

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50031306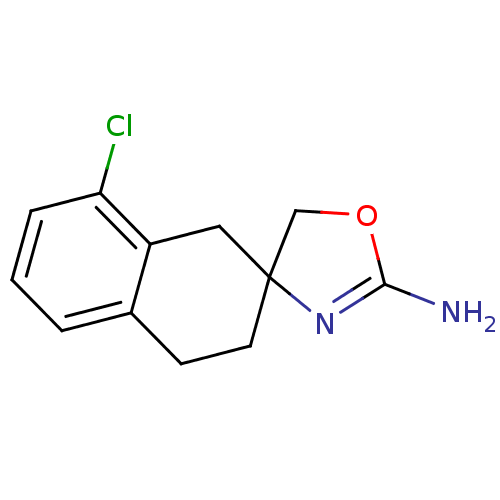 (8-chlorospiro[1,2,3,4-tetrahydronaphthalene-2,4'-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier Curated by ChEMBL | Assay Description Binding affinity towards Alpha-2A adrenergic receptor in human platelets using [3H]-RX-821002 as radioligand | J Med Chem 38: 4056-69 (1995) BindingDB Entry DOI: 10.7270/Q2DV1HXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50031324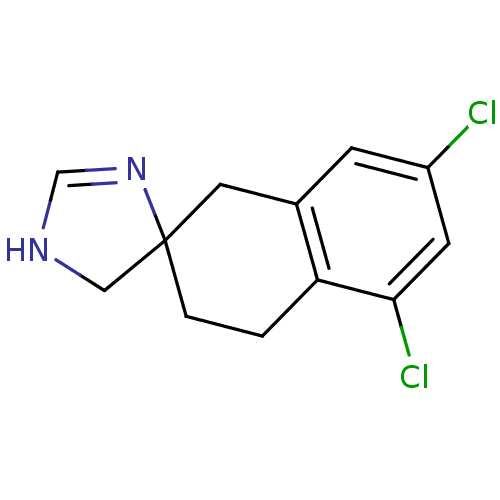 (5',7'-dichlorospiro[4,5-dihydro-1H-imidazole-4,2'-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier Curated by ChEMBL | Assay Description Binding affinity towards Alpha-2A adrenergic receptor in human platelets using [3H]-RX-821002 as radioligand | J Med Chem 38: 4056-69 (1995) BindingDB Entry DOI: 10.7270/Q2DV1HXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50031311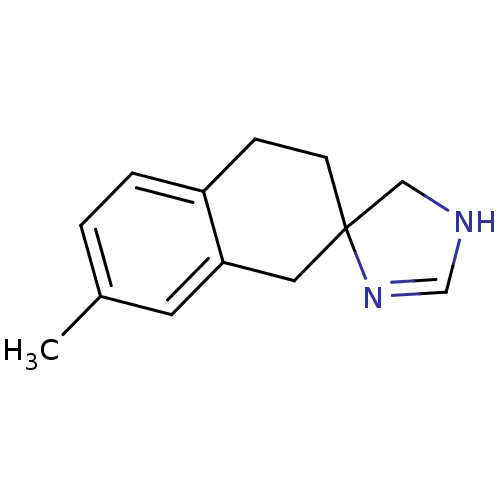 (7'-methylspiro[4,5-dihydro-1H-imidazole-4,2'-(1',2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier Curated by ChEMBL | Assay Description Binding affinity towards Alpha-2A adrenergic receptor in human platelets using [3H]-RX-821002 as radioligand | J Med Chem 38: 4056-69 (1995) BindingDB Entry DOI: 10.7270/Q2DV1HXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50031316 (5'-methoxyspiro[4,5-dihydro-1H-imidazole-4,2'-(1',...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier Curated by ChEMBL | Assay Description Binding affinity towards Alpha-2A adrenergic receptor in human platelets using [3H]-RX-821002 as radioligand | J Med Chem 38: 4056-69 (1995) BindingDB Entry DOI: 10.7270/Q2DV1HXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50031301 (8'-fluorospiro[4,5-dihydro-1H-imidazole-4,2'-(1',2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier Curated by ChEMBL | Assay Description Binding affinity towards Alpha-2A adrenergic receptor in human platelets using [3H]-RX-821002 as radioligand | J Med Chem 38: 4056-69 (1995) BindingDB Entry DOI: 10.7270/Q2DV1HXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (NEONATAL RAT) | BDBM50031306 (8-chlorospiro[1,2,3,4-tetrahydronaphthalene-2,4'-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier Curated by ChEMBL | Assay Description Binding affinity towards Alpha-2B adrenergic receptor in neonatal rat lung using [3H]-RX-821002 as radioligand | J Med Chem 38: 4056-69 (1995) BindingDB Entry DOI: 10.7270/Q2DV1HXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50031319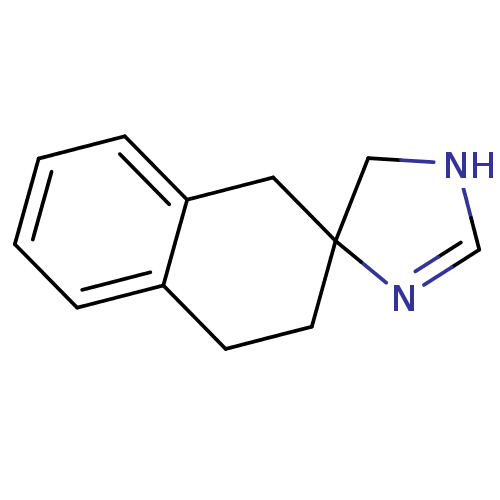 (CHEMBL130853 | spiro[4,5-dihydro-1H-imidazole-4,2'...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier Curated by ChEMBL | Assay Description Binding affinity towards Alpha-2A adrenergic receptor in human platelets using [3H]-RX-821002 as radioligand | J Med Chem 38: 4056-69 (1995) BindingDB Entry DOI: 10.7270/Q2DV1HXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (NEONATAL RAT) | BDBM50031311 (7'-methylspiro[4,5-dihydro-1H-imidazole-4,2'-(1',2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier Curated by ChEMBL | Assay Description Binding affinity towards Alpha-2B adrenergic receptor in neonatal rat lung using [3H]-RX-821002 as radioligand | J Med Chem 38: 4056-69 (1995) BindingDB Entry DOI: 10.7270/Q2DV1HXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50031308 (7'-methoxyspiro[4,5-dihydro-1H-imidazole-4,2'-(1',...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier Curated by ChEMBL | Assay Description Binding affinity towards Alpha-2A adrenergic receptor in human platelets using [3H]-RX-821002 as radioligand | J Med Chem 38: 4056-69 (1995) BindingDB Entry DOI: 10.7270/Q2DV1HXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50031330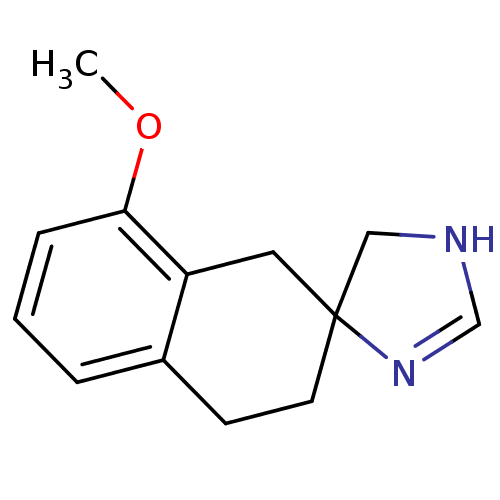 (8'-methoxyspiro[4,5-dihydro-1H-imidazole-4,2'-(1',...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier Curated by ChEMBL | Assay Description Binding affinity towards Alpha-2A adrenergic receptor in human platelets using [3H]-RX-821002 as radioligand | J Med Chem 38: 4056-69 (1995) BindingDB Entry DOI: 10.7270/Q2DV1HXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50031326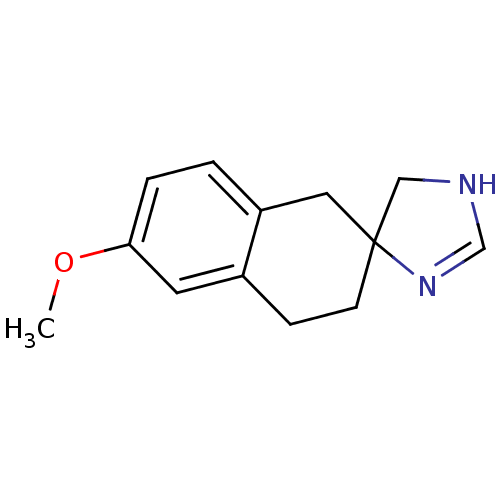 (6'-methoxyspiro[4,5-dihydro-1H-imidazole-4,2'-(1',...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier Curated by ChEMBL | Assay Description Binding affinity towards Alpha-2A adrenergic receptor in human platelets using [3H]-RX-821002 as radioligand | J Med Chem 38: 4056-69 (1995) BindingDB Entry DOI: 10.7270/Q2DV1HXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (NEONATAL RAT) | BDBM50031324 (5',7'-dichlorospiro[4,5-dihydro-1H-imidazole-4,2'-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier Curated by ChEMBL | Assay Description Binding affinity towards Alpha-2B adrenergic receptor in neonatal rat lung using [3H]-RX-821002 as radioligand | J Med Chem 38: 4056-69 (1995) BindingDB Entry DOI: 10.7270/Q2DV1HXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (NEONATAL RAT) | BDBM50031330 (8'-methoxyspiro[4,5-dihydro-1H-imidazole-4,2'-(1',...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier Curated by ChEMBL | Assay Description Binding affinity towards Alpha-2B adrenergic receptor in neonatal rat lung using [3H]-RX-821002 as radioligand | J Med Chem 38: 4056-69 (1995) BindingDB Entry DOI: 10.7270/Q2DV1HXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (NEONATAL RAT) | BDBM50031308 (7'-methoxyspiro[4,5-dihydro-1H-imidazole-4,2'-(1',...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier Curated by ChEMBL | Assay Description Binding affinity towards Alpha-2B adrenergic receptor in neonatal rat lung using [3H]-RX-821002 as radioligand | J Med Chem 38: 4056-69 (1995) BindingDB Entry DOI: 10.7270/Q2DV1HXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (NEONATAL RAT) | BDBM50031301 (8'-fluorospiro[4,5-dihydro-1H-imidazole-4,2'-(1',2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 152 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier Curated by ChEMBL | Assay Description Binding affinity towards Alpha-2B adrenergic receptor in neonatal rat lung using [3H]-RX-821002 as radioligand | J Med Chem 38: 4056-69 (1995) BindingDB Entry DOI: 10.7270/Q2DV1HXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (NEONATAL RAT) | BDBM50031316 (5'-methoxyspiro[4,5-dihydro-1H-imidazole-4,2'-(1',...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 159 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier Curated by ChEMBL | Assay Description Binding affinity towards Alpha-2B adrenergic receptor in neonatal rat lung using [3H]-RX-821002 as radioligand | J Med Chem 38: 4056-69 (1995) BindingDB Entry DOI: 10.7270/Q2DV1HXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (NEONATAL RAT) | BDBM50027067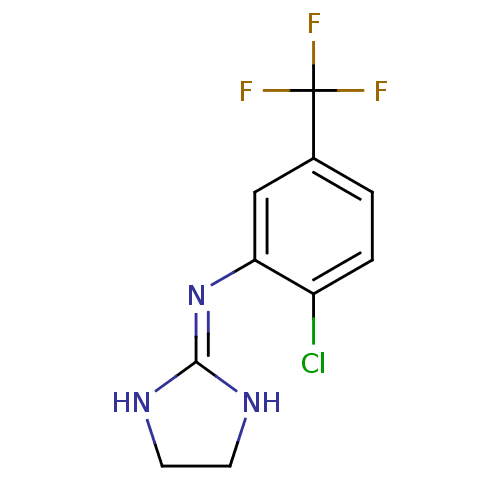 ((2-Chloro-5-trifluoromethyl-phenyl)-(4,5-dihydro-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 179 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier Curated by ChEMBL | Assay Description Binding affinity towards Alpha-2B adrenergic receptor in neonatal rat lung using [3H]-RX-821002 as radioligand | J Med Chem 38: 4056-69 (1995) BindingDB Entry DOI: 10.7270/Q2DV1HXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (NEONATAL RAT) | BDBM50031319 (CHEMBL130853 | spiro[4,5-dihydro-1H-imidazole-4,2'...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 275 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier Curated by ChEMBL | Assay Description Binding affinity towards Alpha-2B adrenergic receptor in neonatal rat lung using [3H]-RX-821002 as radioligand | J Med Chem 38: 4056-69 (1995) BindingDB Entry DOI: 10.7270/Q2DV1HXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (NEONATAL RAT) | BDBM50031326 (6'-methoxyspiro[4,5-dihydro-1H-imidazole-4,2'-(1',...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier Curated by ChEMBL | Assay Description Binding affinity towards Alpha-2B adrenergic receptor in neonatal rat lung using [3H]-RX-821002 as radioligand | J Med Chem 38: 4056-69 (1995) BindingDB Entry DOI: 10.7270/Q2DV1HXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50027067 ((2-Chloro-5-trifluoromethyl-phenyl)-(4,5-dihydro-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier Curated by ChEMBL | Assay Description Binding affinity towards Alpha-2A adrenergic receptor in human platelets using [3H]-RX-821002 as radioligand | J Med Chem 38: 4056-69 (1995) BindingDB Entry DOI: 10.7270/Q2DV1HXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50367254 (ENALAPRILAT) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles Curated by ChEMBL | Assay Description In vitro activity against angiotensin I converting enzyme especially against Hip-His-Leu residues | J Med Chem 34: 663-9 (1991) BindingDB Entry DOI: 10.7270/Q21V5FKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50011365 (1-[2-(1-Carboxy-butylamino)-propionyl]-octahydro-i...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles Curated by ChEMBL | Assay Description In vitro activity against angiotensin I converting enzyme especially against Hip-His-Leu residues | J Med Chem 34: 663-9 (1991) BindingDB Entry DOI: 10.7270/Q21V5FKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50011362 (1-(3-Mercapto-2-methyl-propionyl)-pyrrolidine-2-ca...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles Curated by ChEMBL | Assay Description In vitro activity against angiotensin I converting enzyme especially against Hip-His-Leu residues | J Med Chem 34: 663-9 (1991) BindingDB Entry DOI: 10.7270/Q21V5FKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50011361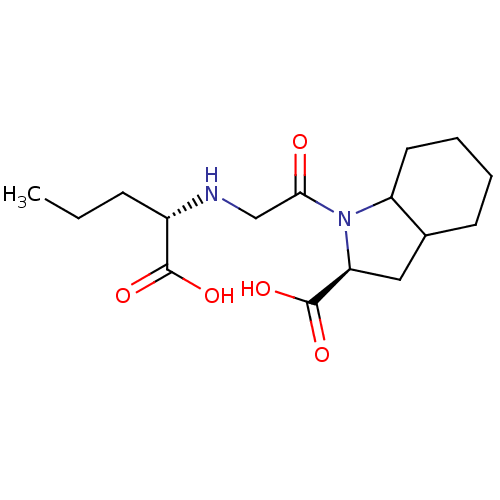 (1-[2-(1-Carboxy-butylamino)-acetyl]-octahydro-indo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles Curated by ChEMBL | Assay Description In vitro activity against angiotensin I converting enzyme especially against Hip-His-Leu residues | J Med Chem 34: 663-9 (1991) BindingDB Entry DOI: 10.7270/Q21V5FKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50011367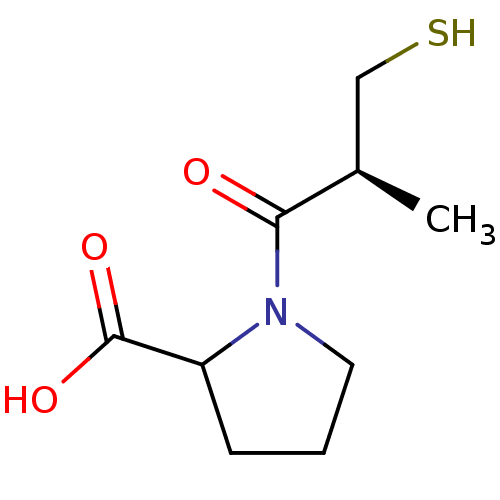 (1-(3-Mercapto-2-methyl-propionyl)-pyrrolidine-2-ca...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles Curated by ChEMBL | Assay Description In vitro activity against angiotensin I converting enzyme especially against Hip-His-Leu residues | J Med Chem 34: 663-9 (1991) BindingDB Entry DOI: 10.7270/Q21V5FKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50011366 ((S)1-(3-Mercapto-propionyl)-pyrrolidine-2-carboxyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles Curated by ChEMBL | Assay Description In vitro activity against angiotensin I converting enzyme especially against Hip-His-Leu residues | J Med Chem 34: 663-9 (1991) BindingDB Entry DOI: 10.7270/Q21V5FKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50011364 (1-[2-(1-Carboxy-3-phenyl-propylamino)-acetyl]-pyrr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles Curated by ChEMBL | Assay Description In vitro activity against angiotensin I converting enzyme especially against Hip-His-Leu residues | J Med Chem 34: 663-9 (1991) BindingDB Entry DOI: 10.7270/Q21V5FKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50011363 (1-[2-(1-Carboxy-butylamino)-propionyl]-octahydro-i...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles Curated by ChEMBL | Assay Description In vitro activity against angiotensin I converting enzyme especially against Hip-His-Leu residues | J Med Chem 34: 663-9 (1991) BindingDB Entry DOI: 10.7270/Q21V5FKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50011359 (1-[2-(1-Carboxy-3-phenyl-propylamino)-propionyl]-p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles Curated by ChEMBL | Assay Description In vitro activity against angiotensin I converting enzyme especially against Hip-His-Leu residues | J Med Chem 34: 663-9 (1991) BindingDB Entry DOI: 10.7270/Q21V5FKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||