

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50366780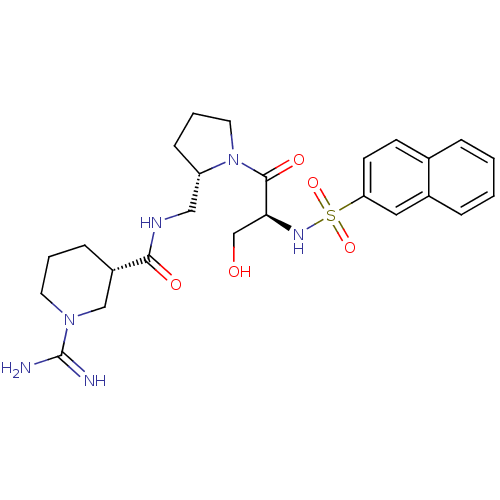 (BMS-189090 | CHEMBL138877) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro reversible inhibition of thrombin catalytic activity | Bioorg Med Chem Lett 12: 41-4 (2001) BindingDB Entry DOI: 10.7270/Q2MP53T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50039010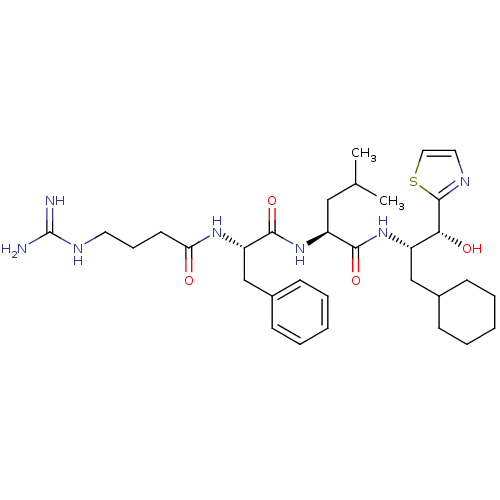 ((S)-2-[(S)-2-(4-Guanidino-butyrylamino)-3-phenyl-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for thrombin was reported | J Med Chem 37: 2122-4 (1994) BindingDB Entry DOI: 10.7270/Q2FQ9VPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50287156 (2-Benzyl-1H-indole-5-carboxamidine | CHEMBL287401) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of human alpha-thrombin catalytic activity | Bioorg Med Chem Lett 6: 1339-1344 (1996) Article DOI: 10.1016/0960-894X(96)00229-6 BindingDB Entry DOI: 10.7270/Q2TM7B2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50228863 ((S)-1-((R)-2-Methylamino-3-phenyl-propionyl)-pyrro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro activity against trypsin was determined | J Med Chem 37: 2122-4 (1994) BindingDB Entry DOI: 10.7270/Q2FQ9VPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50228863 ((S)-1-((R)-2-Methylamino-3-phenyl-propionyl)-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro activity against thrombin was determined | J Med Chem 37: 2122-4 (1994) BindingDB Entry DOI: 10.7270/Q2FQ9VPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107445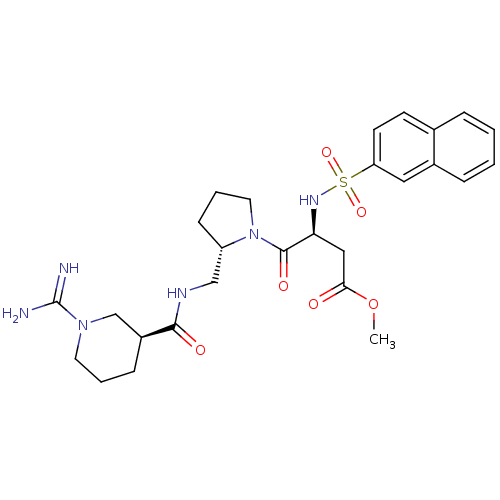 ((S)-4-((S)-2-{[((S)-1-Carbamimidoyl-piperidine-3-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation. | Bioorg Med Chem Lett 12: 41-4 (2001) BindingDB Entry DOI: 10.7270/Q2MP53T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107455 (4-Carbamimidoyl-N-{(S)-1-[(S)-3-hydroxy-2-(naphtha...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation. | Bioorg Med Chem Lett 12: 41-4 (2001) BindingDB Entry DOI: 10.7270/Q2MP53T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50366780 (BMS-189090 | CHEMBL138877) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation. | Bioorg Med Chem Lett 12: 41-4 (2001) BindingDB Entry DOI: 10.7270/Q2MP53T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50366780 (BMS-189090 | CHEMBL138877) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation. | Bioorg Med Chem Lett 12: 41-4 (2001) BindingDB Entry DOI: 10.7270/Q2MP53T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50228863 ((S)-1-((R)-2-Methylamino-3-phenyl-propionyl)-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of thrombin catalytic activity using s-2238 substrate at 10 uM was measured at rt after 3 min incubation with compound | Bioorg Med Chem Lett 12: 41-4 (2001) BindingDB Entry DOI: 10.7270/Q2MP53T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107446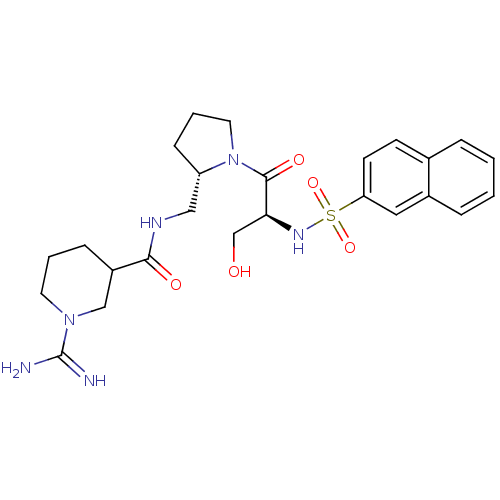 (1-Carbamimidoyl-piperidine-3-carboxylic acid {(S)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation. | Bioorg Med Chem Lett 12: 41-4 (2001) BindingDB Entry DOI: 10.7270/Q2MP53T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50038001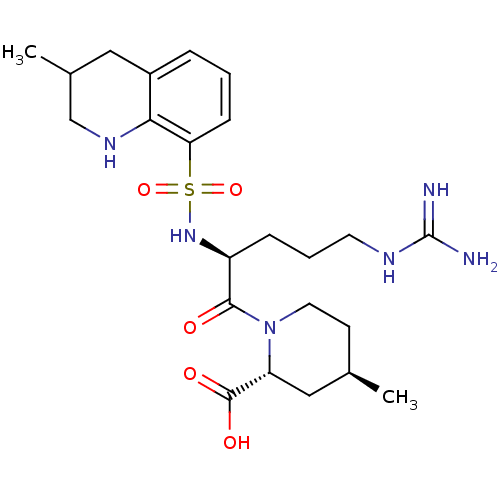 ((2R,4R)-1-((S)-5-(diaminomethyleneamino)-2-(3-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of thrombin catalytic activity using s-2238 substrate at 10 uM was measured at rat after 3 min incubation with compound | Bioorg Med Chem Lett 12: 41-4 (2001) BindingDB Entry DOI: 10.7270/Q2MP53T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107442 (6-Guanidino-hexanoic acid {(S)-1-[(S)-3-hydroxy-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation. | Bioorg Med Chem Lett 12: 41-4 (2001) BindingDB Entry DOI: 10.7270/Q2MP53T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107434 (1-Carbamimidoyl-piperidine-3-carboxylic acid {(S)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation. | Bioorg Med Chem Lett 12: 41-4 (2001) BindingDB Entry DOI: 10.7270/Q2MP53T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107453 (5-Guanidino-pentanoic acid {(S)-1-[(S)-3-hydroxy-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation. | Bioorg Med Chem Lett 12: 41-4 (2001) BindingDB Entry DOI: 10.7270/Q2MP53T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107449 (1-Carbamimidoyl-piperidine-4-carboxylic acid {(S)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation. | Bioorg Med Chem Lett 12: 41-4 (2001) BindingDB Entry DOI: 10.7270/Q2MP53T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50039010 ((S)-2-[(S)-2-(4-Guanidino-butyrylamino)-3-phenyl-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro activity against thrombin was determined | J Med Chem 37: 2122-4 (1994) BindingDB Entry DOI: 10.7270/Q2FQ9VPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107447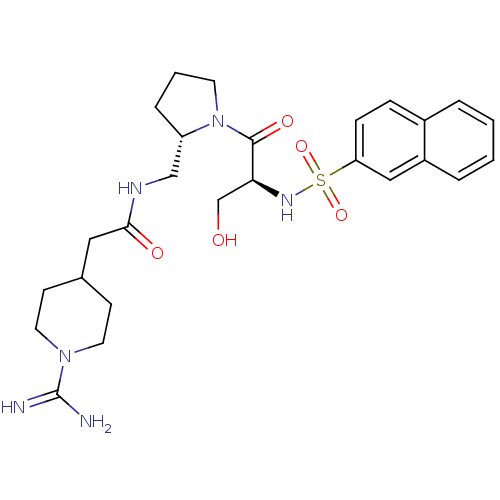 (2-(1-Carbamimidoyl-piperidin-4-yl)-N-{(S)-1-[(S)-3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation. | Bioorg Med Chem Lett 12: 41-4 (2001) BindingDB Entry DOI: 10.7270/Q2MP53T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50228863 ((S)-1-((R)-2-Methylamino-3-phenyl-propionyl)-pyrro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro activity against plasmid was determined | J Med Chem 37: 2122-4 (1994) BindingDB Entry DOI: 10.7270/Q2FQ9VPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107439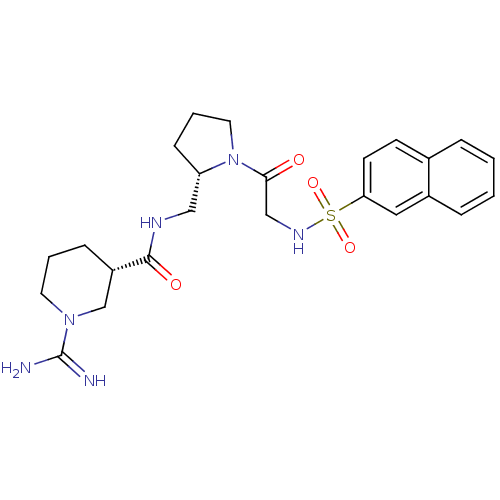 (1-Carbamimidoyl-piperidine-3-carboxylic acid {(S)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation. | Bioorg Med Chem Lett 12: 41-4 (2001) BindingDB Entry DOI: 10.7270/Q2MP53T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107444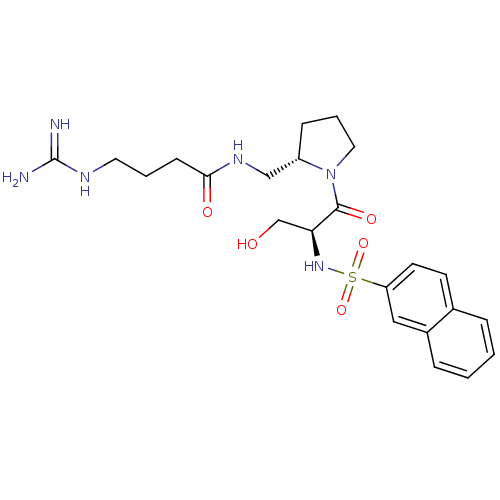 (4-Guanidino-N-{(S)-1-[(S)-3-hydroxy-2-(naphthalene...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation. | Bioorg Med Chem Lett 12: 41-4 (2001) BindingDB Entry DOI: 10.7270/Q2MP53T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50451350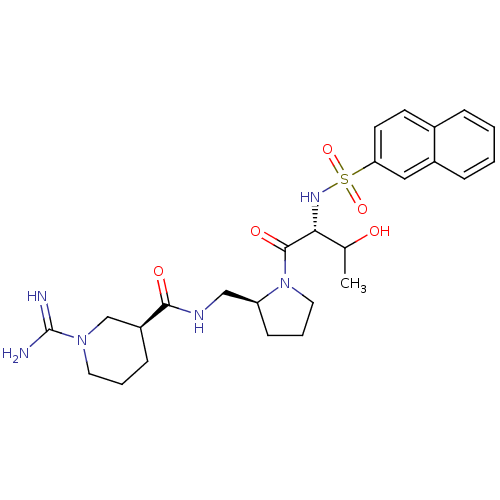 (CHEMBL2111938) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of thrombin catalytic activity using s-2238 substrate at 10 uM was measured at rat after 3 min incubation with compound | Bioorg Med Chem Lett 12: 41-4 (2001) BindingDB Entry DOI: 10.7270/Q2MP53T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107450 ((R)-1-Carbamimidoyl-piperidine-3-carboxylic acid {...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation. | Bioorg Med Chem Lett 12: 41-4 (2001) BindingDB Entry DOI: 10.7270/Q2MP53T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107441 ((2S,4S)-1-[(S)-3-Hydroxy-2-(naphthalene-2-sulfonyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation. | Bioorg Med Chem Lett 12: 41-4 (2001) BindingDB Entry DOI: 10.7270/Q2MP53T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107448 (1-Carbamimidoyl-piperidine-3-carboxylic acid {(S)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation. | Bioorg Med Chem Lett 12: 41-4 (2001) BindingDB Entry DOI: 10.7270/Q2MP53T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50287156 (2-Benzyl-1H-indole-5-carboxamidine | CHEMBL287401) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of human alpha-thrombin catalytic activity. | Bioorg Med Chem Lett 6: 1339-1344 (1996) Article DOI: 10.1016/0960-894X(96)00229-6 BindingDB Entry DOI: 10.7270/Q2TM7B2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107440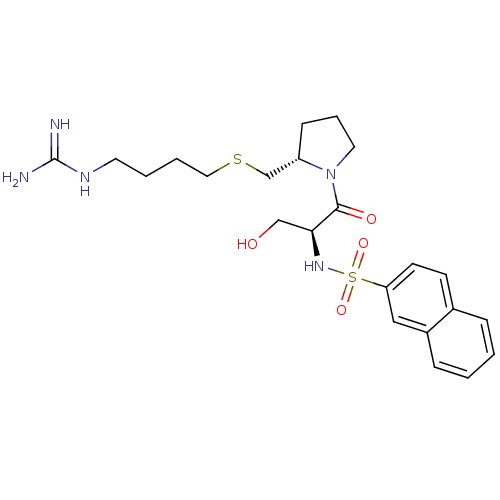 (CHEMBL136076 | Naphthalene-2-sulfonic acid {(S)-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation. | Bioorg Med Chem Lett 12: 41-4 (2001) BindingDB Entry DOI: 10.7270/Q2MP53T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50039007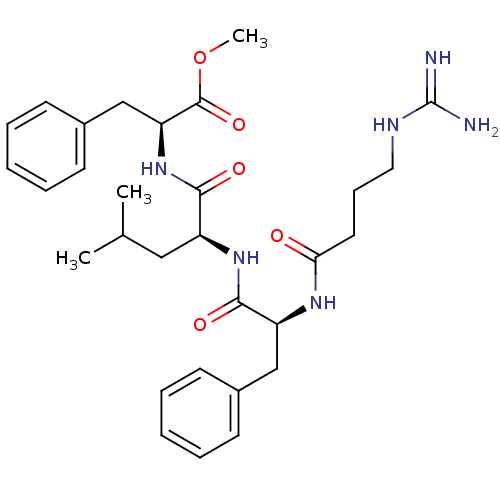 ((S)-2-{(S)-2-[(S)-2-(4-Guanidino-butyrylamino)-3-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro activity against thrombin with 10 uM substrate s-2238 (D-Phe-Pip-Arg-pNA) | J Med Chem 37: 2122-4 (1994) BindingDB Entry DOI: 10.7270/Q2FQ9VPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50039010 ((S)-2-[(S)-2-(4-Guanidino-butyrylamino)-3-phenyl-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro activity against trypsin was determined | J Med Chem 37: 2122-4 (1994) BindingDB Entry DOI: 10.7270/Q2FQ9VPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107451 ((2R,4R)-1-[(S)-3-Hydroxy-2-(naphthalene-2-sulfonyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation. | Bioorg Med Chem Lett 12: 41-4 (2001) BindingDB Entry DOI: 10.7270/Q2MP53T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50287159 (2-(3-Phenoxy-benzyl)-1H-indole-5-carboxamidine | C...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of human alpha-thrombin catalytic activity. | Bioorg Med Chem Lett 6: 1339-1344 (1996) Article DOI: 10.1016/0960-894X(96)00229-6 BindingDB Entry DOI: 10.7270/Q2TM7B2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50287154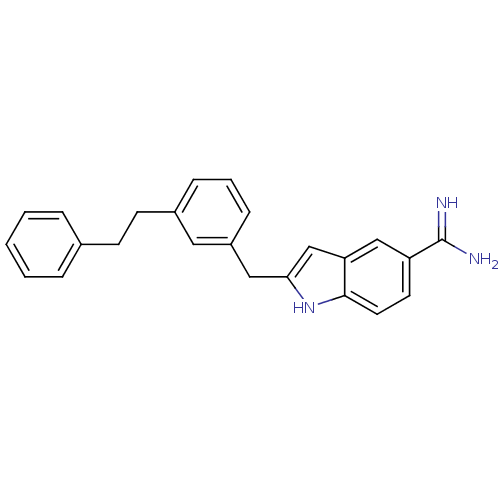 (2-(3-Phenethyl-benzyl)-1H-indole-5-carboxamidine |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of human alpha-thrombin catalytic activity. | Bioorg Med Chem Lett 6: 1339-1344 (1996) Article DOI: 10.1016/0960-894X(96)00229-6 BindingDB Entry DOI: 10.7270/Q2TM7B2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50039021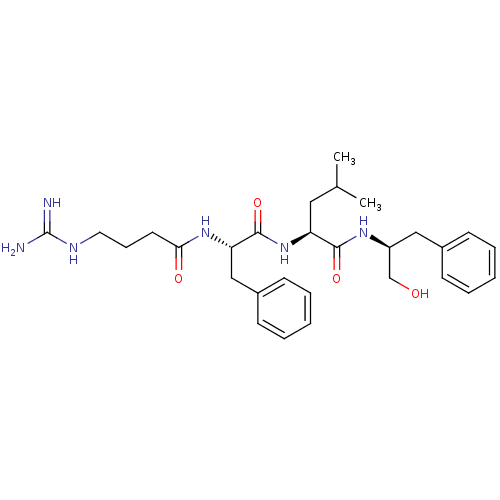 ((S)-2-[(S)-2-(4-Guanidino-butyrylamino)-3-phenyl-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro activity against thrombin with 10 uM substrate s-2238 (D-Phe-Pip-Arg-pNA) | J Med Chem 37: 2122-4 (1994) BindingDB Entry DOI: 10.7270/Q2FQ9VPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107438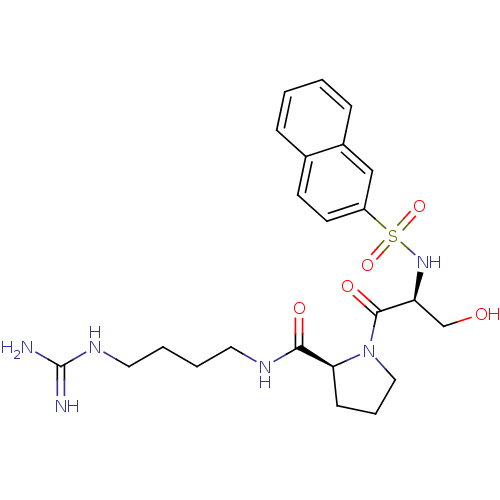 ((S)-1-[(S)-3-Hydroxy-2-(naphthalene-2-sulfonylamin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation. | Bioorg Med Chem Lett 12: 41-4 (2001) BindingDB Entry DOI: 10.7270/Q2MP53T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107443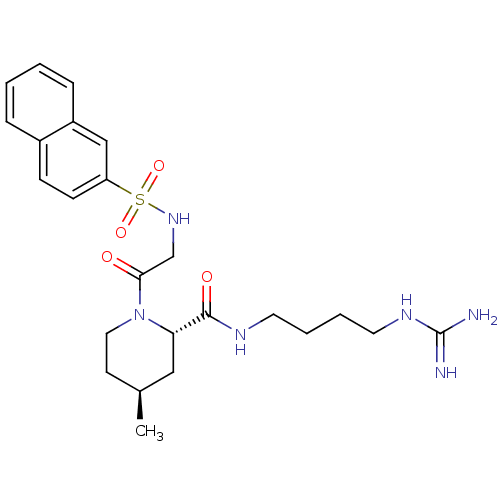 ((2S,4S)-4-Methyl-1-[2-(naphthalene-2-sulfonylamino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of thrombin catalytic activity using s-2238 substrate at 10 uM was measured at rt after 3 min incubation with compound | Bioorg Med Chem Lett 12: 41-4 (2001) BindingDB Entry DOI: 10.7270/Q2MP53T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50228863 ((S)-1-((R)-2-Methylamino-3-phenyl-propionyl)-pyrro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro activity of the compound against Factor Xa was determined | J Med Chem 37: 2122-4 (1994) BindingDB Entry DOI: 10.7270/Q2FQ9VPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50039010 ((S)-2-[(S)-2-(4-Guanidino-butyrylamino)-3-phenyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro activity against Factor Xa was determined | J Med Chem 37: 2122-4 (1994) BindingDB Entry DOI: 10.7270/Q2FQ9VPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107436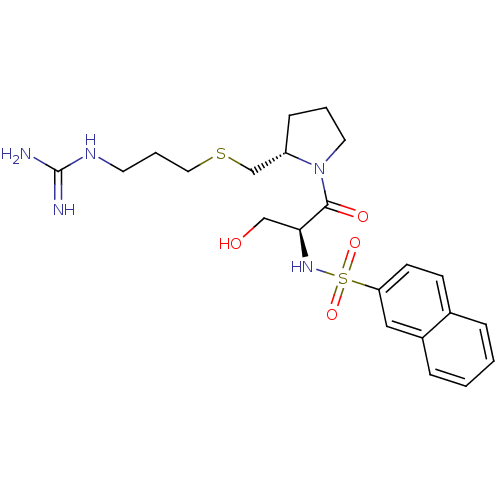 (CHEMBL344825 | Naphthalene-2-sulfonic acid {(S)-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation. | Bioorg Med Chem Lett 12: 41-4 (2001) BindingDB Entry DOI: 10.7270/Q2MP53T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50039018 (6-Amino-hexanoic acid {(S)-1-[(S)-1-((1S,2R)-1-cyc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro activity against thrombin with 10 uM substrate s-2238 (D-Phe-Pip-Arg-pNA) | J Med Chem 37: 2122-4 (1994) BindingDB Entry DOI: 10.7270/Q2FQ9VPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107454 (7-Guanidino-heptanoic acid {(S)-1-[(S)-3-hydroxy-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation. | Bioorg Med Chem Lett 12: 41-4 (2001) BindingDB Entry DOI: 10.7270/Q2MP53T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50287152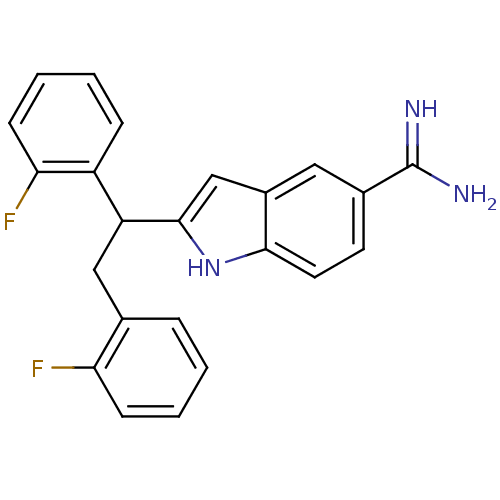 (2-[1,2-Bis-(2-fluoro-phenyl)-ethyl]-1H-indole-5-ca...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of human alpha-thrombin catalytic activity. | Bioorg Med Chem Lett 6: 1339-1344 (1996) Article DOI: 10.1016/0960-894X(96)00229-6 BindingDB Entry DOI: 10.7270/Q2TM7B2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50039020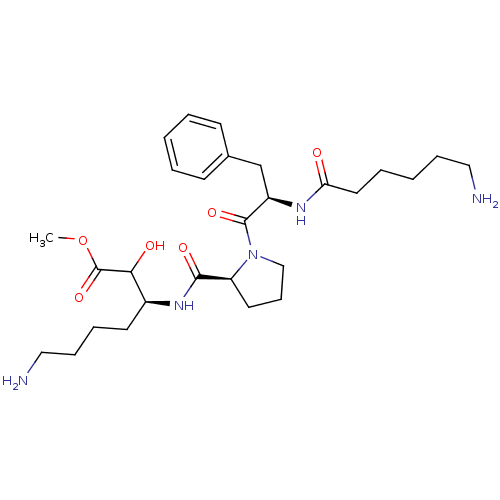 ((S)-7-Amino-3-({(S)-1-[(R)-2-(6-amino-hexanoylamin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro activity against thrombin with 10 uM substrate s-2238 (D-Phe-Pip-Arg-pNA) | J Med Chem 37: 2122-4 (1994) BindingDB Entry DOI: 10.7270/Q2FQ9VPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50287160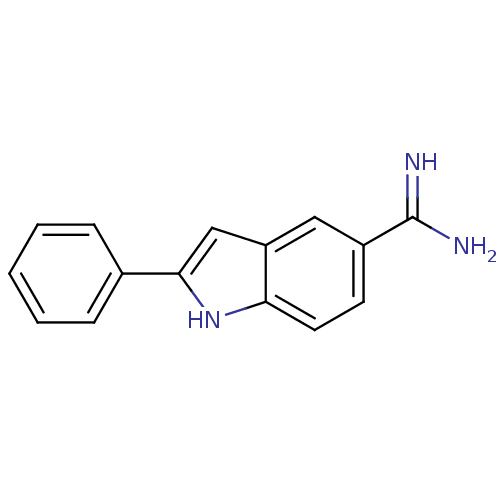 (2-Phenyl-1H-indole-5-carboxamidine | 2-phenyl-1H-i...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of human alpha-thrombin catalytic activity. | Bioorg Med Chem Lett 6: 1339-1344 (1996) Article DOI: 10.1016/0960-894X(96)00229-6 BindingDB Entry DOI: 10.7270/Q2TM7B2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50287157 (6,7,8,9-Tetrahydro-5H-carbazole-3-carboxamidine | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of human alpha-thrombin catalytic activity. | Bioorg Med Chem Lett 6: 1339-1344 (1996) Article DOI: 10.1016/0960-894X(96)00229-6 BindingDB Entry DOI: 10.7270/Q2TM7B2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50027306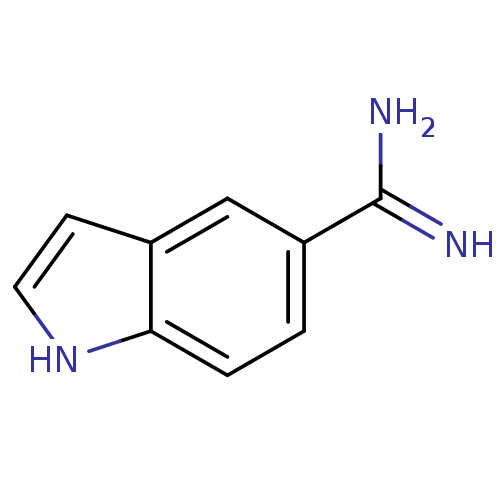 (1H-Indole-5-carboxamidine | CHEMBL26490) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of human alpha-thrombin catalytic activity. | Bioorg Med Chem Lett 6: 1339-1344 (1996) Article DOI: 10.1016/0960-894X(96)00229-6 BindingDB Entry DOI: 10.7270/Q2TM7B2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107437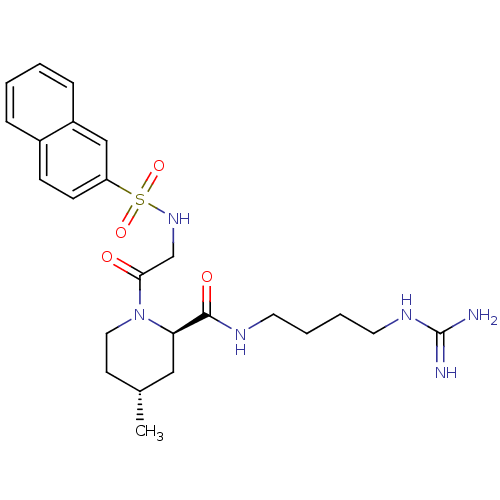 ((2R,4R)-4-Methyl-1-[2-(naphthalene-2-sulfonylamino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation. | Bioorg Med Chem Lett 12: 41-4 (2001) BindingDB Entry DOI: 10.7270/Q2MP53T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50039016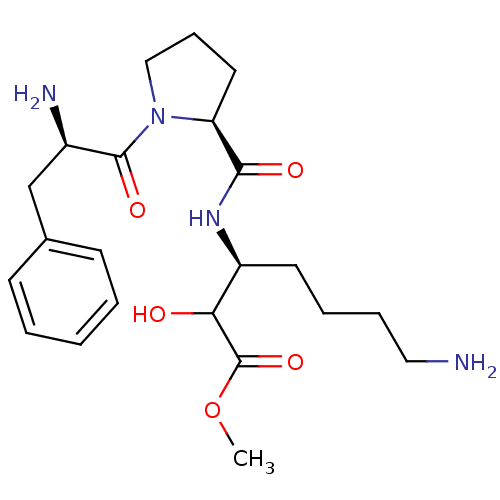 ((S)-7-Amino-3-{[(S)-1-((R)-2-amino-3-phenyl-propio...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro activity against thrombin was determined, using a 10 microM substrate s-2238 (D-Phe-Pip-Arg-pNA); 20-30 | J Med Chem 37: 2122-4 (1994) BindingDB Entry DOI: 10.7270/Q2FQ9VPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50287153 (2-(2-Fluoro-benzyl)-1H-indole-5-carboxamidine | CH...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of human alpha-thrombin catalytic activity. | Bioorg Med Chem Lett 6: 1339-1344 (1996) Article DOI: 10.1016/0960-894X(96)00229-6 BindingDB Entry DOI: 10.7270/Q2TM7B2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50102768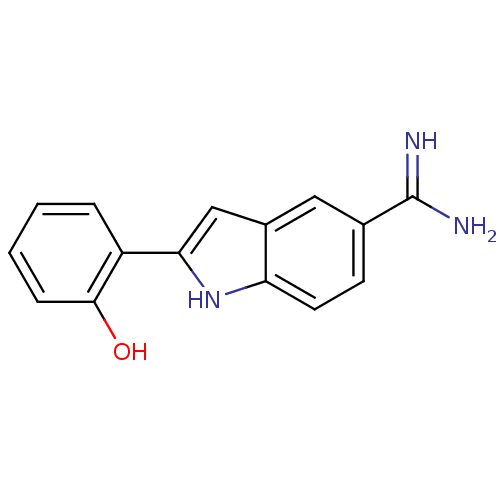 (2-(2-Hydroxy-phenyl)-1H-indole-5-carboxamidine | C...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of human alpha-thrombin catalytic activity. | Bioorg Med Chem Lett 6: 1339-1344 (1996) Article DOI: 10.1016/0960-894X(96)00229-6 BindingDB Entry DOI: 10.7270/Q2TM7B2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50039011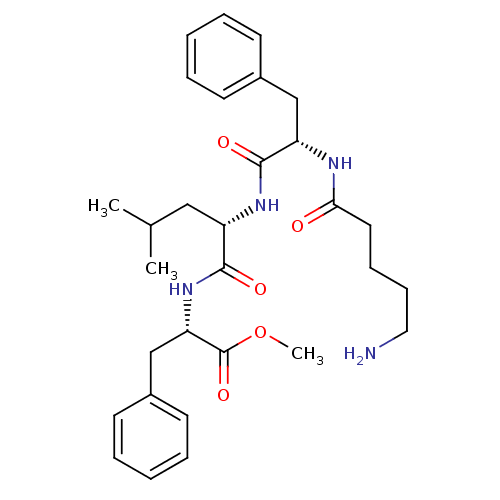 ((S)-2-{(S)-2-[(S)-2-(5-Amino-pentanoylamino)-3-phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro activity against thrombin with 10 uM substrate s-2238 (D-Phe-Pip-Arg-pNA) | J Med Chem 37: 2122-4 (1994) BindingDB Entry DOI: 10.7270/Q2FQ9VPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 61 total ) | Next | Last >> |