

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50109179 (4-[3-(3-{3-[4-(3-Hydroxy-phenyl)-piperidin-1-yl]-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-PYY radioligand to human neuropeptide Y1 receptor in SK-N-MC cell membrane | Bioorg Med Chem Lett 12: 379-82 (2002) BindingDB Entry DOI: 10.7270/Q2DJ5DZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50109122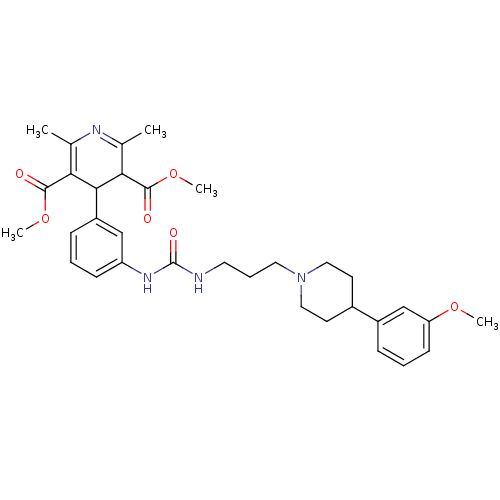 (4-[3-(3-{3-[4-(3-Methoxy-phenyl)-piperidin-1-yl]-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-PYY radioligand to human neuropeptide Y1 receptor in SK-N-MC cell membrane | Bioorg Med Chem Lett 12: 379-82 (2002) BindingDB Entry DOI: 10.7270/Q2DJ5DZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50109188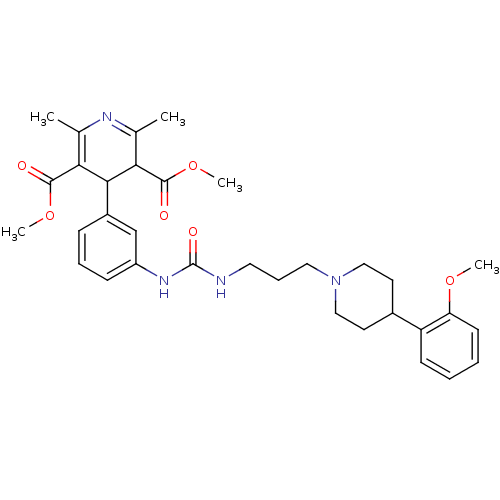 (4-[3-(3-{3-[4-(2-Methoxy-phenyl)-piperidin-1-yl]-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-PYY radioligand to human neuropeptide Y1 receptor in SK-N-MC cell membrane | Bioorg Med Chem Lett 12: 379-82 (2002) BindingDB Entry DOI: 10.7270/Q2DJ5DZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50109182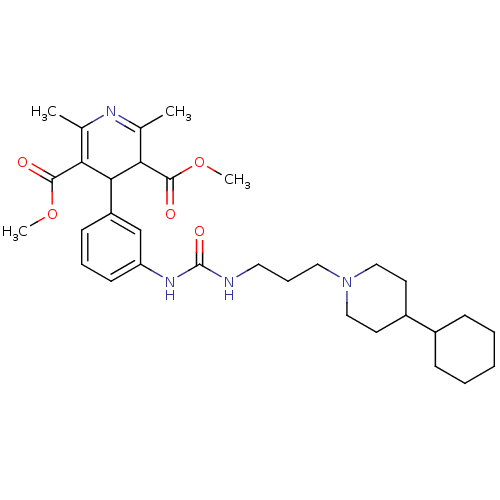 (4-(3-{3-[3-(4-Cyclohexyl-piperidin-1-yl)-propyl]-u...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-PYY radioligand to human neuropeptide Y1 receptor in SK-N-MC cell membrane | Bioorg Med Chem Lett 12: 379-82 (2002) BindingDB Entry DOI: 10.7270/Q2DJ5DZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50109186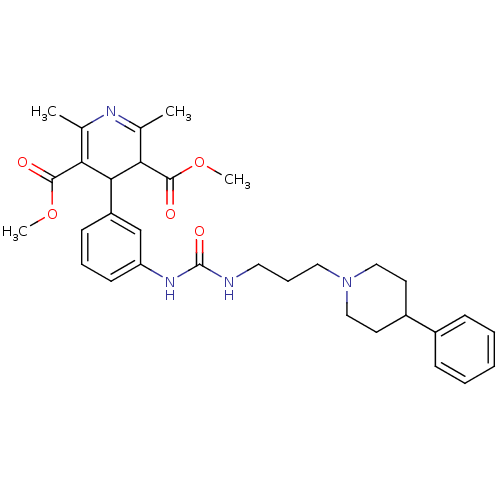 (2,6-Dimethyl-4-(3-{3-[3-(4-phenyl-piperidin-1-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-PYY radioligand to human neuropeptide Y1 receptor in SK-N-MC cell membrane | Bioorg Med Chem Lett 12: 379-82 (2002) BindingDB Entry DOI: 10.7270/Q2DJ5DZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50109185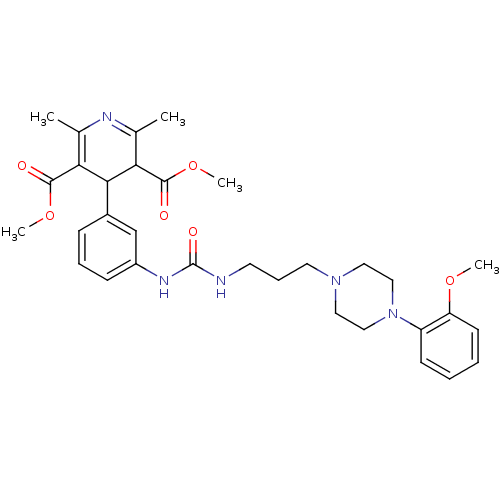 (4-[3-(3-{3-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-PYY radioligand to human neuropeptide Y1 receptor in SK-N-MC cell membrane | Bioorg Med Chem Lett 12: 379-82 (2002) BindingDB Entry DOI: 10.7270/Q2DJ5DZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50060728 ((R)-2-(2,2-diphenylacetamido)-5-guanidino-N-(4-hyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-PYY radioligand to human neuropeptide Y1 receptor in SK-N-MC cell membrane | Bioorg Med Chem Lett 12: 379-82 (2002) BindingDB Entry DOI: 10.7270/Q2DJ5DZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50109194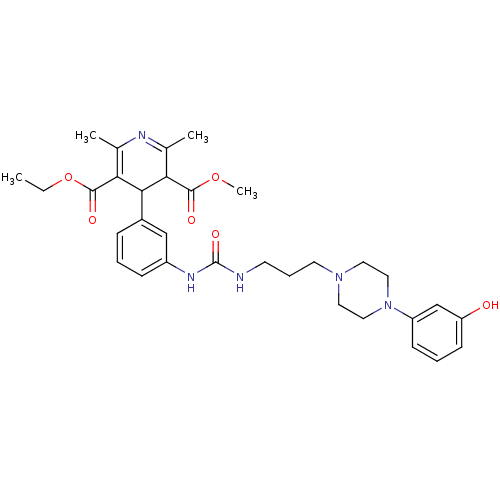 (4-[3-(3-{3-[4-(3-Hydroxy-phenyl)-piperazin-1-yl]-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-PYY radioligand to human neuropeptide Y1 receptor in SK-N-MC cell membrane | Bioorg Med Chem Lett 12: 379-82 (2002) BindingDB Entry DOI: 10.7270/Q2DJ5DZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50109173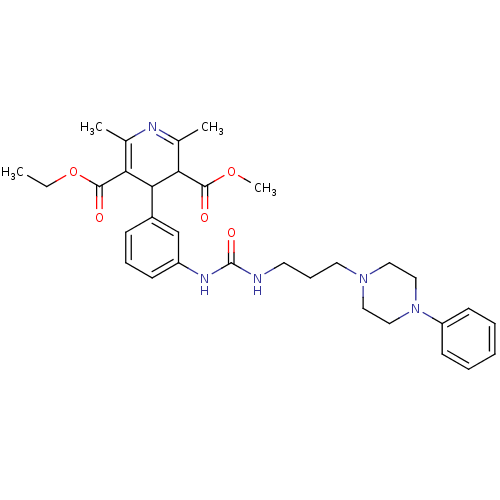 (2,6-Dimethyl-4-(3-{3-[3-(4-phenyl-piperazin-1-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-PYY radioligand to human neuropeptide Y1 receptor in SK-N-MC cell membrane | Bioorg Med Chem Lett 12: 379-82 (2002) BindingDB Entry DOI: 10.7270/Q2DJ5DZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50109193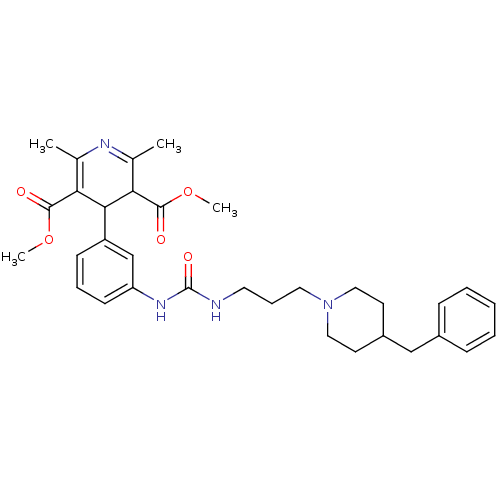 (4-(3-{3-[3-(4-Benzyl-piperidin-1-yl)-propyl]-ureid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-PYY radioligand to human neuropeptide Y1 receptor in SK-N-MC cell membrane | Bioorg Med Chem Lett 12: 379-82 (2002) BindingDB Entry DOI: 10.7270/Q2DJ5DZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50109178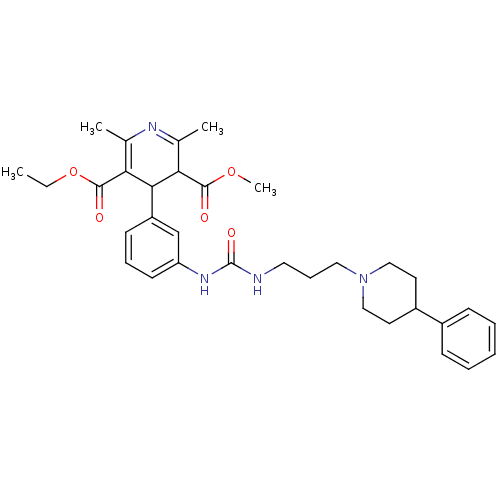 (2,6-Dimethyl-4-(3-{3-[3-(4-phenyl-piperidin-1-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-PYY radioligand to human neuropeptide Y1 receptor in SK-N-MC cell membrane | Bioorg Med Chem Lett 12: 379-82 (2002) BindingDB Entry DOI: 10.7270/Q2DJ5DZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50109183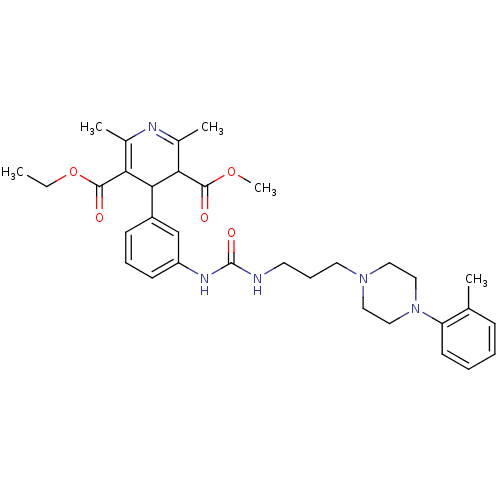 (2,6-Dimethyl-4-(3-{3-[3-(4-o-tolyl-piperazin-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-PYY radioligand to human neuropeptide Y1 receptor in SK-N-MC cell membrane | Bioorg Med Chem Lett 12: 379-82 (2002) BindingDB Entry DOI: 10.7270/Q2DJ5DZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50109192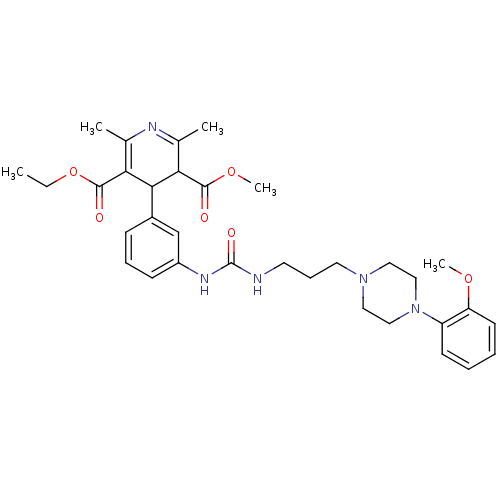 (4-[3-(3-{3-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-PYY radioligand to human neuropeptide Y1 receptor in SK-N-MC cell membrane | Bioorg Med Chem Lett 12: 379-82 (2002) BindingDB Entry DOI: 10.7270/Q2DJ5DZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50109180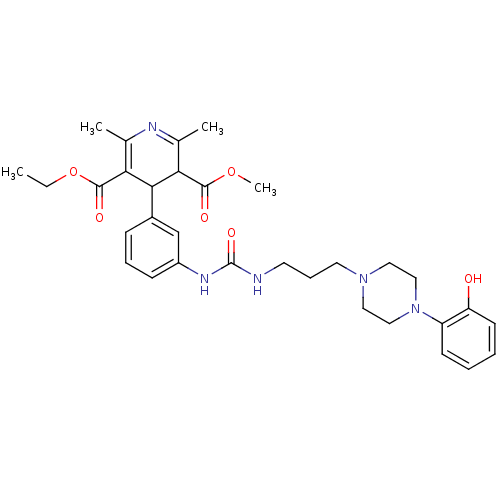 (4-[3-(3-{3-[4-(2-Hydroxy-phenyl)-piperazin-1-yl]-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 108 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-PYY radioligand to human neuropeptide Y1 receptor in SK-N-MC cell membrane | Bioorg Med Chem Lett 12: 379-82 (2002) BindingDB Entry DOI: 10.7270/Q2DJ5DZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50109181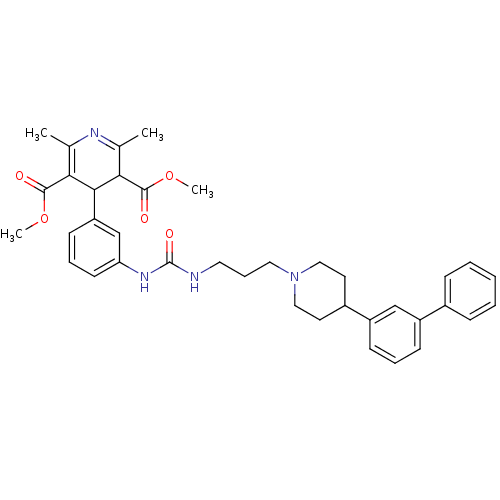 (4-(3-{3-[3-(4-Biphenyl-3-yl-piperidin-1-yl)-propyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 188 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-PYY radioligand to human neuropeptide Y1 receptor in SK-N-MC cell membrane | Bioorg Med Chem Lett 12: 379-82 (2002) BindingDB Entry DOI: 10.7270/Q2DJ5DZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50109190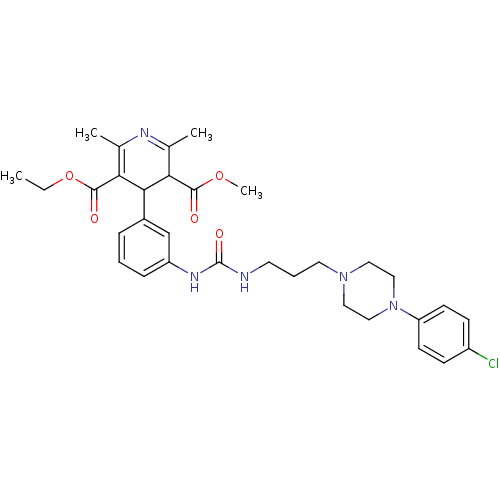 (4-[3-(3-{3-[4-(4-Chloro-phenyl)-piperazin-1-yl]-pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 322 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-PYY radioligand to human neuropeptide Y1 receptor in SK-N-MC cell membrane | Bioorg Med Chem Lett 12: 379-82 (2002) BindingDB Entry DOI: 10.7270/Q2DJ5DZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50109177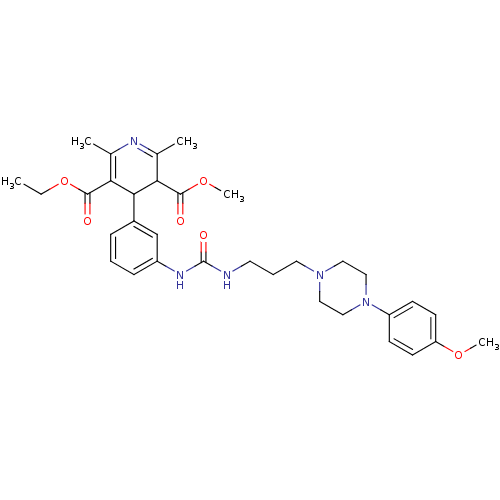 (4-[3-(3-{3-[4-(4-Methoxy-phenyl)-piperazin-1-yl]-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 405 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-PYY radioligand to human neuropeptide Y1 receptor in SK-N-MC cell membrane | Bioorg Med Chem Lett 12: 379-82 (2002) BindingDB Entry DOI: 10.7270/Q2DJ5DZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50109184 (2,6-Dimethyl-4-(3-{3-[3-(4-p-tolyl-piperazin-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 503 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-PYY radioligand to human neuropeptide Y1 receptor in SK-N-MC cell membrane | Bioorg Med Chem Lett 12: 379-82 (2002) BindingDB Entry DOI: 10.7270/Q2DJ5DZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50109176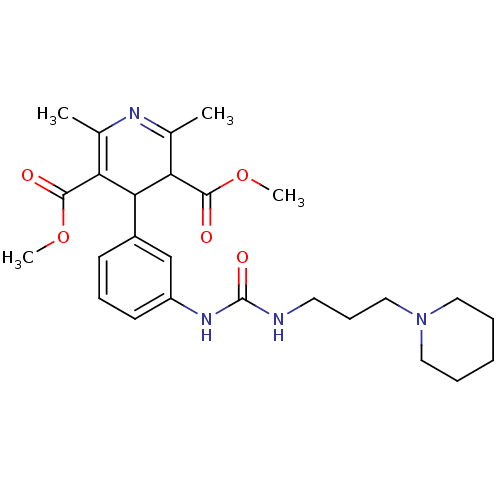 (2,6-Dimethyl-4-{3-[3-(3-piperidin-1-yl-propyl)-ure...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 523 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-PYY radioligand to human neuropeptide Y1 receptor in SK-N-MC cell membrane | Bioorg Med Chem Lett 12: 379-82 (2002) BindingDB Entry DOI: 10.7270/Q2DJ5DZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50109189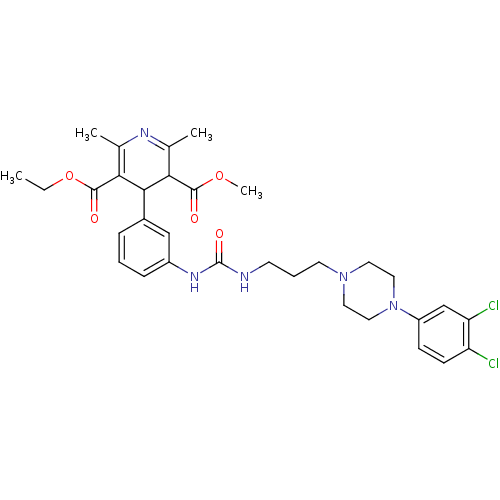 (4-[3-(3-{3-[4-(3,4-Dichloro-phenyl)-piperazin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 677 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-PYY radioligand to human neuropeptide Y1 receptor in SK-N-MC cell membrane | Bioorg Med Chem Lett 12: 379-82 (2002) BindingDB Entry DOI: 10.7270/Q2DJ5DZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50109191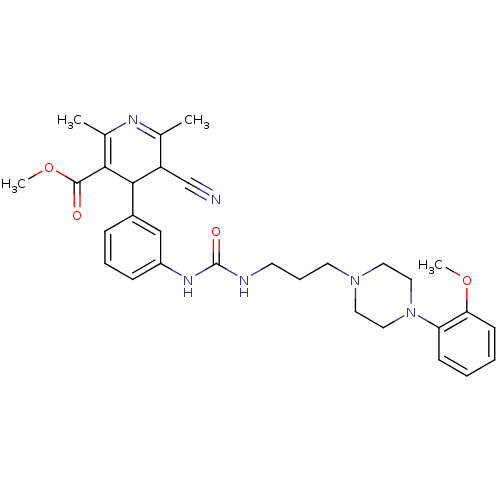 (5-Cyano-4-[3-(3-{3-[4-(2-methoxy-phenyl)-piperazin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 684 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-PYY radioligand to human neuropeptide Y1 receptor in SK-N-MC cell membrane | Bioorg Med Chem Lett 12: 379-82 (2002) BindingDB Entry DOI: 10.7270/Q2DJ5DZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50109175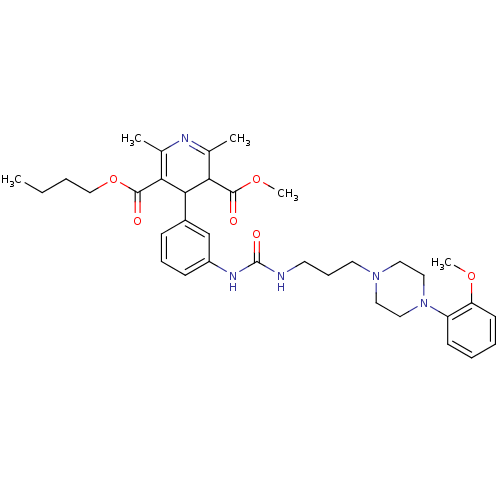 (4-[3-(3-{3-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 995 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-PYY radioligand to human neuropeptide Y1 receptor in SK-N-MC cell membrane | Bioorg Med Chem Lett 12: 379-82 (2002) BindingDB Entry DOI: 10.7270/Q2DJ5DZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50109174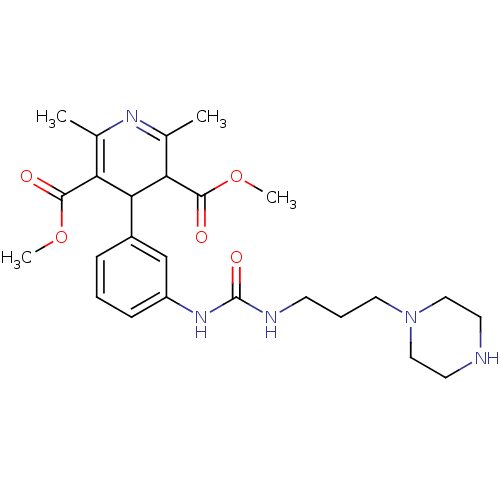 (2,6-Dimethyl-4-{3-[3-(3-piperazin-1-yl-propyl)-ure...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-PYY radioligand to human neuropeptide Y1 receptor in SK-N-MC cell membrane | Bioorg Med Chem Lett 12: 379-82 (2002) BindingDB Entry DOI: 10.7270/Q2DJ5DZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50109187 (4-[3-(3-{3-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-PYY radioligand to human neuropeptide Y1 receptor in SK-N-MC cell membrane | Bioorg Med Chem Lett 12: 379-82 (2002) BindingDB Entry DOI: 10.7270/Q2DJ5DZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50240412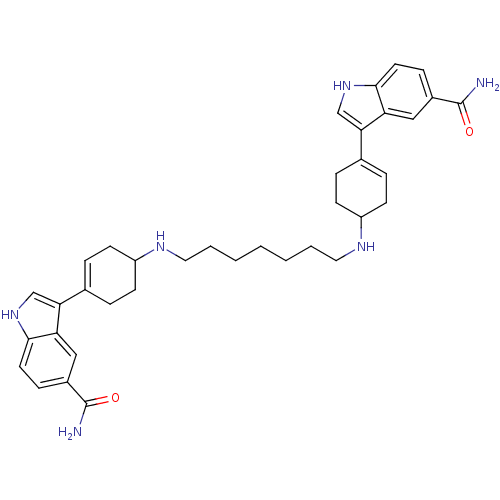 (3-(4-{7-[4-(5-carbamoyl-1H-3-indolyl)-3-cyclohexen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1D receptor subtype | Bioorg Med Chem Lett 5: 123-126 (1995) Article DOI: 10.1016/0960-894X(94)00470-Z BindingDB Entry DOI: 10.7270/Q2KP824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50285314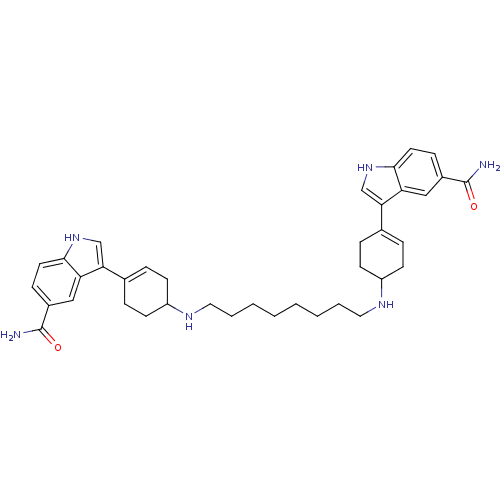 (3-(4-{8-[4-(5-carbamoyl-1H-3-indolyl)-3-cyclohexen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1D receptor subtype | Bioorg Med Chem Lett 5: 123-126 (1995) Article DOI: 10.1016/0960-894X(94)00470-Z BindingDB Entry DOI: 10.7270/Q2KP824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50285320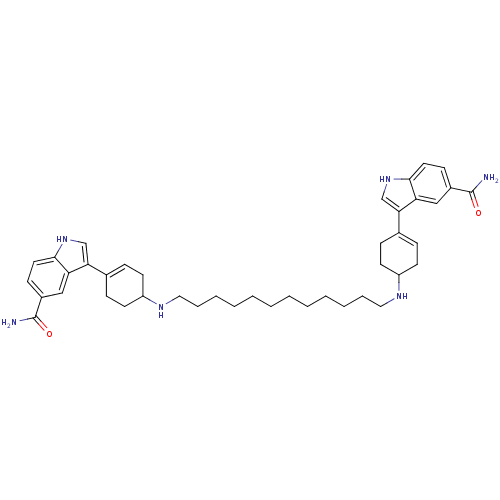 (3-(4-{12-[4-(5-carbamoyl-1H-3-indolyl)-3-cyclohexe...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1A receptor receptor subtype | Bioorg Med Chem Lett 5: 123-126 (1995) Article DOI: 10.1016/0960-894X(94)00470-Z BindingDB Entry DOI: 10.7270/Q2KP824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50285313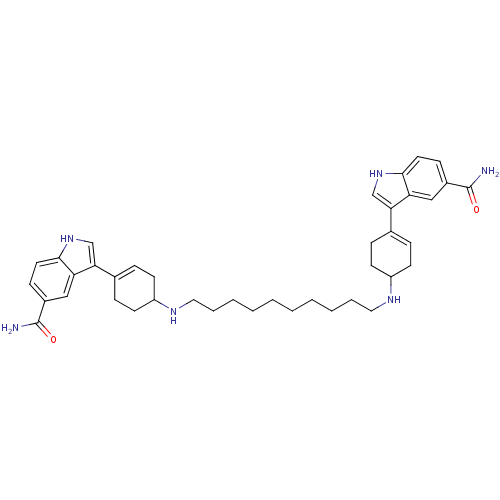 (3-(4-{10-[4-(5-carbamoyl-1H-3-indolyl)-3-cyclohexe...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1A receptor receptor subtype | Bioorg Med Chem Lett 5: 123-126 (1995) Article DOI: 10.1016/0960-894X(94)00470-Z BindingDB Entry DOI: 10.7270/Q2KP824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50285320 (3-(4-{12-[4-(5-carbamoyl-1H-3-indolyl)-3-cyclohexe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1D receptor subtype | Bioorg Med Chem Lett 5: 123-126 (1995) Article DOI: 10.1016/0960-894X(94)00470-Z BindingDB Entry DOI: 10.7270/Q2KP824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50285316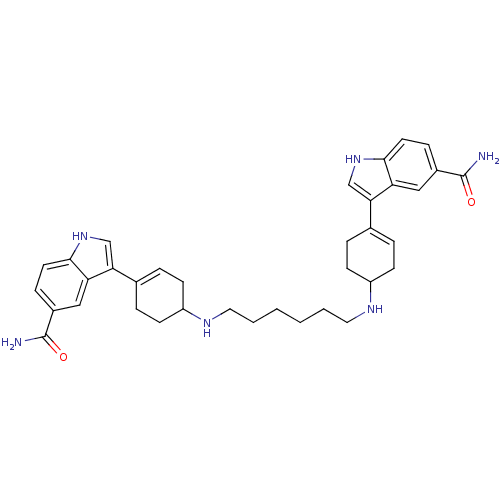 (3-(4-{6-[4-(5-carbamoyl-1H-3-indolyl)-3-cyclohexen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1D receptor subtype | Bioorg Med Chem Lett 5: 123-126 (1995) Article DOI: 10.1016/0960-894X(94)00470-Z BindingDB Entry DOI: 10.7270/Q2KP824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50285319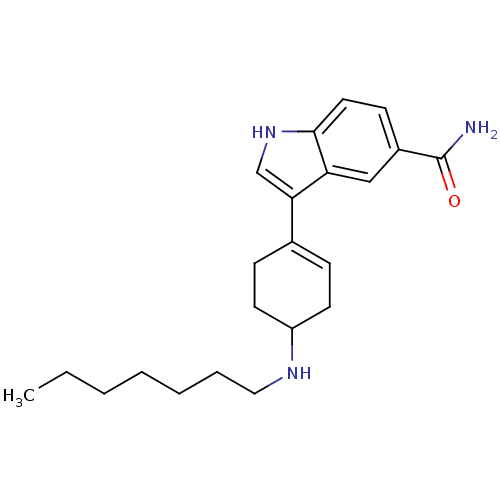 (3-(4-Heptylamino-cyclohex-1-enyl)-1H-indole-5-carb...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1A receptor receptor subtype | Bioorg Med Chem Lett 5: 123-126 (1995) Article DOI: 10.1016/0960-894X(94)00470-Z BindingDB Entry DOI: 10.7270/Q2KP824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50240412 (3-(4-{7-[4-(5-carbamoyl-1H-3-indolyl)-3-cyclohexen...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.83 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1A receptor receptor subtype | Bioorg Med Chem Lett 5: 123-126 (1995) Article DOI: 10.1016/0960-894X(94)00470-Z BindingDB Entry DOI: 10.7270/Q2KP824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50285319 (3-(4-Heptylamino-cyclohex-1-enyl)-1H-indole-5-carb...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.98 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1D receptor subtype | Bioorg Med Chem Lett 5: 123-126 (1995) Article DOI: 10.1016/0960-894X(94)00470-Z BindingDB Entry DOI: 10.7270/Q2KP824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50285315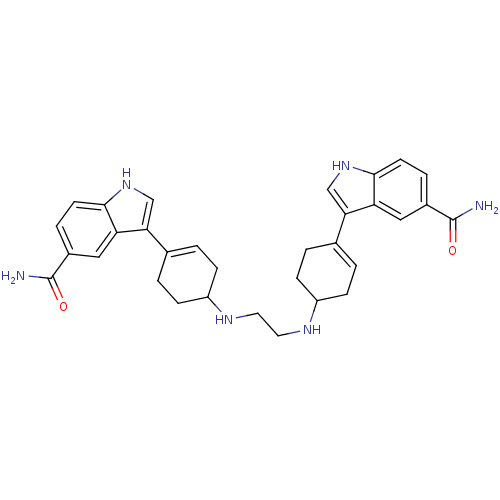 (3-(4-{2-[4-(5-carbamoyl-1H-3-indolyl)-3-cyclohexen...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1A receptor receptor subtype | Bioorg Med Chem Lett 5: 123-126 (1995) Article DOI: 10.1016/0960-894X(94)00470-Z BindingDB Entry DOI: 10.7270/Q2KP824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50285314 (3-(4-{8-[4-(5-carbamoyl-1H-3-indolyl)-3-cyclohexen...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1A receptor receptor subtype | Bioorg Med Chem Lett 5: 123-126 (1995) Article DOI: 10.1016/0960-894X(94)00470-Z BindingDB Entry DOI: 10.7270/Q2KP824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50285316 (3-(4-{6-[4-(5-carbamoyl-1H-3-indolyl)-3-cyclohexen...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1A receptor receptor subtype | Bioorg Med Chem Lett 5: 123-126 (1995) Article DOI: 10.1016/0960-894X(94)00470-Z BindingDB Entry DOI: 10.7270/Q2KP824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50285313 (3-(4-{10-[4-(5-carbamoyl-1H-3-indolyl)-3-cyclohexe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.63 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1D receptor subtype | Bioorg Med Chem Lett 5: 123-126 (1995) Article DOI: 10.1016/0960-894X(94)00470-Z BindingDB Entry DOI: 10.7270/Q2KP824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50285318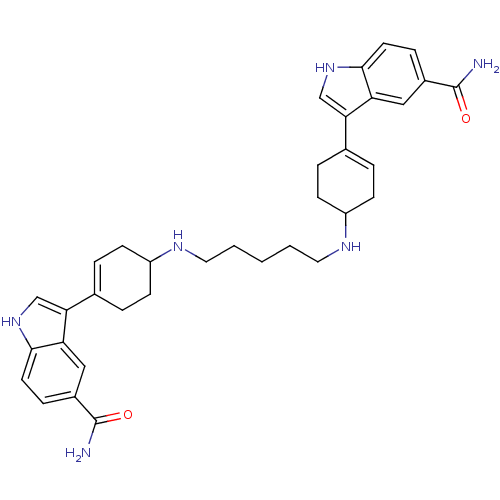 (3-(4-{5-[4-(5-carbamoyl-1H-3-indolyl)-3-cyclohexen...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.86 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1A receptor receptor subtype | Bioorg Med Chem Lett 5: 123-126 (1995) Article DOI: 10.1016/0960-894X(94)00470-Z BindingDB Entry DOI: 10.7270/Q2KP824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50285318 (3-(4-{5-[4-(5-carbamoyl-1H-3-indolyl)-3-cyclohexen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1D receptor subtype | Bioorg Med Chem Lett 5: 123-126 (1995) Article DOI: 10.1016/0960-894X(94)00470-Z BindingDB Entry DOI: 10.7270/Q2KP824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50285315 (3-(4-{2-[4-(5-carbamoyl-1H-3-indolyl)-3-cyclohexen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1D receptor subtype | Bioorg Med Chem Lett 5: 123-126 (1995) Article DOI: 10.1016/0960-894X(94)00470-Z BindingDB Entry DOI: 10.7270/Q2KP824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50285314 (3-(4-{8-[4-(5-carbamoyl-1H-3-indolyl)-3-cyclohexen...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 12.4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit Serotonin transporter | Bioorg Med Chem Lett 5: 123-126 (1995) Article DOI: 10.1016/0960-894X(94)00470-Z BindingDB Entry DOI: 10.7270/Q2KP824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50285315 (3-(4-{2-[4-(5-carbamoyl-1H-3-indolyl)-3-cyclohexen...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit Serotonin transporter | Bioorg Med Chem Lett 5: 123-126 (1995) Article DOI: 10.1016/0960-894X(94)00470-Z BindingDB Entry DOI: 10.7270/Q2KP824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50285312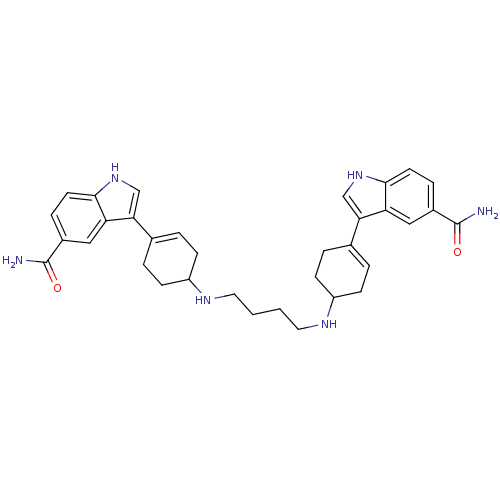 (3-(4-{4-[4-(5-carbamoyl-1H-3-indolyl)-3-cyclohexen...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 14.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1A receptor receptor subtype | Bioorg Med Chem Lett 5: 123-126 (1995) Article DOI: 10.1016/0960-894X(94)00470-Z BindingDB Entry DOI: 10.7270/Q2KP824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50240412 (3-(4-{7-[4-(5-carbamoyl-1H-3-indolyl)-3-cyclohexen...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 17.7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit Serotonin transporter | Bioorg Med Chem Lett 5: 123-126 (1995) Article DOI: 10.1016/0960-894X(94)00470-Z BindingDB Entry DOI: 10.7270/Q2KP824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50285317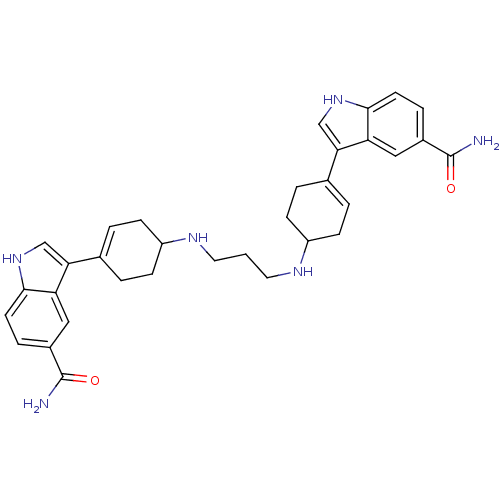 (3-(4-{3-[4-(5-carbamoyl-1H-3-indolyl)-3-cyclohexen...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1A receptor receptor subtype | Bioorg Med Chem Lett 5: 123-126 (1995) Article DOI: 10.1016/0960-894X(94)00470-Z BindingDB Entry DOI: 10.7270/Q2KP824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50285312 (3-(4-{4-[4-(5-carbamoyl-1H-3-indolyl)-3-cyclohexen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 20.3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1D receptor subtype | Bioorg Med Chem Lett 5: 123-126 (1995) Article DOI: 10.1016/0960-894X(94)00470-Z BindingDB Entry DOI: 10.7270/Q2KP824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50285317 (3-(4-{3-[4-(5-carbamoyl-1H-3-indolyl)-3-cyclohexen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 27.3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1D receptor subtype | Bioorg Med Chem Lett 5: 123-126 (1995) Article DOI: 10.1016/0960-894X(94)00470-Z BindingDB Entry DOI: 10.7270/Q2KP824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50285316 (3-(4-{6-[4-(5-carbamoyl-1H-3-indolyl)-3-cyclohexen...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 33.9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit Serotonin transporter | Bioorg Med Chem Lett 5: 123-126 (1995) Article DOI: 10.1016/0960-894X(94)00470-Z BindingDB Entry DOI: 10.7270/Q2KP824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50285318 (3-(4-{5-[4-(5-carbamoyl-1H-3-indolyl)-3-cyclohexen...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 47.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit Serotonin transporter | Bioorg Med Chem Lett 5: 123-126 (1995) Article DOI: 10.1016/0960-894X(94)00470-Z BindingDB Entry DOI: 10.7270/Q2KP824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50285312 (3-(4-{4-[4-(5-carbamoyl-1H-3-indolyl)-3-cyclohexen...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 47.8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit Serotonin transporter | Bioorg Med Chem Lett 5: 123-126 (1995) Article DOI: 10.1016/0960-894X(94)00470-Z BindingDB Entry DOI: 10.7270/Q2KP824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 58 total ) | Next | Last >> |