

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50005681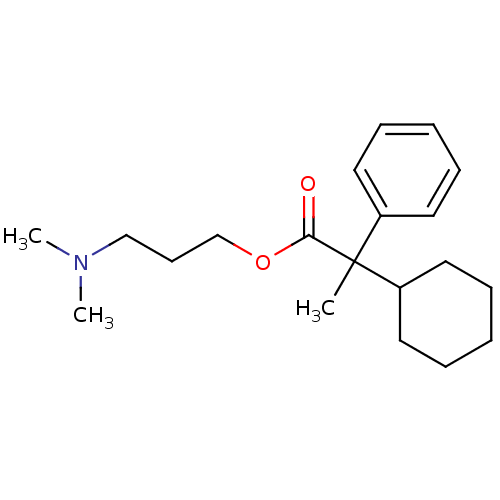 (2-Cyclohexyl-2-phenyl-propionic acid 3-dimethylami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Antimuscurinic potency and subset specificity of the compound was characterised by its inhibition of the [3H]-NMS Binding to Muscarinic acetylcholine... | J Med Chem 35: 1290-5 (1992) BindingDB Entry DOI: 10.7270/Q28914T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50403547 (ATROPEN | ATROPINE) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of carbachol-induced release of alpha-amylase from pancreatic acinar cells from that of rat ileum contained the M2 receptor subtypes | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50005685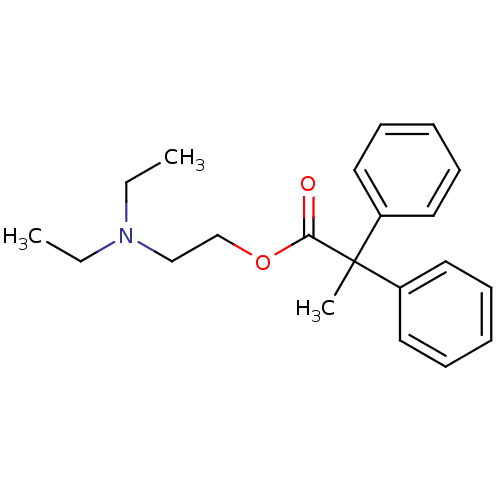 (2,2-Diphenyl-propionic acid 2-diethylamino-ethyl e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of carbachol-induced release of alpha-amylase from pancreatic acinar cells from that of rat ileum contained the M2 receptor subtypes | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50005681 (2-Cyclohexyl-2-phenyl-propionic acid 3-dimethylami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Antimuscurinic potency and subset specificity of the compound was characterised by its inhibition of the [3H]-NMS Binding to Muscarinic acetylcholine... | J Med Chem 35: 1290-5 (1992) BindingDB Entry DOI: 10.7270/Q28914T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50005681 (2-Cyclohexyl-2-phenyl-propionic acid 3-dimethylami...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Antimuscurinic potency and subset specificity of the compound was characterised by its inhibition of the [3H]-NMS Binding to Muscarinic acetylcholine... | J Med Chem 35: 1290-5 (1992) BindingDB Entry DOI: 10.7270/Q28914T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50018230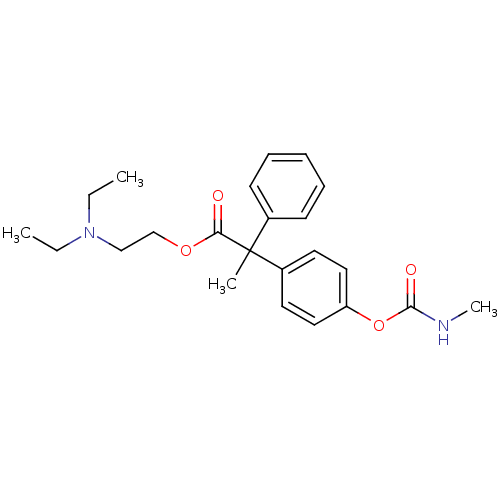 (2-(4-Methylcarbamoyloxy-phenyl)-2-phenyl-propionic...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Binding affinity was determined from the inhibition of contraction of guinea pig ileum which has Muscarinic acetylcholine receptor M2 subtype. | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50005685 (2,2-Diphenyl-propionic acid 2-diethylamino-ethyl e...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Antimuscurinic potency and subset specificity of the compound was characterised by inhibition of the [3H]-NMS Binding to Muscarinic acetylcholine rec... | J Med Chem 35: 1290-5 (1992) BindingDB Entry DOI: 10.7270/Q28914T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM50403547 (ATROPEN | ATROPINE) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to cerebral cortex membranes which contain predominantly the Muscarinic acetylcholine receptor M1 subtypes | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50005684 (2-Cyclohexyl-2-phenyl-propionic acid 3-diethylamin...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Antimuscurinic potency and subset specificity of the compound was characterised by its inhibition of the [3H]-NMS Binding to Muscarinic acetylcholine... | J Med Chem 35: 1290-5 (1992) BindingDB Entry DOI: 10.7270/Q28914T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50005684 (2-Cyclohexyl-2-phenyl-propionic acid 3-diethylamin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Antimuscurinic potency and subset specificity of the compound was characterised by its inhibition of the [3H]-NMS Binding to Muscarinic acetylcholine... | J Med Chem 35: 1290-5 (1992) BindingDB Entry DOI: 10.7270/Q28914T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50005686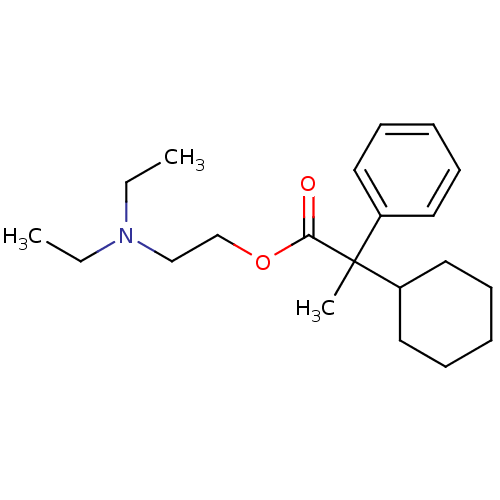 (2-Cyclohexyl-2-phenyl-propionic acid 2-diethylamin...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Antimuscurinic potency and subset specificity of the compound was characterised by its inhibition of the [3H]-NMS Binding to Muscarinic acetylcholine... | J Med Chem 35: 1290-5 (1992) BindingDB Entry DOI: 10.7270/Q28914T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50005684 (2-Cyclohexyl-2-phenyl-propionic acid 3-diethylamin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Antimuscurinic potency and subset specificity of the compound was characterised by its inhibition of the [3H]-NMS Binding to Muscarinic acetylcholine... | J Med Chem 35: 1290-5 (1992) BindingDB Entry DOI: 10.7270/Q28914T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50018229 (2-(4-Hydroxy-phenyl)-2-phenyl-propionic acid 2-die...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of carbachol-induced release of alpha-amylase from pancreatic acinar cells from that of rat ileum contained the M2 receptor subtypes | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50005685 (2,2-Diphenyl-propionic acid 2-diethylamino-ethyl e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Antimuscurinic potency and subset specificity of the compound was characterised by its inhibition of the [3H]-NMS Binding to Muscarinic acetylcholine... | J Med Chem 35: 1290-5 (1992) BindingDB Entry DOI: 10.7270/Q28914T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50005685 (2,2-Diphenyl-propionic acid 2-diethylamino-ethyl e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Antimuscurinic potency and subset specificity of the compound was characterised by inhibition of the [3H]-NMS Binding to Muscarinic acetylcholine rec... | J Med Chem 35: 1290-5 (1992) BindingDB Entry DOI: 10.7270/Q28914T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50005685 (2,2-Diphenyl-propionic acid 2-diethylamino-ethyl e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Antimuscurinic potency and subset specificity of the compound was characterised by inhibition of the [3H]-NMS Binding to Muscarinic acetylcholine rec... | J Med Chem 35: 1290-5 (1992) BindingDB Entry DOI: 10.7270/Q28914T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50005686 (2-Cyclohexyl-2-phenyl-propionic acid 2-diethylamin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Antimuscurinic potency and subset specificity of the compound was characterised by its inhibition of the [3H]-NMS Muscarinic acetylcholine receptor M... | J Med Chem 35: 1290-5 (1992) BindingDB Entry DOI: 10.7270/Q28914T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50005686 (2-Cyclohexyl-2-phenyl-propionic acid 2-diethylamin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Antimuscurinic potency and subset specificity of the compound was characterised by its inhibition of the [3H]-NMS Binding to Muscarinic acetylcholine... | J Med Chem 35: 1290-5 (1992) BindingDB Entry DOI: 10.7270/Q28914T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50005684 (2-Cyclohexyl-2-phenyl-propionic acid 3-diethylamin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Antimuscurinic potency and subset specificity of the compound was characterised by its inhibition of the [3H]-NMS Binding to Muscarinic acetylcholine... | J Med Chem 35: 1290-5 (1992) BindingDB Entry DOI: 10.7270/Q28914T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50005686 (2-Cyclohexyl-2-phenyl-propionic acid 2-diethylamin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Antimuscurinic potency and subset specificity of the compound was characterised by its inhibition of the [3H]-NMS Binding to Muscarinic acetylcholine... | J Med Chem 35: 1290-5 (1992) BindingDB Entry DOI: 10.7270/Q28914T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM50018230 (2-(4-Methylcarbamoyloxy-phenyl)-2-phenyl-propionic...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to cerebral cortex membranes which contain predominantly the Muscarinic acetylcholine receptor M1 subtypes | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50005681 (2-Cyclohexyl-2-phenyl-propionic acid 3-dimethylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Antimuscurinic potency and subset specificity of the compound was characterised by its inhibition of the [3H]-NMS Binding to Muscarinic acetylcholine... | J Med Chem 35: 1290-5 (1992) BindingDB Entry DOI: 10.7270/Q28914T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50005682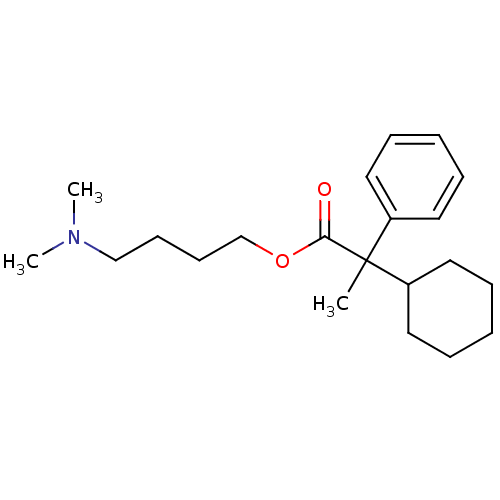 (2-Cyclohexyl-2-phenyl-propionic acid 4-dimethylami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Antimuscurinic potency and subset specificity of the compound was characterised by its inhibition of the [3H]-NMS Binding to Muscarinic acetylcholine... | J Med Chem 35: 1290-5 (1992) BindingDB Entry DOI: 10.7270/Q28914T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50005682 (2-Cyclohexyl-2-phenyl-propionic acid 4-dimethylami...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Antimuscurinic potency and subset specificity of the compound was characterised by its inhibition of the [3H]-NMS Binding to Muscarinic acetylcholine... | J Med Chem 35: 1290-5 (1992) BindingDB Entry DOI: 10.7270/Q28914T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50018225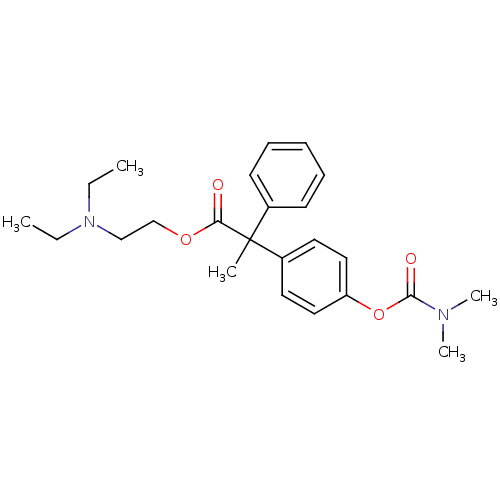 (2-(4-Dimethylcarbamoyloxy-phenyl)-2-phenyl-propion...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Binding affinity was determined from the inhibition of contraction of guinea pig ileum which has Muscarinic acetylcholine receptor M2 subtype. | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM50005685 (2,2-Diphenyl-propionic acid 2-diethylamino-ethyl e...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to cerebral cortex membranes which contain predominantly the Muscarinic acetylcholine receptor M1 subtypes | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50005682 (2-Cyclohexyl-2-phenyl-propionic acid 4-dimethylami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Antimuscurinic potency and subset specificity of the compound was characterised by its inhibition of the [3H]-NMS Binding to Muscarinic acetylcholine... | J Med Chem 35: 1290-5 (1992) BindingDB Entry DOI: 10.7270/Q28914T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM50018229 (2-(4-Hydroxy-phenyl)-2-phenyl-propionic acid 2-die...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to cerebral cortex membranes which contain predominantly the Muscarinic acetylcholine receptor M1 subtypes | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50005683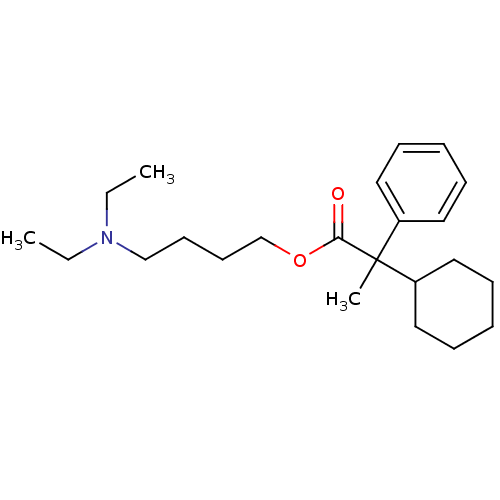 (2-Cyclohexyl-2-phenyl-propionic acid 4-diethylamin...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 111 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Antimuscurinic potency and subset specificity of the compound was characterised by its inhibition of the [3H]-NMS Binding to Muscarinic acetylcholine... | J Med Chem 35: 1290-5 (1992) BindingDB Entry DOI: 10.7270/Q28914T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM39341 (11-[(4-methylpiperazin-1-yl)acetyl]-5,11-dihydro-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of carbachol-induced release of alpha-amylase from pancreatic acinar cells from that of rat ileum contained the muscarinic acetylcholine r... | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM50018225 (2-(4-Dimethylcarbamoyloxy-phenyl)-2-phenyl-propion...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to cerebral cortex membranes which contain predominantly the Muscarinic acetylcholine receptor M1 subtypes | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50005683 (2-Cyclohexyl-2-phenyl-propionic acid 4-diethylamin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Antimuscurinic potency and subset specificity of the compound was characterised by its inhibition of the [3H]-NMS Binding to Muscarinic acetylcholine... | J Med Chem 35: 1290-5 (1992) BindingDB Entry DOI: 10.7270/Q28914T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM39341 (11-[(4-methylpiperazin-1-yl)acetyl]-5,11-dihydro-6...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to cerebral cortex membranes which contain predominantly the muscarinic acetylcholine receptor M1 | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50005680 (2-Cyclohexyl-2-phenyl-propionic acid 5-diethylamin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 173 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Antimuscurinic potency and subset specificity of the compound was characterised by its inhibition of the [3H]-NMS Binding to Muscarinic acetylcholine... | J Med Chem 35: 1290-5 (1992) BindingDB Entry DOI: 10.7270/Q28914T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50005680 (2-Cyclohexyl-2-phenyl-propionic acid 5-diethylamin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 178 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Antimuscurinic potency and subset specificity of the compound was characterised by its inhibition of the [3H]-NMS Binding to Muscarinic acetylcholine... | J Med Chem 35: 1290-5 (1992) BindingDB Entry DOI: 10.7270/Q28914T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50005683 (2-Cyclohexyl-2-phenyl-propionic acid 4-diethylamin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 209 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Antimuscurinic potency and subset specificity of the compound was characterised by its inhibition of the [3H]-NMS Binding to Muscarinic acetylcholine... | J Med Chem 35: 1290-5 (1992) BindingDB Entry DOI: 10.7270/Q28914T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50005680 (2-Cyclohexyl-2-phenyl-propionic acid 5-diethylamin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 246 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Antimuscurinic potency and subset specificity of the compound was characterised by its inhibition of the [3H]-NMS Binding to Muscarinic acetylcholine... | J Med Chem 35: 1290-5 (1992) BindingDB Entry DOI: 10.7270/Q28914T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50005683 (2-Cyclohexyl-2-phenyl-propionic acid 4-diethylamin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 248 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Antimuscurinic potency and subset specificity of the compound was characterised by its inhibition of the [3H]-NMS Binding to Muscarinic acetylcholine... | J Med Chem 35: 1290-5 (1992) BindingDB Entry DOI: 10.7270/Q28914T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50005682 (2-Cyclohexyl-2-phenyl-propionic acid 4-dimethylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 258 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Antimuscurinic potency and subset specificity of the compound was characterised by its inhibition of the [3H]-NMS Binding to Muscarinic acetylcholine... | J Med Chem 35: 1290-5 (1992) BindingDB Entry DOI: 10.7270/Q28914T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50005680 (2-Cyclohexyl-2-phenyl-propionic acid 5-diethylamin...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 298 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Antimuscurinic potency and subset specificity of the compound was characterised by its inhibition of the [3H]-NMS Binding to Muscarinic acetylcholine... | J Med Chem 35: 1290-5 (1992) BindingDB Entry DOI: 10.7270/Q28914T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50018226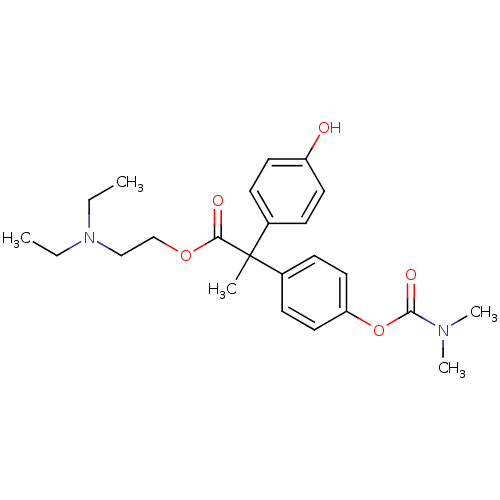 (2-(4-Dimethylcarbamoyloxy-phenyl)-2-(4-hydroxy-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of anticholinesterase activity by their ability to inactivate human serum butyrylcholinesterase | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50018225 (2-(4-Dimethylcarbamoyloxy-phenyl)-2-phenyl-propion...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of anticholinesterase activity by their ability to inactivate human serum butyrylcholinesterase | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50018230 (2-(4-Methylcarbamoyloxy-phenyl)-2-phenyl-propionic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Anticholinesterase activity by their ability to inactivate human serum butyrylcholinesterase | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM50018227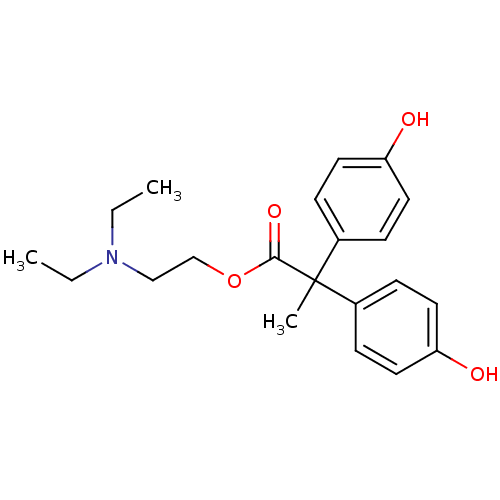 (2,2-Bis-(4-hydroxy-phenyl)-propionic acid 2-diethy...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to cerebral cortex membranes which contain predominantly the Muscarinic acetylcholine receptor M1 subtypes | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50018226 (2-(4-Dimethylcarbamoyloxy-phenyl)-2-(4-hydroxy-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of carbachol-induced release of alpha-amylase from pancreatic acinar cells from that of rat ileum contained the M2 receptor subtypes | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50109574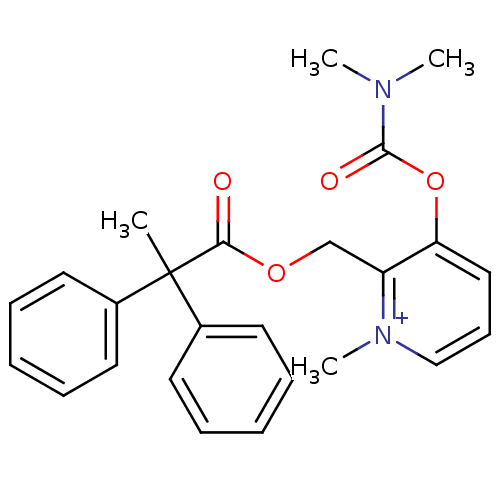 (3-Dimethylcarbamoyloxy-2-(2,2-diphenyl-propionylox...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibitory activity against Butyrylcholinesterase (BChE) | J Med Chem 45: 902-10 (2002) BindingDB Entry DOI: 10.7270/Q22B8ZR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM50018226 (2-(4-Dimethylcarbamoyloxy-phenyl)-2-(4-hydroxy-phe...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to cerebral cortex membranes which contain predominantly the Muscarinic acetylcholine receptor M1 subtypes | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50313079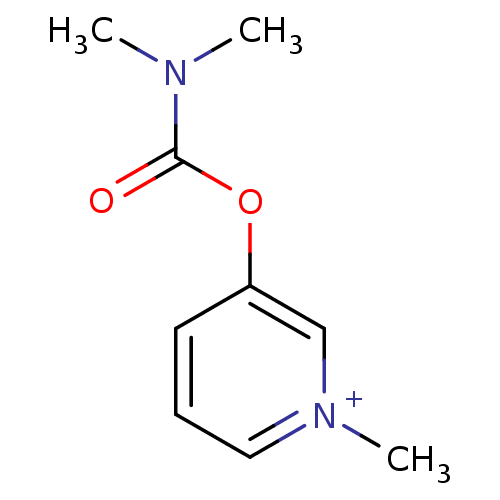 (3-Dimethylcarbamoyloxy-1-methyl-pyridinium; bromid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of anticholinesterase activity by their ability to inactivate human serum butyrylcholinesterase | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50018228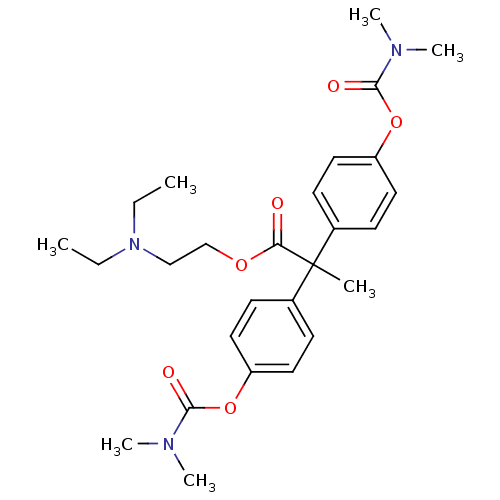 (2,2-Bis-(4-dimethylcarbamoyloxy-phenyl)-propionic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of anticholinesterase activity by their ability to inactivate human serum butyrylcholinesterase | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50018227 (2,2-Bis-(4-hydroxy-phenyl)-propionic acid 2-diethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of carbachol-induced release of alpha-amylase from pancreatic acinar cells from that of rat ileum contained the M2 receptor subtypes | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 71 total ) | Next | Last >> |