 Found 625 hits with Last Name = 'lark' and Initial = 'ma'
Found 625 hits with Last Name = 'lark' and Initial = 'ma' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin K
(Homo sapiens (Human)) | BDBM19769

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H30N4O6S/c1-17(2)14-20(29-26(33)23-15-18-8-3-4-10-22(18)36-23)25(32)28-19-9-7-13-30(16-21(19)31)37(34,35)24-11-5-6-12-27-24/h3-6,8,10-12,15,17,19-20H,7,9,13-14,16H2,1-2H3,(H,28,32)(H,29,33)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin K |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin S
(Homo sapiens (Human)) | BDBM50084650
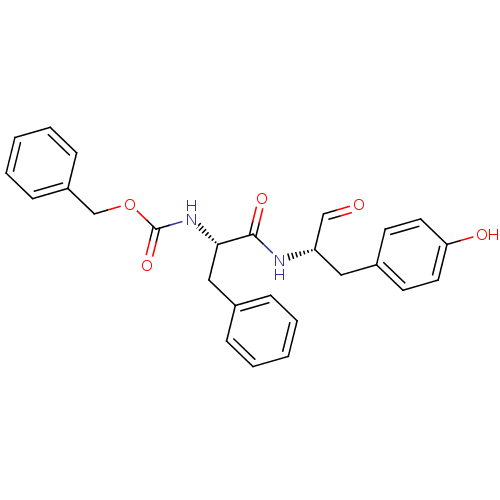
(CHEMBL177914 | {(S)-1-[(S)-1-Formyl-2-(4-hydroxy-p...)Show SMILES Oc1ccc(C[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)OCc2ccccc2)C=O)cc1 Show InChI InChI=1S/C26H26N2O5/c29-17-22(15-20-11-13-23(30)14-12-20)27-25(31)24(16-19-7-3-1-4-8-19)28-26(32)33-18-21-9-5-2-6-10-21/h1-14,17,22,24,30H,15-16,18H2,(H,27,31)(H,28,32)/t22-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin S |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50002402
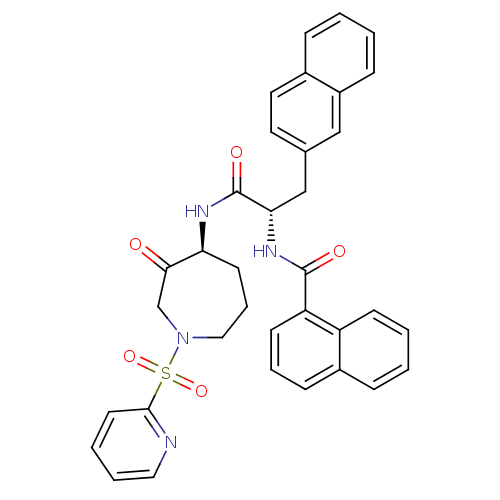
(CHEMBL194643)Show SMILES O=C(N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1)[C@H](Cc1ccc2ccccc2c1)NC(=O)c1cccc2ccccc12 Show InChI InChI=1S/C35H32N4O5S/c40-32-23-39(45(43,44)33-16-5-6-19-36-33)20-8-15-30(32)37-35(42)31(22-24-17-18-25-9-1-2-11-27(25)21-24)38-34(41)29-14-7-12-26-10-3-4-13-28(26)29/h1-7,9-14,16-19,21,30-31H,8,15,20,22-23H2,(H,37,42)(H,38,41)/t30-,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin L |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50084650

(CHEMBL177914 | {(S)-1-[(S)-1-Formyl-2-(4-hydroxy-p...)Show SMILES Oc1ccc(C[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)OCc2ccccc2)C=O)cc1 Show InChI InChI=1S/C26H26N2O5/c29-17-22(15-20-11-13-23(30)14-12-20)27-25(31)24(16-19-7-3-1-4-8-19)28-26(32)33-18-21-9-5-2-6-10-21/h1-14,17,22,24,30H,15-16,18H2,(H,27,31)(H,28,32)/t22-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin L |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50002399
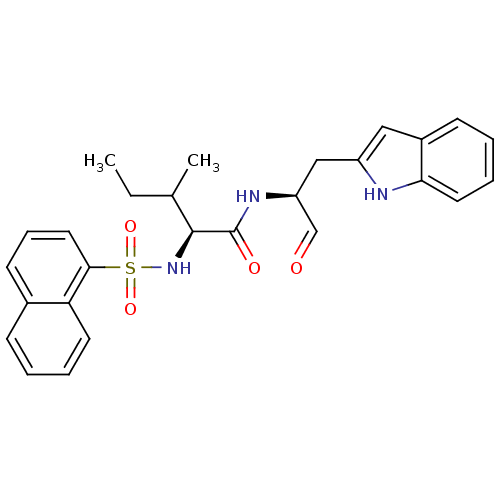
(CHEMBL197958)Show SMILES CCC(C)[C@H](NS(=O)(=O)c1cccc2ccccc12)C(=O)N[C@@H](Cc1cc2ccccc2[nH]1)C=O Show InChI InChI=1S/C27H29N3O4S/c1-3-18(2)26(30-35(33,34)25-14-8-11-19-9-4-6-12-23(19)25)27(32)29-22(17-31)16-21-15-20-10-5-7-13-24(20)28-21/h4-15,17-18,22,26,28,30H,3,16H2,1-2H3,(H,29,32)/t18?,22-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin S |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50002374
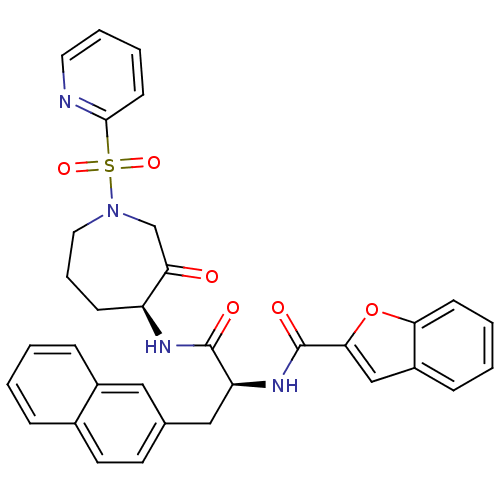
(CHEMBL194861)Show SMILES O=C(N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1)[C@H](Cc1ccc2ccccc2c1)NC(=O)c1cc2ccccc2o1 Show InChI InChI=1S/C33H30N4O6S/c38-28-21-37(44(41,42)31-13-5-6-16-34-31)17-7-11-26(28)35-32(39)27(19-22-14-15-23-8-1-2-9-24(23)18-22)36-33(40)30-20-25-10-3-4-12-29(25)43-30/h1-6,8-10,12-16,18,20,26-27H,7,11,17,19,21H2,(H,35,39)(H,36,40)/t26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin L |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50084650

(CHEMBL177914 | {(S)-1-[(S)-1-Formyl-2-(4-hydroxy-p...)Show SMILES Oc1ccc(C[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)OCc2ccccc2)C=O)cc1 Show InChI InChI=1S/C26H26N2O5/c29-17-22(15-20-11-13-23(30)14-12-20)27-25(31)24(16-19-7-3-1-4-8-19)28-26(32)33-18-21-9-5-2-6-10-21/h1-14,17,22,24,30H,15-16,18H2,(H,27,31)(H,28,32)/t22-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin K |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM50034711
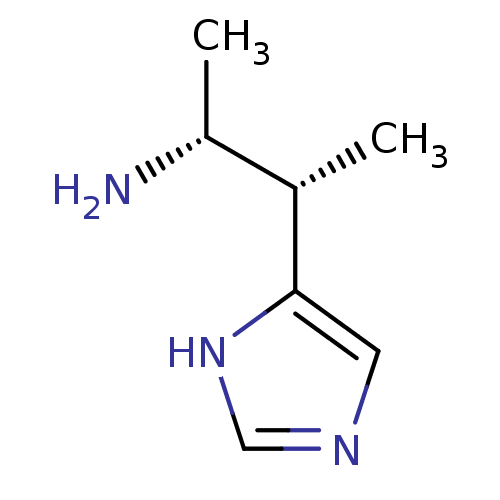
((1R,2S)-2-(1H-Imidazol-4-yl)-1-methyl-propylamine ...)Show InChI InChI=1S/C7H13N3/c1-5(6(2)8)7-3-9-4-10-7/h3-6H,8H2,1-2H3,(H,9,10)/t5-,6+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding afinity against Histamine H3 receptor |
J Med Chem 38: 1593-9 (1995)
BindingDB Entry DOI: 10.7270/Q2BK1BC1 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50002399

(CHEMBL197958)Show SMILES CCC(C)[C@H](NS(=O)(=O)c1cccc2ccccc12)C(=O)N[C@@H](Cc1cc2ccccc2[nH]1)C=O Show InChI InChI=1S/C27H29N3O4S/c1-3-18(2)26(30-35(33,34)25-14-8-11-19-9-4-6-12-23(19)25)27(32)29-22(17-31)16-21-15-20-10-5-7-13-24(20)28-21/h4-15,17-18,22,26,28,30H,3,16H2,1-2H3,(H,29,32)/t18?,22-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin L |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50002366
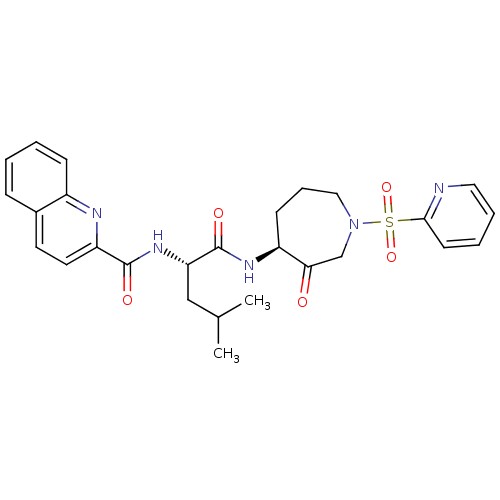
(CHEMBL433985)Show SMILES CC(C)C[C@H](NC(=O)c1ccc2ccccc2n1)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 Show InChI InChI=1S/C27H31N5O5S/c1-18(2)16-23(31-26(34)22-13-12-19-8-3-4-9-20(19)29-22)27(35)30-21-10-7-15-32(17-24(21)33)38(36,37)25-11-5-6-14-28-25/h3-6,8-9,11-14,18,21,23H,7,10,15-17H2,1-2H3,(H,30,35)(H,31,34)/t21-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin K |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50002400
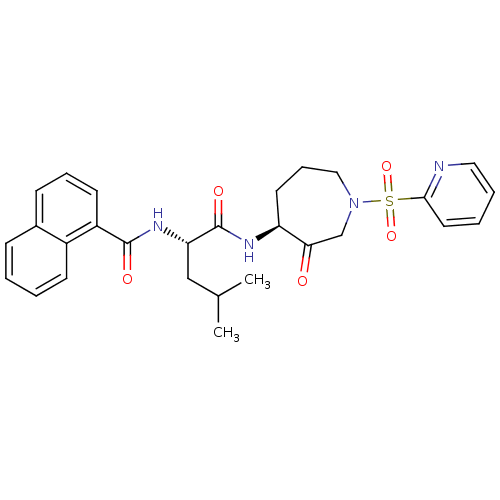
(CHEMBL382286)Show SMILES CC(C)C[C@H](NC(=O)c1cccc2ccccc12)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 Show InChI InChI=1S/C28H32N4O5S/c1-19(2)17-24(31-27(34)22-12-7-10-20-9-3-4-11-21(20)22)28(35)30-23-13-8-16-32(18-25(23)33)38(36,37)26-14-5-6-15-29-26/h3-7,9-12,14-15,19,23-24H,8,13,16-18H2,1-2H3,(H,30,35)(H,31,34)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin L |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM22904

((2R)-1-(1H-imidazol-5-yl)propan-2-amine | (R)-alph...)Show InChI InChI=1S/C6H11N3/c1-5(7)2-6-3-8-4-9-6/h3-5H,2,7H2,1H3,(H,8,9)/t5-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding afinity against Histamine H3 receptor |
J Med Chem 38: 1593-9 (1995)
BindingDB Entry DOI: 10.7270/Q2BK1BC1 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50002367
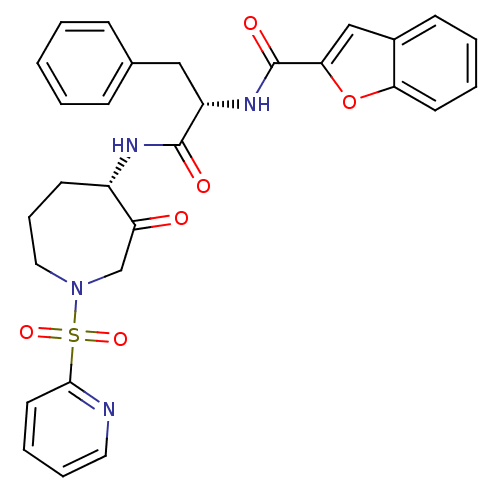
(CHEMBL407633)Show SMILES O=C(N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1)[C@H](Cc1ccccc1)NC(=O)c1cc2ccccc2o1 Show InChI InChI=1S/C29H28N4O6S/c34-24-19-33(40(37,38)27-14-6-7-15-30-27)16-8-12-22(24)31-28(35)23(17-20-9-2-1-3-10-20)32-29(36)26-18-21-11-4-5-13-25(21)39-26/h1-7,9-11,13-15,18,22-23H,8,12,16-17,19H2,(H,31,35)(H,32,36)/t22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin L |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50002399

(CHEMBL197958)Show SMILES CCC(C)[C@H](NS(=O)(=O)c1cccc2ccccc12)C(=O)N[C@@H](Cc1cc2ccccc2[nH]1)C=O Show InChI InChI=1S/C27H29N3O4S/c1-3-18(2)26(30-35(33,34)25-14-8-11-19-9-4-6-12-23(19)25)27(32)29-22(17-31)16-21-15-20-10-5-7-13-24(20)28-21/h4-15,17-18,22,26,28,30H,3,16H2,1-2H3,(H,29,32)/t18?,22-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin K |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19769

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H30N4O6S/c1-17(2)14-20(29-26(33)23-15-18-8-3-4-10-22(18)36-23)25(32)28-19-9-7-13-30(16-21(19)31)37(34,35)24-11-5-6-12-27-24/h3-6,8,10-12,15,17,19-20H,7,9,13-14,16H2,1-2H3,(H,28,32)(H,29,33)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin L |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM50034708
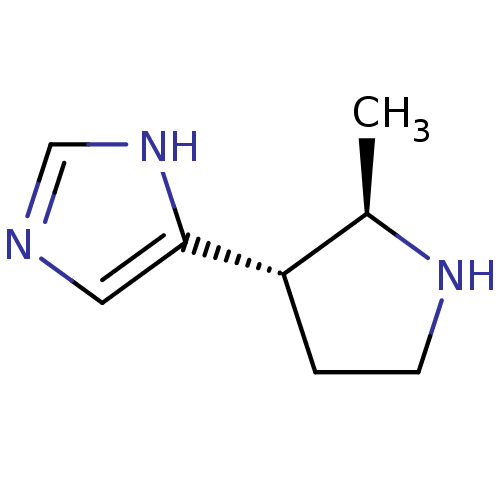
(4-((2R,3S)-2-Methyl-pyrrolidin-3-yl)-1H-imidazole ...)Show InChI InChI=1S/C8H13N3/c1-6-7(2-3-10-6)8-4-9-5-11-8/h4-7,10H,2-3H2,1H3,(H,9,11)/t6-,7+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding afinity against Histamine H3 receptor |
J Med Chem 38: 1593-9 (1995)
BindingDB Entry DOI: 10.7270/Q2BK1BC1 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM50034708

(4-((2R,3S)-2-Methyl-pyrrolidin-3-yl)-1H-imidazole ...)Show InChI InChI=1S/C8H13N3/c1-6-7(2-3-10-6)8-4-9-5-11-8/h4-7,10H,2-3H2,1H3,(H,9,11)/t6-,7+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding afinity against Histamine H3 receptor |
J Med Chem 38: 1593-9 (1995)
BindingDB Entry DOI: 10.7270/Q2BK1BC1 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50002374

(CHEMBL194861)Show SMILES O=C(N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1)[C@H](Cc1ccc2ccccc2c1)NC(=O)c1cc2ccccc2o1 Show InChI InChI=1S/C33H30N4O6S/c38-28-21-37(44(41,42)31-13-5-6-16-34-31)17-7-11-26(28)35-32(39)27(19-22-14-15-23-8-1-2-9-24(23)18-22)36-33(40)30-20-25-10-3-4-12-29(25)43-30/h1-6,8-10,12-16,18,20,26-27H,7,11,17,19,21H2,(H,35,39)(H,36,40)/t26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin S |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM19769

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H30N4O6S/c1-17(2)14-20(29-26(33)23-15-18-8-3-4-10-22(18)36-23)25(32)28-19-9-7-13-30(16-21(19)31)37(34,35)24-11-5-6-12-27-24/h3-6,8,10-12,15,17,19-20H,7,9,13-14,16H2,1-2H3,(H,28,32)(H,29,33)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin S |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50002366

(CHEMBL433985)Show SMILES CC(C)C[C@H](NC(=O)c1ccc2ccccc2n1)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 Show InChI InChI=1S/C27H31N5O5S/c1-18(2)16-23(31-26(34)22-13-12-19-8-3-4-9-20(19)29-22)27(35)30-21-10-7-15-32(17-24(21)33)38(36,37)25-11-5-6-14-28-25/h3-6,8-9,11-14,18,21,23H,7,10,15-17H2,1-2H3,(H,30,35)(H,31,34)/t21-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin L |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50002400

(CHEMBL382286)Show SMILES CC(C)C[C@H](NC(=O)c1cccc2ccccc12)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 Show InChI InChI=1S/C28H32N4O5S/c1-19(2)17-24(31-27(34)22-12-7-10-20-9-3-4-11-21(20)22)28(35)30-23-13-8-16-32(18-25(23)33)38(36,37)26-14-5-6-15-29-26/h3-7,9-12,14-15,19,23-24H,8,13,16-18H2,1-2H3,(H,30,35)(H,31,34)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin S |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50002398
(CHEMBL196023)Show SMILES CC(C)C[C@H](NC(=O)c1cccc2cccnc12)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 Show InChI InChI=1S/C27H31N5O5S/c1-18(2)16-22(31-26(34)20-10-5-8-19-9-6-14-29-25(19)20)27(35)30-21-11-7-15-32(17-23(21)33)38(36,37)24-12-3-4-13-28-24/h3-6,8-10,12-14,18,21-22H,7,11,15-17H2,1-2H3,(H,30,35)(H,31,34)/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin L |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50002367

(CHEMBL407633)Show SMILES O=C(N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1)[C@H](Cc1ccccc1)NC(=O)c1cc2ccccc2o1 Show InChI InChI=1S/C29H28N4O6S/c34-24-19-33(40(37,38)27-14-6-7-15-30-27)16-8-12-22(24)31-28(35)23(17-20-9-2-1-3-10-20)32-29(36)26-18-21-11-4-5-13-25(21)39-26/h1-7,9-11,13-15,18,22-23H,8,12,16-17,19H2,(H,31,35)(H,32,36)/t22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin S |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM50034706
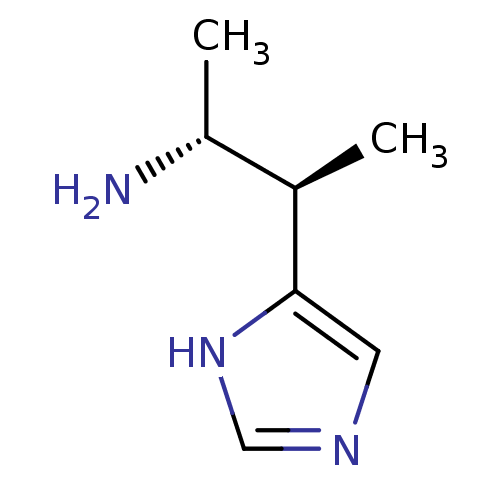
((1R,2R)-2-(1H-Imidazol-4-yl)-1-methyl-propylamine ...)Show InChI InChI=1S/C7H13N3/c1-5(6(2)8)7-3-9-4-10-7/h3-6H,8H2,1-2H3,(H,9,10)/t5-,6-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding afinity against Histamine H3 receptor |
J Med Chem 38: 1593-9 (1995)
BindingDB Entry DOI: 10.7270/Q2BK1BC1 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50002367

(CHEMBL407633)Show SMILES O=C(N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1)[C@H](Cc1ccccc1)NC(=O)c1cc2ccccc2o1 Show InChI InChI=1S/C29H28N4O6S/c34-24-19-33(40(37,38)27-14-6-7-15-30-27)16-8-12-22(24)31-28(35)23(17-20-9-2-1-3-10-20)32-29(36)26-18-21-11-4-5-13-25(21)39-26/h1-7,9-11,13-15,18,22-23H,8,12,16-17,19H2,(H,31,35)(H,32,36)/t22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin K |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50002400

(CHEMBL382286)Show SMILES CC(C)C[C@H](NC(=O)c1cccc2ccccc12)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 Show InChI InChI=1S/C28H32N4O5S/c1-19(2)17-24(31-27(34)22-12-7-10-20-9-3-4-11-21(20)22)28(35)30-23-13-8-16-32(18-25(23)33)38(36,37)26-14-5-6-15-29-26/h3-7,9-12,14-15,19,23-24H,8,13,16-18H2,1-2H3,(H,30,35)(H,31,34)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin K |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50002402

(CHEMBL194643)Show SMILES O=C(N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1)[C@H](Cc1ccc2ccccc2c1)NC(=O)c1cccc2ccccc12 Show InChI InChI=1S/C35H32N4O5S/c40-32-23-39(45(43,44)33-16-5-6-19-36-33)20-8-15-30(32)37-35(42)31(22-24-17-18-25-9-1-2-11-27(25)21-24)38-34(41)29-14-7-12-26-10-3-4-13-28(26)29/h1-7,9-14,16-19,21,30-31H,8,15,20,22-23H2,(H,37,42)(H,38,41)/t30-,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin S |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50002374

(CHEMBL194861)Show SMILES O=C(N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1)[C@H](Cc1ccc2ccccc2c1)NC(=O)c1cc2ccccc2o1 Show InChI InChI=1S/C33H30N4O6S/c38-28-21-37(44(41,42)31-13-5-6-16-34-31)17-7-11-26(28)35-32(39)27(19-22-14-15-23-8-1-2-9-24(23)18-22)36-33(40)30-20-25-10-3-4-12-29(25)43-30/h1-6,8-10,12-16,18,20,26-27H,7,11,17,19,21H2,(H,35,39)(H,36,40)/t26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin B |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50002366

(CHEMBL433985)Show SMILES CC(C)C[C@H](NC(=O)c1ccc2ccccc2n1)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 Show InChI InChI=1S/C27H31N5O5S/c1-18(2)16-23(31-26(34)22-13-12-19-8-3-4-9-20(19)29-22)27(35)30-21-10-7-15-32(17-24(21)33)38(36,37)25-11-5-6-14-28-25/h3-6,8-9,11-14,18,21,23H,7,10,15-17H2,1-2H3,(H,30,35)(H,31,34)/t21-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin S |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM50034707
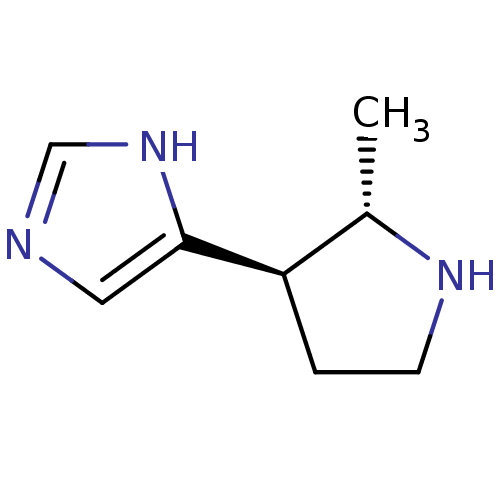
(4-((2S,3R)-2-Methyl-pyrrolidin-3-yl)-1H-imidazole ...)Show InChI InChI=1S/C8H13N3/c1-6-7(2-3-10-6)8-4-9-5-11-8/h4-7,10H,2-3H2,1H3,(H,9,11)/t6-,7+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding afinity against Histamine H3 receptor |
J Med Chem 38: 1593-9 (1995)
BindingDB Entry DOI: 10.7270/Q2BK1BC1 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50002398

(CHEMBL196023)Show SMILES CC(C)C[C@H](NC(=O)c1cccc2cccnc12)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 Show InChI InChI=1S/C27H31N5O5S/c1-18(2)16-22(31-26(34)20-10-5-8-19-9-6-14-29-25(19)20)27(35)30-21-11-7-15-32(17-23(21)33)38(36,37)24-12-3-4-13-28-24/h3-6,8-10,12-14,18,21-22H,7,11,15-17H2,1-2H3,(H,30,35)(H,31,34)/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin S |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50002398

(CHEMBL196023)Show SMILES CC(C)C[C@H](NC(=O)c1cccc2cccnc12)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 Show InChI InChI=1S/C27H31N5O5S/c1-18(2)16-22(31-26(34)20-10-5-8-19-9-6-14-29-25(19)20)27(35)30-21-11-7-15-32(17-23(21)33)38(36,37)24-12-3-4-13-28-24/h3-6,8-10,12-14,18,21-22H,7,11,15-17H2,1-2H3,(H,30,35)(H,31,34)/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin K |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50002402

(CHEMBL194643)Show SMILES O=C(N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1)[C@H](Cc1ccc2ccccc2c1)NC(=O)c1cccc2ccccc12 Show InChI InChI=1S/C35H32N4O5S/c40-32-23-39(45(43,44)33-16-5-6-19-36-33)20-8-15-30(32)37-35(42)31(22-24-17-18-25-9-1-2-11-27(25)21-24)38-34(41)29-14-7-12-26-10-3-4-13-28(26)29/h1-7,9-14,16-19,21,30-31H,8,15,20,22-23H2,(H,37,42)(H,38,41)/t30-,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin B |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50002367

(CHEMBL407633)Show SMILES O=C(N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1)[C@H](Cc1ccccc1)NC(=O)c1cc2ccccc2o1 Show InChI InChI=1S/C29H28N4O6S/c34-24-19-33(40(37,38)27-14-6-7-15-30-27)16-8-12-22(24)31-28(35)23(17-20-9-2-1-3-10-20)32-29(36)26-18-21-11-4-5-13-25(21)39-26/h1-7,9-11,13-15,18,22-23H,8,12,16-17,19H2,(H,31,35)(H,32,36)/t22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 193 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin B |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50002374

(CHEMBL194861)Show SMILES O=C(N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1)[C@H](Cc1ccc2ccccc2c1)NC(=O)c1cc2ccccc2o1 Show InChI InChI=1S/C33H30N4O6S/c38-28-21-37(44(41,42)31-13-5-6-16-34-31)17-7-11-26(28)35-32(39)27(19-22-14-15-23-8-1-2-9-24(23)18-22)36-33(40)30-20-25-10-3-4-12-29(25)43-30/h1-6,8-10,12-16,18,20,26-27H,7,11,17,19,21H2,(H,35,39)(H,36,40)/t26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 295 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin K |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM19769

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H30N4O6S/c1-17(2)14-20(29-26(33)23-15-18-8-3-4-10-22(18)36-23)25(32)28-19-9-7-13-30(16-21(19)31)37(34,35)24-11-5-6-12-27-24/h3-6,8,10-12,15,17,19-20H,7,9,13-14,16H2,1-2H3,(H,28,32)(H,29,33)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin B |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM50034709

((1S,2S)-2-(1H-Imidazol-4-yl)-cyclopentylamine | CH...)Show InChI InChI=1S/C8H13N3/c9-7-3-1-2-6(7)8-4-10-5-11-8/h4-7H,1-3,9H2,(H,10,11)/t6-,7-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding afinity against Histamine H3 receptor |
J Med Chem 38: 1593-9 (1995)
BindingDB Entry DOI: 10.7270/Q2BK1BC1 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50002366

(CHEMBL433985)Show SMILES CC(C)C[C@H](NC(=O)c1ccc2ccccc2n1)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 Show InChI InChI=1S/C27H31N5O5S/c1-18(2)16-23(31-26(34)22-13-12-19-8-3-4-9-20(19)29-22)27(35)30-21-10-7-15-32(17-24(21)33)38(36,37)25-11-5-6-14-28-25/h3-6,8-9,11-14,18,21,23H,7,10,15-17H2,1-2H3,(H,30,35)(H,31,34)/t21-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin B |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50002398

(CHEMBL196023)Show SMILES CC(C)C[C@H](NC(=O)c1cccc2cccnc12)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 Show InChI InChI=1S/C27H31N5O5S/c1-18(2)16-22(31-26(34)20-10-5-8-19-9-6-14-29-25(19)20)27(35)30-21-11-7-15-32(17-23(21)33)38(36,37)24-12-3-4-13-28-24/h3-6,8-10,12-14,18,21-22H,7,11,15-17H2,1-2H3,(H,30,35)(H,31,34)/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin B |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50568221
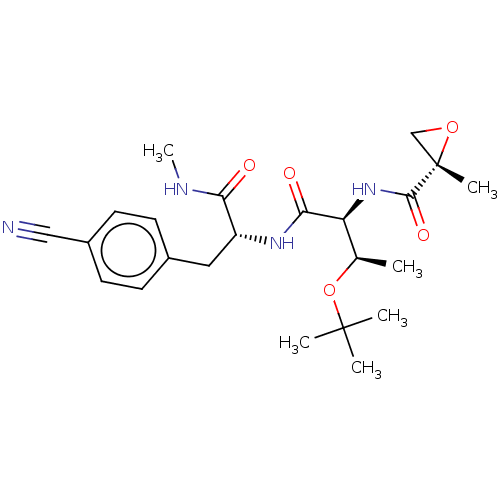
(CHEMBL4854947)Show SMILES CNC(=O)[C@@H](Cc1ccc(cc1)C#N)NC(=O)[C@@H](NC(=O)[C@]1(C)CO1)[C@@H](C)OC(C)(C)C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His-tagged BTK expressed in baculovirus infected in Sf9 cells assessed as inhibitory constant incubated fo... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116223
BindingDB Entry DOI: 10.7270/Q2M04964 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50568222
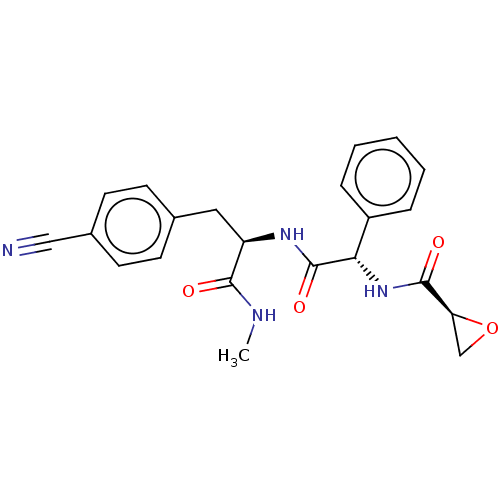
(CHEMBL4867490)Show SMILES CNC(=O)[C@@H](Cc1ccc(cc1)C#N)NC(=O)[C@@H](NC(=O)[C@@H]1CO1)c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His-tagged BTK expressed in baculovirus infected in Sf9 cells assessed as inhibitory constant incubated fo... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116223
BindingDB Entry DOI: 10.7270/Q2M04964 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM50034713
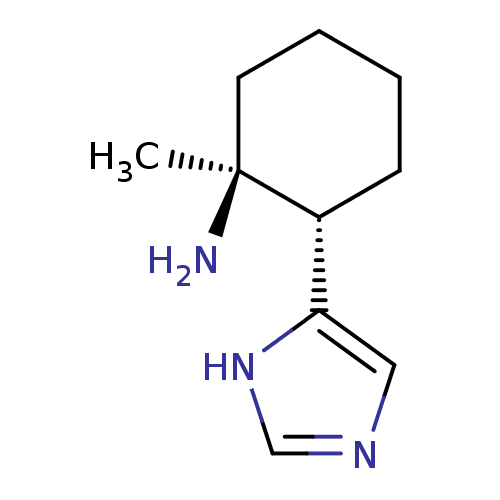
((1S,2S)-2-(1H-Imidazol-4-yl)-1-methyl-cyclohexylam...)Show InChI InChI=1S/C10H17N3/c1-10(11)5-3-2-4-8(10)9-6-12-7-13-9/h6-8H,2-5,11H2,1H3,(H,12,13)/t8-,10+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding afinity against Histamine H3 receptor |
J Med Chem 38: 1593-9 (1995)
BindingDB Entry DOI: 10.7270/Q2BK1BC1 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM50034710

((1R,2S)-2-(1H-Imidazol-4-yl)-1-methyl-cyclohexylam...)Show InChI InChI=1S/C10H17N3/c1-10(11)5-3-2-4-8(10)9-6-12-7-13-9/h6-8H,2-5,11H2,1H3,(H,12,13)/t8-,10-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding afinity against Histamine H3 receptor |
J Med Chem 38: 1593-9 (1995)
BindingDB Entry DOI: 10.7270/Q2BK1BC1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50568220
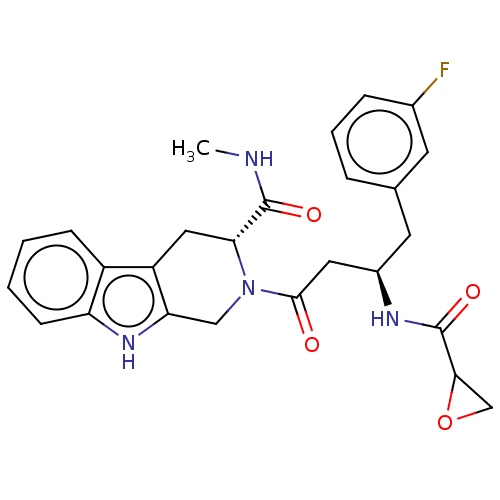
(CHEMBL4873655)Show SMILES CNC(=O)[C@H]1Cc2c(CN1C(=O)C[C@@H](Cc1cccc(F)c1)NC(=O)C1CO1)[nH]c1ccccc21 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His-tagged BTK expressed in baculovirus infected in Sf9 cells assessed as inhibitory constant incubated fo... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116223
BindingDB Entry DOI: 10.7270/Q2M04964 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Cavia porcellus (domestic guinea pig)) | BDBM22904

((2R)-1-(1H-imidazol-5-yl)propan-2-amine | (R)-alph...)Show InChI InChI=1S/C6H11N3/c1-5(7)2-6-3-8-4-9-6/h3-5H,2,7H2,1H3,(H,8,9)/t5-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding afinity against H1 receptor |
J Med Chem 38: 1593-9 (1995)
BindingDB Entry DOI: 10.7270/Q2BK1BC1 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50034708

(4-((2R,3S)-2-Methyl-pyrrolidin-3-yl)-1H-imidazole ...)Show InChI InChI=1S/C8H13N3/c1-6-7(2-3-10-6)8-4-9-5-11-8/h4-7,10H,2-3H2,1H3,(H,9,11)/t6-,7+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding afinity against H1 receptor |
J Med Chem 38: 1593-9 (1995)
BindingDB Entry DOI: 10.7270/Q2BK1BC1 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50034707

(4-((2S,3R)-2-Methyl-pyrrolidin-3-yl)-1H-imidazole ...)Show InChI InChI=1S/C8H13N3/c1-6-7(2-3-10-6)8-4-9-5-11-8/h4-7,10H,2-3H2,1H3,(H,9,11)/t6-,7+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding afinity against H1 receptor |
J Med Chem 38: 1593-9 (1995)
BindingDB Entry DOI: 10.7270/Q2BK1BC1 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50002402

(CHEMBL194643)Show SMILES O=C(N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1)[C@H](Cc1ccc2ccccc2c1)NC(=O)c1cccc2ccccc12 Show InChI InChI=1S/C35H32N4O5S/c40-32-23-39(45(43,44)33-16-5-6-19-36-33)20-8-15-30(32)37-35(42)31(22-24-17-18-25-9-1-2-11-27(25)21-24)38-34(41)29-14-7-12-26-10-3-4-13-28(26)29/h1-7,9-14,16-19,21,30-31H,8,15,20,22-23H2,(H,37,42)(H,38,41)/t30-,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin K |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM225238
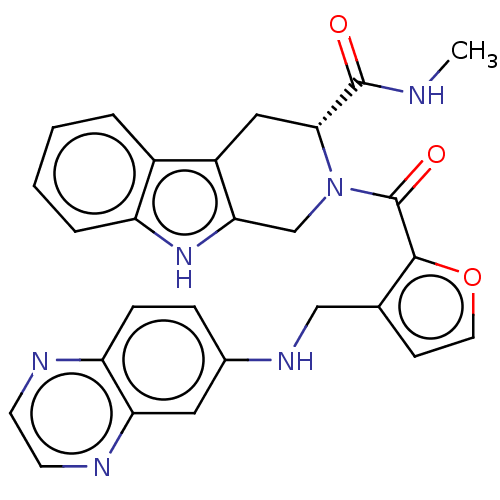
(BTK inhibitor, 3)Show SMILES CNC(=O)[C@H]1Cc2c(CN1C(=O)c1occc1CNc1ccc3nccnc3c1)[nH]c1ccccc21 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant wild type human N-terminal His6-tagged BTK expressed in baculovirus incubated for 20 mins by TR-FRET based competitive bind... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116223
BindingDB Entry DOI: 10.7270/Q2M04964 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM225238

(BTK inhibitor, 3)Show SMILES CNC(=O)[C@H]1Cc2c(CN1C(=O)c1occc1CNc1ccc3nccnc3c1)[nH]c1ccccc21 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | 30 |
X-Chem Pharmaceuticals
| Assay Description
The BTK time-resolved FRET-based competitive binding assay and cell-based BTK assays have been previously described.[Xu et al., J.Pharmacol. Exp. The... |
Chembiochem 18: 864-871 (2017)
Article DOI: 10.1002/cbic.201600573
BindingDB Entry DOI: 10.7270/Q22J69Q5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data