-86-74(81)82)91-63(104)51(23-15-33-85-73(79)80)92-66(107)55(37-43(2)3)97-68(109)57(40-46-19-9-8-10-20-46)90-60(102)42-88-59(101)41-89-62(103)49(78)39-47-27-29-48(100)30-28-47/h8-10,19-20,27-30,43-45,49-58,61,100H,7,11-18,21-26,31-42,76-78H2,1-6H3,(H,88,101)(H,89,103)(H,90,102)(H,91,104)(H,92,107)(H,93,110)(H,94,111)(H,95,108)(H,96,105)(H,97,109)(H,98,106)(H,113,114)(H4,79,80,85)(H4,81,82,86)(H4,83,84,87)/t45-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,61-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human KOR expressed in mouse HN9.10 cell membranes incubated for 2 hrs by liquid scintillation counting based radiol... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZG6WWT |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50439330
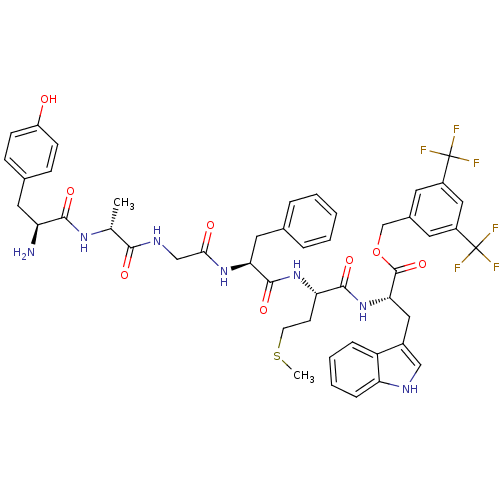
(CHEMBL2419541)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C48H51F6N7O8S/c1-27(58-43(65)36(55)20-29-12-14-34(62)15-13-29)42(64)57-25-41(63)59-39(21-28-8-4-3-5-9-28)45(67)60-38(16-17-70-2)44(66)61-40(22-31-24-56-37-11-7-6-10-35(31)37)46(68)69-26-30-18-32(47(49,50)51)23-33(19-30)48(52,53)54/h3-15,18-19,23-24,27,36,38-40,56,62H,16-17,20-22,25-26,55H2,1-2H3,(H,57,64)(H,58,65)(H,59,63)(H,60,67)(H,61,66)/t27-,36+,38+,39+,40+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 23: 4975-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.065
BindingDB Entry DOI: 10.7270/Q2222W64 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50439328
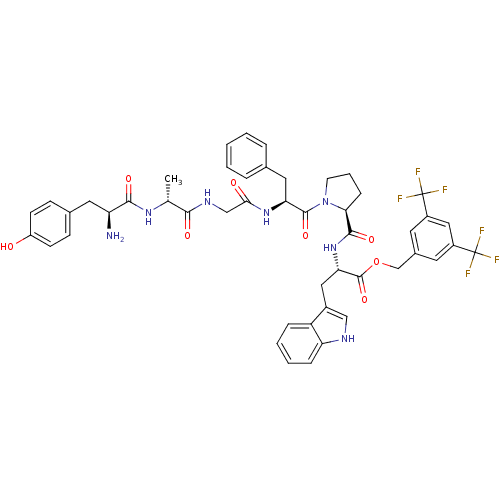
(CHEMBL2419543)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C48H49F6N7O8/c1-27(58-43(65)36(55)20-29-13-15-34(62)16-14-29)42(64)57-25-41(63)59-38(21-28-8-3-2-4-9-28)45(67)61-17-7-12-40(61)44(66)60-39(22-31-24-56-37-11-6-5-10-35(31)37)46(68)69-26-30-18-32(47(49,50)51)23-33(19-30)48(52,53)54/h2-6,8-11,13-16,18-19,23-24,27,36,38-40,56,62H,7,12,17,20-22,25-26,55H2,1H3,(H,57,64)(H,58,65)(H,59,63)(H,60,66)/t27-,36+,38+,39+,40+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 23: 4975-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.065
BindingDB Entry DOI: 10.7270/Q2222W64 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50010704
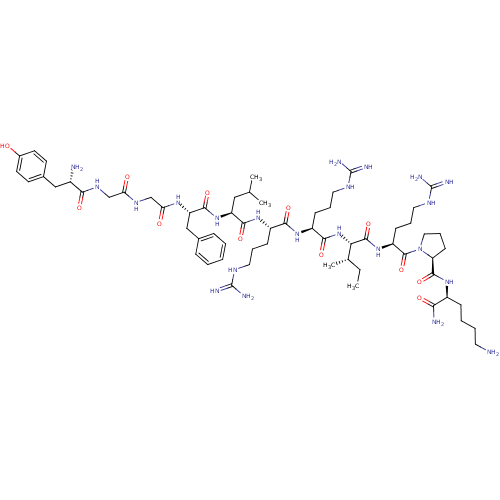
(CHEMBL216640 | Dyn A(1-11)-NH2 | Dynorphin A analo...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(N)=O Show InChI InChI=1S/C63H104N22O12/c1-5-37(4)51(59(96)82-45(20-13-29-75-63(71)72)60(97)85-30-14-21-48(85)58(95)79-42(52(66)89)17-9-10-26-64)84-55(92)44(19-12-28-74-62(69)70)80-54(91)43(18-11-27-73-61(67)68)81-56(93)46(31-36(2)3)83-57(94)47(33-38-15-7-6-8-16-38)78-50(88)35-76-49(87)34-77-53(90)41(65)32-39-22-24-40(86)25-23-39/h6-8,15-16,22-25,36-37,41-48,51,86H,5,9-14,17-21,26-35,64-65H2,1-4H3,(H2,66,89)(H,76,87)(H,77,90)(H,78,88)(H,79,95)(H,80,91)(H,81,93)(H,82,96)(H,83,94)(H,84,92)(H4,67,68,73)(H4,69,70,74)(H4,71,72,75)/t37-,41-,42-,43-,44-,45-,46-,47-,48-,51-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human KOR expressed in mouse HN9.10 cell membranes incubated for 2 hrs by liquid scintillation counting based radiol... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZG6WWT |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50122099
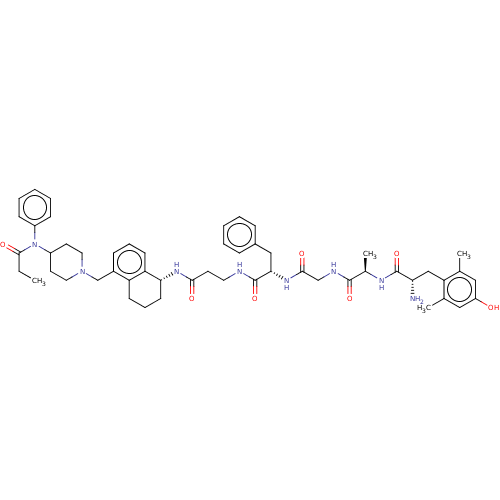
(CHEMBL3617471)Show SMILES CCC(=O)N(C1CCN(Cc2cccc3[C@@H](CCCc23)NC(=O)CCNC(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc2c(C)cc(O)cc2C)CC1)c1ccccc1 |r| Show InChI InChI=1S/C53H68N8O7/c1-5-50(65)61(39-17-10-7-11-18-39)40-23-26-60(27-24-40)33-38-16-12-20-43-42(38)19-13-21-46(43)58-48(63)22-25-55-53(68)47(30-37-14-8-6-9-15-37)59-49(64)32-56-51(66)36(4)57-52(67)45(54)31-44-34(2)28-41(62)29-35(44)3/h6-12,14-18,20,28-29,36,40,45-47,62H,5,13,19,21-27,30-33,54H2,1-4H3,(H,55,68)(H,56,66)(H,57,67)(H,58,63)(H,59,64)/t36-,45+,46-,47+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H] DAMGO from rat mu-opioid receptor |
Bioorg Med Chem Lett 25: 4683-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.064
BindingDB Entry DOI: 10.7270/Q2N29ZR2 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50040123
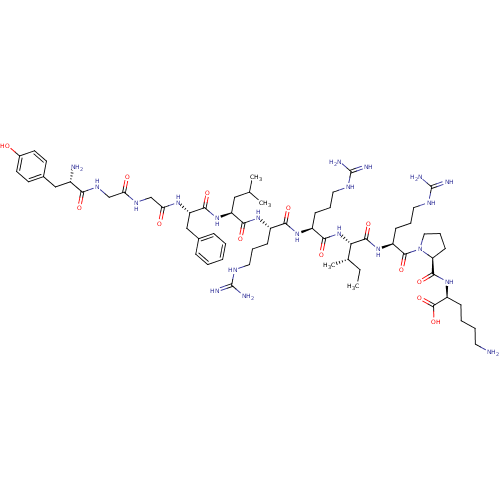
(CHEMBL438223 | HTry-Gly-Gly-Phe-Leu-Arg-Arg-lle-Ar...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(O)=O Show InChI InChI=1S/C63H103N21O13/c1-5-37(4)51(58(94)80-44(20-13-29-74-63(70)71)59(95)84-30-14-21-48(84)57(93)81-45(60(96)97)17-9-10-26-64)83-54(90)43(19-12-28-73-62(68)69)78-53(89)42(18-11-27-72-61(66)67)79-55(91)46(31-36(2)3)82-56(92)47(33-38-15-7-6-8-16-38)77-50(87)35-75-49(86)34-76-52(88)41(65)32-39-22-24-40(85)25-23-39/h6-8,15-16,22-25,36-37,41-48,51,85H,5,9-14,17-21,26-35,64-65H2,1-4H3,(H,75,86)(H,76,88)(H,77,87)(H,78,89)(H,79,91)(H,80,94)(H,81,93)(H,82,92)(H,83,90)(H,96,97)(H4,66,67,72)(H4,68,69,73)(H4,70,71,74)/t37-,41-,42-,43-,44-,45-,46-,47-,48-,51-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human KOR expressed in mouse HN9.10 cell membranes incubated for 2 hrs by liquid scintillation counting based radiol... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZG6WWT |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50439324
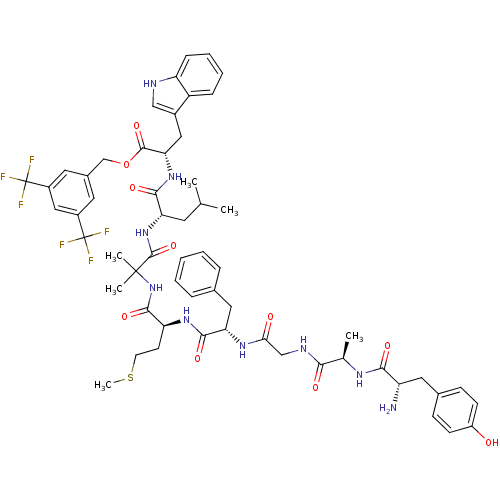
(CHEMBL2419539)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NC(C)(C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C58H69F6N9O10S/c1-32(2)22-45(51(78)71-47(27-37-29-66-43-15-11-10-14-41(37)43)54(81)83-31-36-23-38(57(59,60)61)28-39(24-36)58(62,63)64)72-55(82)56(4,5)73-53(80)44(20-21-84-6)70-52(79)46(26-34-12-8-7-9-13-34)69-48(75)30-67-49(76)33(3)68-50(77)42(65)25-35-16-18-40(74)19-17-35/h7-19,23-24,28-29,32-33,42,44-47,66,74H,20-22,25-27,30-31,65H2,1-6H3,(H,67,76)(H,68,77)(H,69,75)(H,70,79)(H,71,78)(H,72,82)(H,73,80)/t33-,42+,44+,45+,46+,47+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 23: 4975-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.065
BindingDB Entry DOI: 10.7270/Q2222W64 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50545673
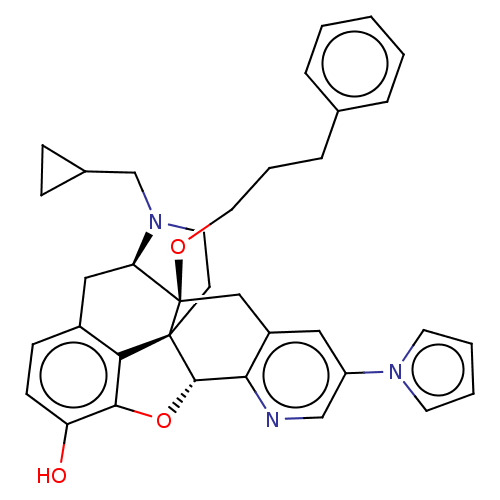
(CHEMBL4634079)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@]5(Cc1cc(cnc21)-n1cccc1)OCCCc1ccccc1)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C36H37N3O3/c40-29-13-12-26-20-30-36(41-18-6-9-24-7-2-1-3-8-24)21-27-19-28(38-15-4-5-16-38)22-37-32(27)34-35(36,31(26)33(29)42-34)14-17-39(30)23-25-10-11-25/h1-5,7-8,12-13,15-16,19,22,25,30,34,40H,6,9-11,14,17-18,20-21,23H2/t30-,34+,35+,36-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.169 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human delta opioid receptor expressed in CHO cell membranes incubated for 1 hr by competition radioligand bin... |
J Med Chem 63: 7663-7694 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00503
BindingDB Entry DOI: 10.7270/Q2B56P91 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50341318

((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-15-(4-hyd...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C61H74F6N10O9S/c1-33(2)21-48(56(83)75-50(27-39-31-69-46-16-11-10-15-43(39)46)55(82)70-30-38-24-40(60(62,63)64)28-41(25-38)61(65,66)67)76-58(85)51-17-12-19-77(51)59(86)47(18-20-87-6)74-57(84)49(26-37-13-8-7-9-14-37)73-52(79)32-71-53(80)36(5)72-54(81)45(68)29-44-34(3)22-42(78)23-35(44)4/h7-11,13-16,22-25,28,31,33,36,45,47-51,69,78H,12,17-21,26-27,29-30,32,68H2,1-6H3,(H,70,82)(H,71,80)(H,72,81)(H,73,79)(H,74,84)(H,75,83)(H,76,85)/t36-,45+,47+,48+,49+,50+,51+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human delta opioid receptor expressed in mouse HN9.10 cells |
J Med Chem 54: 2029-38 (2011)
Article DOI: 10.1021/jm101023r
BindingDB Entry DOI: 10.7270/Q2862GRK |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50500612
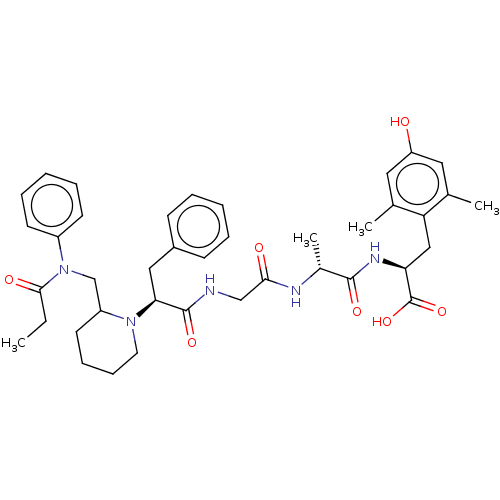
(CHEMBL3754081)Show SMILES CCC(=O)N(CC1CCCCN1[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@H](C)C(=O)N[C@@H](Cc1c(C)cc(O)cc1C)C(O)=O)c1ccccc1 |r| Show InChI InChI=1S/C40H51N5O7/c1-5-37(48)45(30-16-10-7-11-17-30)25-31-18-12-13-19-44(31)35(22-29-14-8-6-9-15-29)39(50)41-24-36(47)42-28(4)38(49)43-34(40(51)52)23-33-26(2)20-32(46)21-27(33)3/h6-11,14-17,20-21,28,31,34-35,46H,5,12-13,18-19,22-25H2,1-4H3,(H,41,50)(H,42,47)(H,43,49)(H,51,52)/t28-,31?,34+,35+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human delta opioid receptor after 180 mins by scintillation counting analysis |
Bioorg Med Chem Lett 26: 222-7 (2016)
Article DOI: 10.1016/j.bmcl.2015.10.081
BindingDB Entry DOI: 10.7270/Q2W66PSM |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50341315
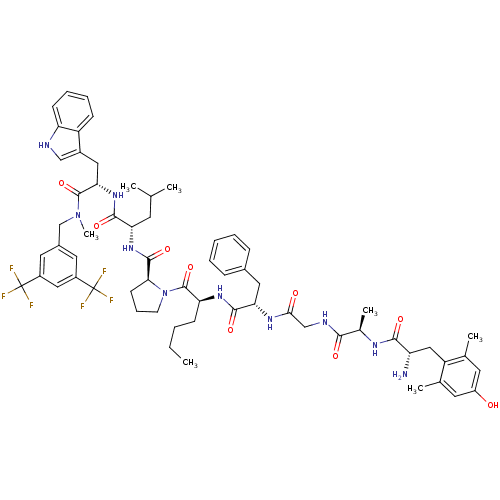
((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-2-butyl-1...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N(C)Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C63H78F6N10O9/c1-8-9-19-49(75-58(85)51(28-39-16-11-10-12-17-39)74-54(81)33-72-55(82)38(6)73-56(83)47(70)31-46-36(4)24-44(80)25-37(46)5)61(88)79-22-15-21-53(79)59(86)76-50(23-35(2)3)57(84)77-52(29-41-32-71-48-20-14-13-18-45(41)48)60(87)78(7)34-40-26-42(62(64,65)66)30-43(27-40)63(67,68)69/h10-14,16-18,20,24-27,30,32,35,38,47,49-53,71,80H,8-9,15,19,21-23,28-29,31,33-34,70H2,1-7H3,(H,72,82)(H,73,83)(H,74,81)(H,75,85)(H,76,86)(H,77,84)/t38-,47+,49+,50+,51+,52+,53+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells |
J Med Chem 54: 2029-38 (2011)
Article DOI: 10.1021/jm101023r
BindingDB Entry DOI: 10.7270/Q2862GRK |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50341315

((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-2-butyl-1...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N(C)Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C63H78F6N10O9/c1-8-9-19-49(75-58(85)51(28-39-16-11-10-12-17-39)74-54(81)33-72-55(82)38(6)73-56(83)47(70)31-46-36(4)24-44(80)25-37(46)5)61(88)79-22-15-21-53(79)59(86)76-50(23-35(2)3)57(84)77-52(29-41-32-71-48-20-14-13-18-45(41)48)60(87)78(7)34-40-26-42(62(64,65)66)30-43(27-40)63(67,68)69/h10-14,16-18,20,24-27,30,32,35,38,47,49-53,71,80H,8-9,15,19,21-23,28-29,31,33-34,70H2,1-7H3,(H,72,82)(H,73,83)(H,74,81)(H,75,85)(H,76,86)(H,77,84)/t38-,47+,49+,50+,51+,52+,53+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells |
J Med Chem 54: 2029-38 (2011)
Article DOI: 10.1021/jm101023r
BindingDB Entry DOI: 10.7270/Q2862GRK |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50439330

(CHEMBL2419541)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C48H51F6N7O8S/c1-27(58-43(65)36(55)20-29-12-14-34(62)15-13-29)42(64)57-25-41(63)59-39(21-28-8-4-3-5-9-28)45(67)60-38(16-17-70-2)44(66)61-40(22-31-24-56-37-11-7-6-10-35(31)37)46(68)69-26-30-18-32(47(49,50)51)23-33(19-30)48(52,53)54/h3-15,18-19,23-24,27,36,38-40,56,62H,16-17,20-22,25-26,55H2,1-2H3,(H,57,64)(H,58,65)(H,59,63)(H,60,67)(H,61,66)/t27-,36+,38+,39+,40+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from rat NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 23: 4975-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.065
BindingDB Entry DOI: 10.7270/Q2222W64 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM21021

(Bifunctional Peptide Ligand, 5 (TY005) | CHEMBL389...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C59H69F6N9O10S/c1-33(2)23-46(53(79)73-48(28-38-30-67-44-14-9-8-13-42(38)44)57(83)84-32-37-24-39(58(60,61)62)29-40(25-37)59(63,64)65)72-55(81)49-15-10-21-74(49)56(82)45(20-22-85-4)71-54(80)47(27-35-11-6-5-7-12-35)70-50(76)31-68-51(77)34(3)69-52(78)43(66)26-36-16-18-41(75)19-17-36/h5-9,11-14,16-19,24-25,29-30,33-34,43,45-49,67,75H,10,15,20-23,26-28,31-32,66H2,1-4H3,(H,68,77)(H,69,78)(H,70,76)(H,71,80)(H,72,81)(H,73,79)/t34-,43+,45+,46+,47+,48+,49+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from rat NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 23: 4975-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.065
BindingDB Entry DOI: 10.7270/Q2222W64 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50558711
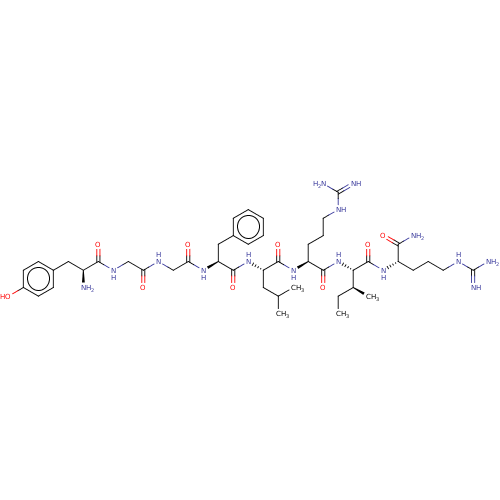
(CHEMBL4754961)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human KOR expressed in mouse HN9.10 cell membranes incubated for 2 hrs by liquid scintillation counting based radiol... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZG6WWT |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50341317
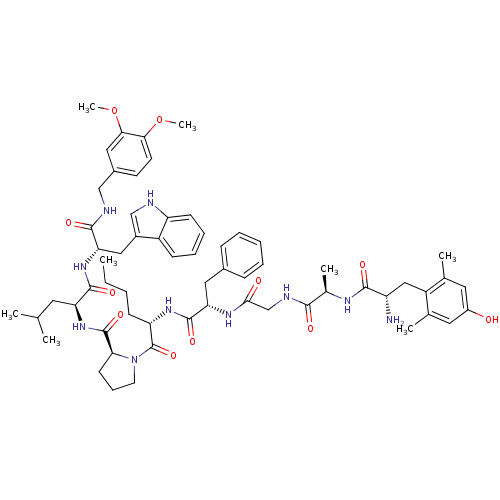
((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-2-butyl-1...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1ccc(OC)c(OC)c1 |r| Show InChI InChI=1S/C62H82N10O11/c1-9-10-20-48(69-60(79)50(29-40-17-12-11-13-18-40)68-55(74)35-66-56(75)39(6)67-57(76)46(63)32-45-37(4)27-43(73)28-38(45)5)62(81)72-25-16-22-52(72)61(80)71-49(26-36(2)3)59(78)70-51(31-42-34-64-47-21-15-14-19-44(42)47)58(77)65-33-41-23-24-53(82-7)54(30-41)83-8/h11-15,17-19,21,23-24,27-28,30,34,36,39,46,48-52,64,73H,9-10,16,20,22,25-26,29,31-33,35,63H2,1-8H3,(H,65,77)(H,66,75)(H,67,76)(H,68,74)(H,69,79)(H,70,78)(H,71,80)/t39-,46+,48+,49+,50+,51+,52+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cells |
J Med Chem 54: 2029-38 (2011)
Article DOI: 10.1021/jm101023r
BindingDB Entry DOI: 10.7270/Q2862GRK |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50558723
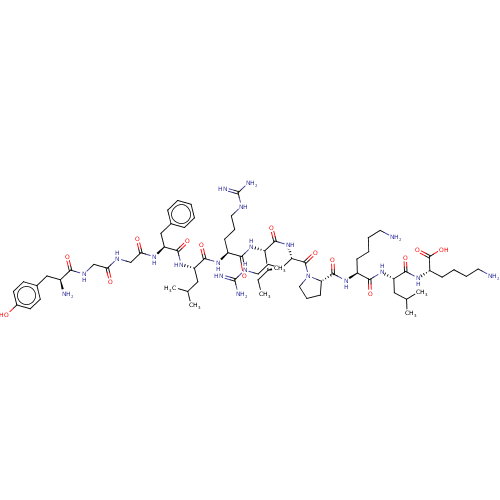
(CHEMBL4760958)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human KOR expressed in mouse HN9.10 cell membranes incubated for 2 hrs by liquid scintillation counting based radiol... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZG6WWT |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50500608
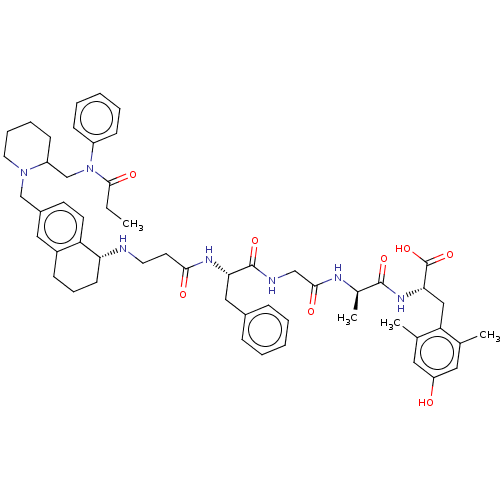
(CHEMBL3753711)Show SMILES CCC(=O)N(CC1CCCCN1Cc1ccc2[C@@H](CCCc2c1)NCCC(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@H](C)C(=O)N[C@@H](Cc1c(C)cc(O)cc1C)C(O)=O)c1ccccc1 |r| Show InChI InChI=1S/C54H69N7O8/c1-5-51(65)61(41-18-10-7-11-19-41)34-42-20-12-13-26-60(42)33-39-22-23-44-40(29-39)17-14-21-46(44)55-25-24-49(63)58-47(30-38-15-8-6-9-16-38)53(67)56-32-50(64)57-37(4)52(66)59-48(54(68)69)31-45-35(2)27-43(62)28-36(45)3/h6-11,15-16,18-19,22-23,27-29,37,42,46-48,55,62H,5,12-14,17,20-21,24-26,30-34H2,1-4H3,(H,56,67)(H,57,64)(H,58,63)(H,59,66)(H,68,69)/t37-,42?,46-,47+,48+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human delta opioid receptor after 180 mins by scintillation counting analysis |
Bioorg Med Chem Lett 26: 222-7 (2016)
Article DOI: 10.1016/j.bmcl.2015.10.081
BindingDB Entry DOI: 10.7270/Q2W66PSM |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50499070
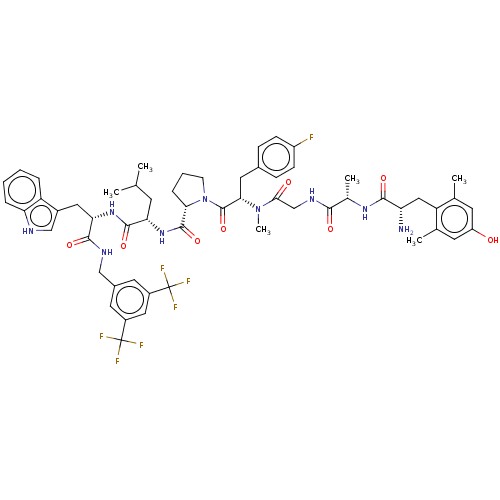
(CHEMBL4299415)Show SMILES CC(C)C[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(F)cc1)N(C)C(=O)CNC(=O)[C@H](C)NC(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C57H66F7N9O8/c1-30(2)18-45(53(79)70-46(24-36-28-66-44-11-8-7-10-41(36)44)52(78)67-27-35-21-37(56(59,60)61)25-38(22-35)57(62,63)64)71-54(80)47-12-9-17-73(47)55(81)48(23-34-13-15-39(58)16-14-34)72(6)49(75)29-68-50(76)33(5)69-51(77)43(65)26-42-31(3)19-40(74)20-32(42)4/h7-8,10-11,13-16,19-22,25,28,30,33,43,45-48,66,74H,9,12,17-18,23-24,26-27,29,65H2,1-6H3,(H,67,78)(H,68,76)(H,69,77)(H,70,79)(H,71,80)/t33-,43-,45-,46-,47-,48-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity to mu opioid receptor (unknown origin) |
J Med Chem 58: 8573-83 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01170
BindingDB Entry DOI: 10.7270/Q2VT1W34 |
More data for this
Ligand-Target Pair | |
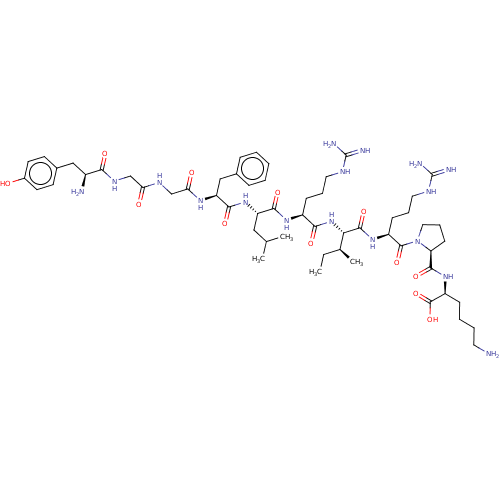
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50558722
(CHEMBL4757601)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human KOR expressed in mouse HN9.10 cell membranes incubated for 2 hrs by liquid scintillation counting based radiol... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZG6WWT |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50341315

((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-2-butyl-1...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N(C)Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C63H78F6N10O9/c1-8-9-19-49(75-58(85)51(28-39-16-11-10-12-17-39)74-54(81)33-72-55(82)38(6)73-56(83)47(70)31-46-36(4)24-44(80)25-37(46)5)61(88)79-22-15-21-53(79)59(86)76-50(23-35(2)3)57(84)77-52(29-41-32-71-48-20-14-13-18-45(41)48)60(87)78(7)34-40-26-42(62(64,65)66)30-43(27-40)63(67,68)69/h10-14,16-18,20,24-27,30,32,35,38,47,49-53,71,80H,8-9,15,19,21-23,28-29,31,33-34,70H2,1-7H3,(H,72,82)(H,73,83)(H,74,81)(H,75,85)(H,76,86)(H,77,84)/t38-,47+,49+,50+,51+,52+,53+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human delta opioid receptor expressed in mouse HN9.10 cells |
J Med Chem 54: 2029-38 (2011)
Article DOI: 10.1021/jm101023r
BindingDB Entry DOI: 10.7270/Q2862GRK |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50122099

(CHEMBL3617471)Show SMILES CCC(=O)N(C1CCN(Cc2cccc3[C@@H](CCCc23)NC(=O)CCNC(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc2c(C)cc(O)cc2C)CC1)c1ccccc1 |r| Show InChI InChI=1S/C53H68N8O7/c1-5-50(65)61(39-17-10-7-11-18-39)40-23-26-60(27-24-40)33-38-16-12-20-43-42(38)19-13-21-46(43)58-48(63)22-25-55-53(68)47(30-37-14-8-6-9-15-37)59-49(64)32-56-51(66)36(4)57-52(67)45(54)31-44-34(2)28-41(62)29-35(44)3/h6-12,14-18,20,28-29,36,40,45-47,62H,5,13,19,21-27,30-33,54H2,1-4H3,(H,55,68)(H,56,66)(H,57,67)(H,58,63)(H,59,64)/t36-,45+,46-,47+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H] DAMGO from human delta opioid receptor |
Bioorg Med Chem Lett 25: 4683-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.064
BindingDB Entry DOI: 10.7270/Q2N29ZR2 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50545677
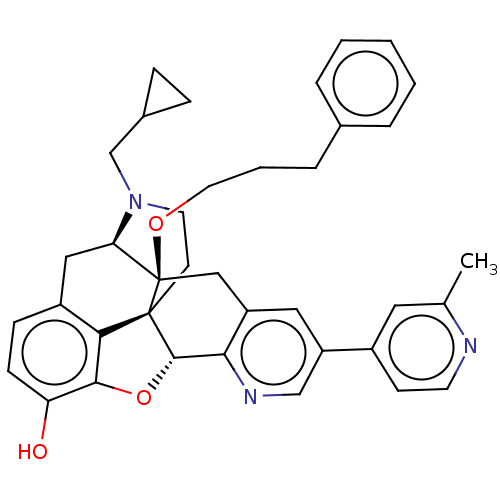
(CHEMBL4645508)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@]5(Cc1cc(cnc21)-c1ccnc(C)c1)OCCCc1ccccc1)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C38H39N3O3/c1-24-18-27(13-15-39-24)30-19-29-21-38(43-17-5-8-25-6-3-2-4-7-25)32-20-28-11-12-31(42)35-33(28)37(38,36(44-35)34(29)40-22-30)14-16-41(32)23-26-9-10-26/h2-4,6-7,11-13,15,18-19,22,26,32,36,42H,5,8-10,14,16-17,20-21,23H2,1H3/t32-,36+,37+,38-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by competition radioligand bin... |
J Med Chem 63: 7663-7694 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00503
BindingDB Entry DOI: 10.7270/Q2B56P91 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50500605
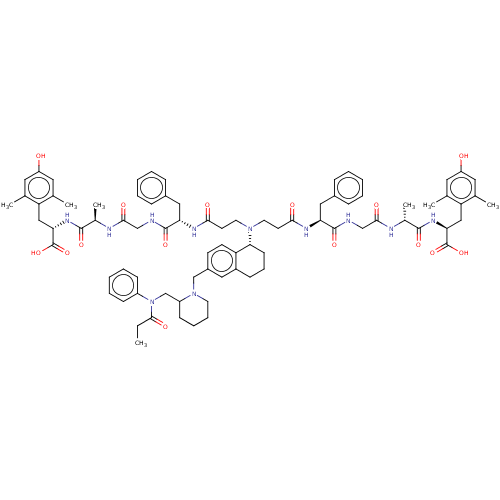
(CHEMBL3752798)Show SMILES CCC(=O)N(CC1CCCCN1Cc1ccc2[C@@H](CCCc2c1)N(CCC(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@H](C)C(=O)N[C@@H](Cc1c(C)cc(O)cc1C)C(O)=O)CCC(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@H](C)C(=O)N[C@@H](Cc1c(C)cc(O)cc1C)C(O)=O)c1ccccc1 |r| Show InChI InChI=1S/C82H103N11O15/c1-8-76(100)93(60-26-16-11-17-27-60)49-61-28-18-19-34-92(61)48-58-30-31-64-59(41-58)25-20-29-71(64)91(35-32-72(96)87-67(42-56-21-12-9-13-22-56)79(103)83-46-74(98)85-54(6)77(101)89-69(81(105)106)44-65-50(2)37-62(94)38-51(65)3)36-33-73(97)88-68(43-57-23-14-10-15-24-57)80(104)84-47-75(99)86-55(7)78(102)90-70(82(107)108)45-66-52(4)39-63(95)40-53(66)5/h9-17,21-24,26-27,30-31,37-41,54-55,61,67-71,94-95H,8,18-20,25,28-29,32-36,42-49H2,1-7H3,(H,83,103)(H,84,104)(H,85,98)(H,86,99)(H,87,96)(H,88,97)(H,89,101)(H,90,102)(H,105,106)(H,107,108)/t54-,55-,61?,67+,68+,69+,70+,71-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human delta opioid receptor after 180 mins by scintillation counting analysis |
Bioorg Med Chem Lett 26: 222-7 (2016)
Article DOI: 10.1016/j.bmcl.2015.10.081
BindingDB Entry DOI: 10.7270/Q2W66PSM |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50500605

(CHEMBL3752798)Show SMILES CCC(=O)N(CC1CCCCN1Cc1ccc2[C@@H](CCCc2c1)N(CCC(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@H](C)C(=O)N[C@@H](Cc1c(C)cc(O)cc1C)C(O)=O)CCC(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@H](C)C(=O)N[C@@H](Cc1c(C)cc(O)cc1C)C(O)=O)c1ccccc1 |r| Show InChI InChI=1S/C82H103N11O15/c1-8-76(100)93(60-26-16-11-17-27-60)49-61-28-18-19-34-92(61)48-58-30-31-64-59(41-58)25-20-29-71(64)91(35-32-72(96)87-67(42-56-21-12-9-13-22-56)79(103)83-46-74(98)85-54(6)77(101)89-69(81(105)106)44-65-50(2)37-62(94)38-51(65)3)36-33-73(97)88-68(43-57-23-14-10-15-24-57)80(104)84-47-75(99)86-55(7)78(102)90-70(82(107)108)45-66-52(4)39-63(95)40-53(66)5/h9-17,21-24,26-27,30-31,37-41,54-55,61,67-71,94-95H,8,18-20,25,28-29,32-36,42-49H2,1-7H3,(H,83,103)(H,84,104)(H,85,98)(H,86,99)(H,87,96)(H,88,97)(H,89,101)(H,90,102)(H,105,106)(H,107,108)/t54-,55-,61?,67+,68+,69+,70+,71-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu-opioid receptor in Sprague-Dawley rat brain membrane after 180 mins by scintillation counting analysis |
Bioorg Med Chem Lett 26: 222-7 (2016)
Article DOI: 10.1016/j.bmcl.2015.10.081
BindingDB Entry DOI: 10.7270/Q2W66PSM |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM21012

((2S)-2-{[(2S)-1-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C54H61F6N9O8/c1-30(2)20-42(50(75)67-43(25-35-28-62-41-13-8-7-12-39(35)41)49(74)63-27-34-21-36(53(55,56)57)26-37(22-34)54(58,59)60)68-51(76)45-14-9-19-69(45)52(77)44(24-32-10-5-4-6-11-32)66-46(71)29-64-47(72)31(3)65-48(73)40(61)23-33-15-17-38(70)18-16-33/h4-8,10-13,15-18,21-22,26,28,30-31,40,42-45,62,70H,9,14,19-20,23-25,27,29,61H2,1-3H3,(H,63,74)(H,64,72)(H,65,73)(H,66,71)(H,67,75)(H,68,76)/t31-,40+,42+,43+,44+,45+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from recombinant human neurokinin-1 receptor expressed in CHO cells incubated for 20 mins by liquid scintillation counting |
J Med Chem 58: 8573-83 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01170
BindingDB Entry DOI: 10.7270/Q2VT1W34 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50500608

(CHEMBL3753711)Show SMILES CCC(=O)N(CC1CCCCN1Cc1ccc2[C@@H](CCCc2c1)NCCC(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@H](C)C(=O)N[C@@H](Cc1c(C)cc(O)cc1C)C(O)=O)c1ccccc1 |r| Show InChI InChI=1S/C54H69N7O8/c1-5-51(65)61(41-18-10-7-11-19-41)34-42-20-12-13-26-60(42)33-39-22-23-44-40(29-39)17-14-21-46(44)55-25-24-49(63)58-47(30-38-15-8-6-9-16-38)53(67)56-32-50(64)57-37(4)52(66)59-48(54(68)69)31-45-35(2)27-43(62)28-36(45)3/h6-11,15-16,18-19,22-23,27-29,37,42,46-48,55,62H,5,12-14,17,20-21,24-26,30-34H2,1-4H3,(H,56,67)(H,57,64)(H,58,63)(H,59,66)(H,68,69)/t37-,42?,46-,47+,48+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu-opioid receptor in Sprague-Dawley rat brain membrane after 180 mins by scintillation counting analysis |
Bioorg Med Chem Lett 26: 222-7 (2016)
Article DOI: 10.1016/j.bmcl.2015.10.081
BindingDB Entry DOI: 10.7270/Q2W66PSM |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM21016
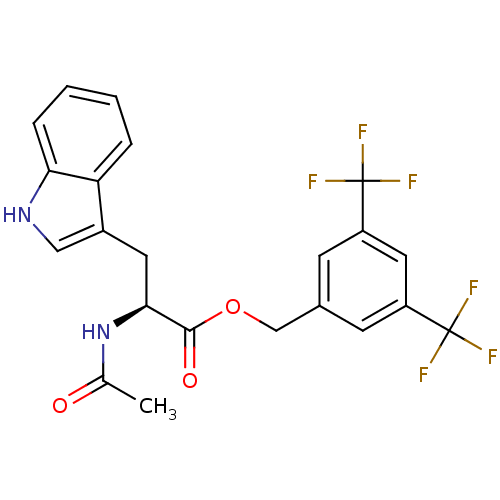
(CHEMBL22870 | L 732138 | L-732,138 | L732138 | N-a...)Show SMILES CC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C22H18F6N2O3/c1-12(31)30-19(8-14-10-29-18-5-3-2-4-17(14)18)20(32)33-11-13-6-15(21(23,24)25)9-16(7-13)22(26,27)28/h2-7,9-10,19,29H,8,11H2,1H3,(H,30,31)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells |
J Med Chem 54: 2029-38 (2011)
Article DOI: 10.1021/jm101023r
BindingDB Entry DOI: 10.7270/Q2862GRK |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM21016

(CHEMBL22870 | L 732138 | L-732,138 | L732138 | N-a...)Show SMILES CC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C22H18F6N2O3/c1-12(31)30-19(8-14-10-29-18-5-3-2-4-17(14)18)20(32)33-11-13-6-15(21(23,24)25)9-16(7-13)22(26,27)28/h2-7,9-10,19,29H,8,11H2,1H3,(H,30,31)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells |
J Med Chem 54: 2029-38 (2011)
Article DOI: 10.1021/jm101023r
BindingDB Entry DOI: 10.7270/Q2862GRK |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50341316
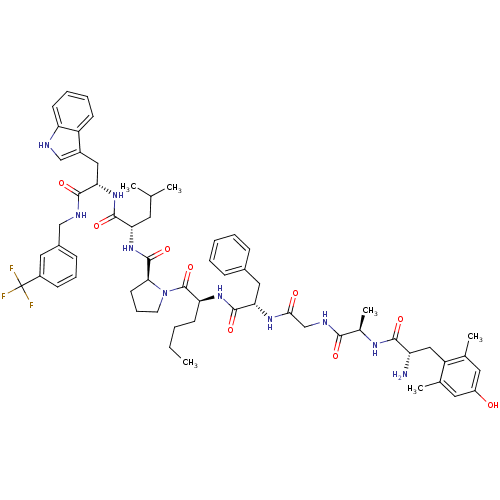
((S)-N-((S)-1-((S)-3-(1H-indol-3-yl)-1-oxo-1-(3-(tr...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C61H77F3N10O9/c1-7-8-21-48(71-58(81)50(29-39-16-10-9-11-17-39)70-53(76)34-68-54(77)38(6)69-55(78)46(65)31-45-36(4)26-43(75)27-37(45)5)60(83)74-24-15-23-52(74)59(82)73-49(25-35(2)3)57(80)72-51(30-41-33-66-47-22-13-12-20-44(41)47)56(79)67-32-40-18-14-19-42(28-40)61(62,63)64/h9-14,16-20,22,26-28,33,35,38,46,48-52,66,75H,7-8,15,21,23-25,29-32,34,65H2,1-6H3,(H,67,79)(H,68,77)(H,69,78)(H,70,76)(H,71,81)(H,72,80)(H,73,82)/t38-,46+,48+,49+,50+,51+,52+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cells |
J Med Chem 54: 2029-38 (2011)
Article DOI: 10.1021/jm101023r
BindingDB Entry DOI: 10.7270/Q2862GRK |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50341316

((S)-N-((S)-1-((S)-3-(1H-indol-3-yl)-1-oxo-1-(3-(tr...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C61H77F3N10O9/c1-7-8-21-48(71-58(81)50(29-39-16-10-9-11-17-39)70-53(76)34-68-54(77)38(6)69-55(78)46(65)31-45-36(4)26-43(75)27-37(45)5)60(83)74-24-15-23-52(74)59(82)73-49(25-35(2)3)57(80)72-51(30-41-33-66-47-22-13-12-20-44(41)47)56(79)67-32-40-18-14-19-42(28-40)61(62,63)64/h9-14,16-20,22,26-28,33,35,38,46,48-52,66,75H,7-8,15,21,23-25,29-32,34,65H2,1-6H3,(H,67,79)(H,68,77)(H,69,78)(H,70,76)(H,71,81)(H,72,80)(H,73,82)/t38-,46+,48+,49+,50+,51+,52+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cells |
J Med Chem 54: 2029-38 (2011)
Article DOI: 10.1021/jm101023r
BindingDB Entry DOI: 10.7270/Q2862GRK |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50500612

(CHEMBL3754081)Show SMILES CCC(=O)N(CC1CCCCN1[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@H](C)C(=O)N[C@@H](Cc1c(C)cc(O)cc1C)C(O)=O)c1ccccc1 |r| Show InChI InChI=1S/C40H51N5O7/c1-5-37(48)45(30-16-10-7-11-17-30)25-31-18-12-13-19-44(31)35(22-29-14-8-6-9-15-29)39(50)41-24-36(47)42-28(4)38(49)43-34(40(51)52)23-33-26(2)20-32(46)21-27(33)3/h6-11,14-17,20-21,28,31,34-35,46H,5,12-13,18-19,22-25H2,1-4H3,(H,41,50)(H,42,47)(H,43,49)(H,51,52)/t28-,31?,34+,35+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu-opioid receptor in Sprague-Dawley rat brain membrane after 180 mins by scintillation counting analysis |
Bioorg Med Chem Lett 26: 222-7 (2016)
Article DOI: 10.1016/j.bmcl.2015.10.081
BindingDB Entry DOI: 10.7270/Q2W66PSM |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50439331
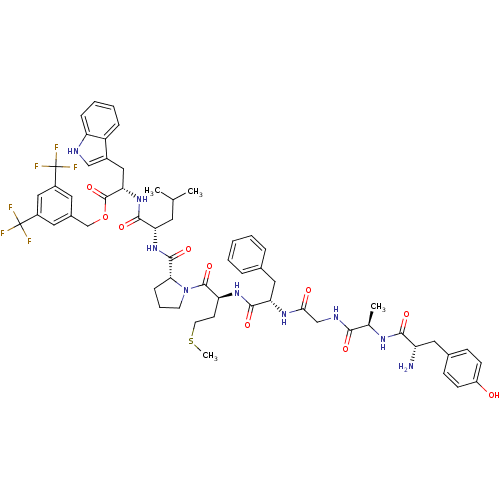
(CHEMBL2419540)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C59H69F6N9O10S/c1-33(2)23-46(53(79)73-48(28-38-30-67-44-14-9-8-13-42(38)44)57(83)84-32-37-24-39(58(60,61)62)29-40(25-37)59(63,64)65)72-55(81)49-15-10-21-74(49)56(82)45(20-22-85-4)71-54(80)47(27-35-11-6-5-7-12-35)70-50(76)31-68-51(77)34(3)69-52(78)43(66)26-36-16-18-41(75)19-17-36/h5-9,11-14,16-19,24-25,29-30,33-34,43,45-49,67,75H,10,15,20-23,26-28,31-32,66H2,1-4H3,(H,68,77)(H,69,78)(H,70,76)(H,71,80)(H,72,81)(H,73,79)/t34-,43+,45+,46+,47+,48+,49-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 23: 4975-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.065
BindingDB Entry DOI: 10.7270/Q2222W64 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50500609
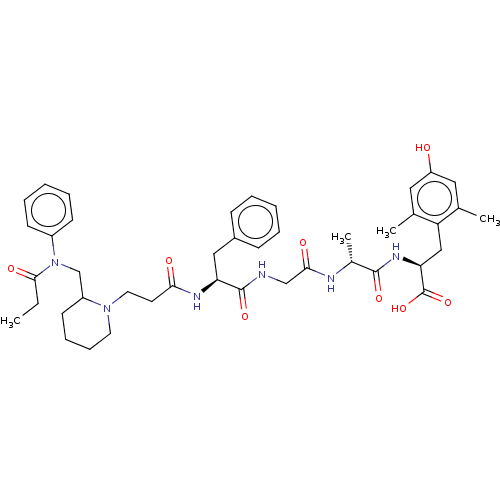
(CHEMBL3754056)Show SMILES CCC(=O)N(CC1CCCCN1CCC(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@H](C)C(=O)N[C@@H](Cc1c(C)cc(O)cc1C)C(O)=O)c1ccccc1 |r| Show InChI InChI=1S/C43H56N6O8/c1-5-40(53)49(32-16-10-7-11-17-32)27-33-18-12-13-20-48(33)21-19-38(51)46-36(24-31-14-8-6-9-15-31)42(55)44-26-39(52)45-30(4)41(54)47-37(43(56)57)25-35-28(2)22-34(50)23-29(35)3/h6-11,14-17,22-23,30,33,36-37,50H,5,12-13,18-21,24-27H2,1-4H3,(H,44,55)(H,45,52)(H,46,51)(H,47,54)(H,56,57)/t30-,33?,36+,37+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu-opioid receptor in Sprague-Dawley rat brain membrane after 180 mins by scintillation counting analysis |
Bioorg Med Chem Lett 26: 222-7 (2016)
Article DOI: 10.1016/j.bmcl.2015.10.081
BindingDB Entry DOI: 10.7270/Q2W66PSM |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50558721

(CHEMBL4784791)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(N)=O |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]diprenorphine from human MOR expressed in mouse NG108-15 cell membranes incubated for 2 hrs by liquid scintillation counting base... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZG6WWT |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50341317

((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-2-butyl-1...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1ccc(OC)c(OC)c1 |r| Show InChI InChI=1S/C62H82N10O11/c1-9-10-20-48(69-60(79)50(29-40-17-12-11-13-18-40)68-55(74)35-66-56(75)39(6)67-57(76)46(63)32-45-37(4)27-43(73)28-38(45)5)62(81)72-25-16-22-52(72)61(80)71-49(26-36(2)3)59(78)70-51(31-42-34-64-47-21-15-14-19-44(42)47)58(77)65-33-41-23-24-53(82-7)54(30-41)83-8/h11-15,17-19,21,23-24,27-28,30,34,36,39,46,48-52,64,73H,9-10,16,20,22,25-26,29,31-33,35,63H2,1-8H3,(H,65,77)(H,66,75)(H,67,76)(H,68,74)(H,69,79)(H,70,78)(H,71,80)/t39-,46+,48+,49+,50+,51+,52+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells |
J Med Chem 54: 2029-38 (2011)
Article DOI: 10.1021/jm101023r
BindingDB Entry DOI: 10.7270/Q2862GRK |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50499069
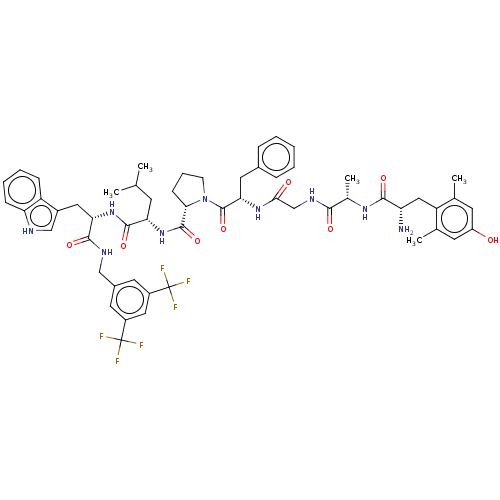
(CHEMBL4299390)Show SMILES CC(C)C[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C56H65F6N9O8/c1-30(2)18-44(52(77)69-45(24-36-28-64-43-15-10-9-14-40(36)43)51(76)65-27-35-21-37(55(57,58)59)25-38(22-35)56(60,61)62)70-53(78)47-16-11-17-71(47)54(79)46(23-34-12-7-6-8-13-34)68-48(73)29-66-49(74)33(5)67-50(75)42(63)26-41-31(3)19-39(72)20-32(41)4/h6-10,12-15,19-22,25,28,30,33,42,44-47,64,72H,11,16-18,23-24,26-27,29,63H2,1-5H3,(H,65,76)(H,66,74)(H,67,75)(H,68,73)(H,69,77)(H,70,78)/t33-,42-,44-,45-,46-,47-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity to delta opioid receptor (unknown origin) |
J Med Chem 58: 8573-83 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01170
BindingDB Entry DOI: 10.7270/Q2VT1W34 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50499071
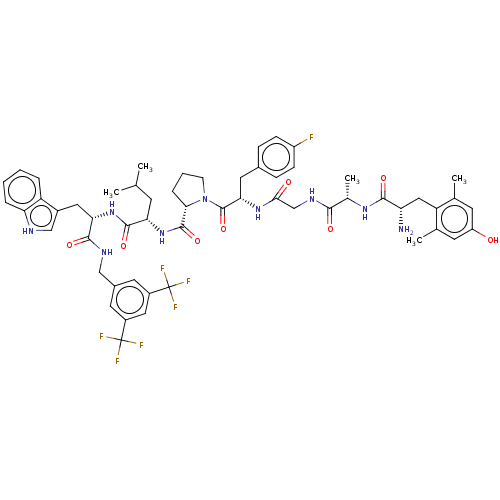
(CHEMBL4299423)Show SMILES CC(C)C[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(F)cc1)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C56H64F7N9O8/c1-29(2)17-44(52(78)70-45(23-35-27-65-43-10-7-6-9-40(35)43)51(77)66-26-34-20-36(55(58,59)60)24-37(21-34)56(61,62)63)71-53(79)47-11-8-16-72(47)54(80)46(22-33-12-14-38(57)15-13-33)69-48(74)28-67-49(75)32(5)68-50(76)42(64)25-41-30(3)18-39(73)19-31(41)4/h6-7,9-10,12-15,18-21,24,27,29,32,42,44-47,65,73H,8,11,16-17,22-23,25-26,28,64H2,1-5H3,(H,66,77)(H,67,75)(H,68,76)(H,69,74)(H,70,78)(H,71,79)/t32-,42-,44-,45-,46-,47-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity to delta opioid receptor (unknown origin) |
J Med Chem 58: 8573-83 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01170
BindingDB Entry DOI: 10.7270/Q2VT1W34 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50499071

(CHEMBL4299423)Show SMILES CC(C)C[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(F)cc1)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C56H64F7N9O8/c1-29(2)17-44(52(78)70-45(23-35-27-65-43-10-7-6-9-40(35)43)51(77)66-26-34-20-36(55(58,59)60)24-37(21-34)56(61,62)63)71-53(79)47-11-8-16-72(47)54(80)46(22-33-12-14-38(57)15-13-33)69-48(74)28-67-49(75)32(5)68-50(76)42(64)25-41-30(3)18-39(73)19-31(41)4/h6-7,9-10,12-15,18-21,24,27,29,32,42,44-47,65,73H,8,11,16-17,22-23,25-26,28,64H2,1-5H3,(H,66,77)(H,67,75)(H,68,76)(H,69,74)(H,70,78)(H,71,79)/t32-,42-,44-,45-,46-,47-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity to mu opioid receptor (unknown origin) |
J Med Chem 58: 8573-83 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01170
BindingDB Entry DOI: 10.7270/Q2VT1W34 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data