 Found 622 hits with Last Name = 'ledeboer' and Initial = 'mw'
Found 622 hits with Last Name = 'ledeboer' and Initial = 'mw' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50300196

(10-(4-hydroxyphenyl)-8,13,15-triazatetracyclo[9.6....)Show SMILES Oc1ccc(cc1)C1c2c[nH]c3nccc(-c4ccccc4NC1=O)c23 Show InChI InChI=1S/C21H15N3O2/c25-13-7-5-12(6-8-13)18-16-11-23-20-19(16)15(9-10-22-20)14-3-1-2-4-17(14)24-21(18)26/h1-11,18,25H,(H,22,23)(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JAK2 |
J Med Chem 52: 7938-41 (2009)
Checked by Author
Article DOI: 10.1021/jm901383u
BindingDB Entry DOI: 10.7270/Q2GF0TK0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093352
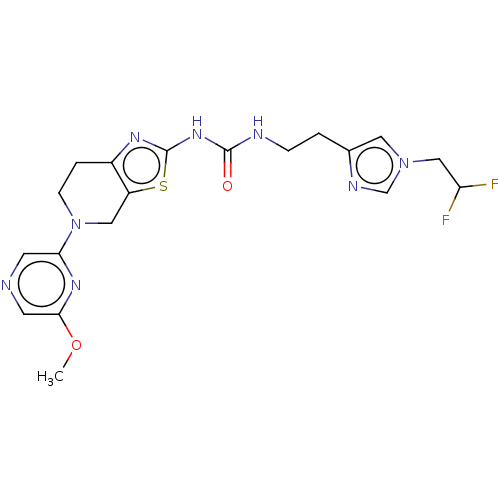
(CHEMBL3586678)Show SMILES COc1cncc(n1)N1CCc2nc(NC(=O)NCCc3cn(CC(F)F)cn3)sc2C1 Show InChI InChI=1S/C19H22F2N8O2S/c1-31-17-7-22-6-16(26-17)29-5-3-13-14(9-29)32-19(25-13)27-18(30)23-4-2-12-8-28(11-24-12)10-15(20)21/h6-8,11,15H,2-5,9-10H2,1H3,(H2,23,25,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093351
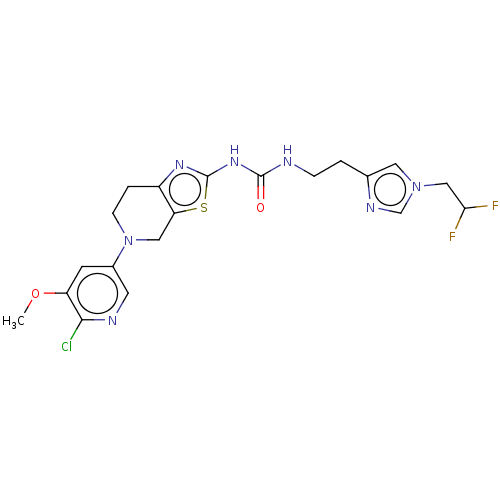
(CHEMBL3585362)Show SMILES COc1cc(cnc1Cl)N1CCc2nc(NC(=O)NCCc3cn(CC(F)F)cn3)sc2C1 Show InChI InChI=1S/C20H22ClF2N7O2S/c1-32-15-6-13(7-25-18(15)21)30-5-3-14-16(9-30)33-20(27-14)28-19(31)24-4-2-12-8-29(11-26-12)10-17(22)23/h6-8,11,17H,2-5,9-10H2,1H3,(H2,24,27,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093355
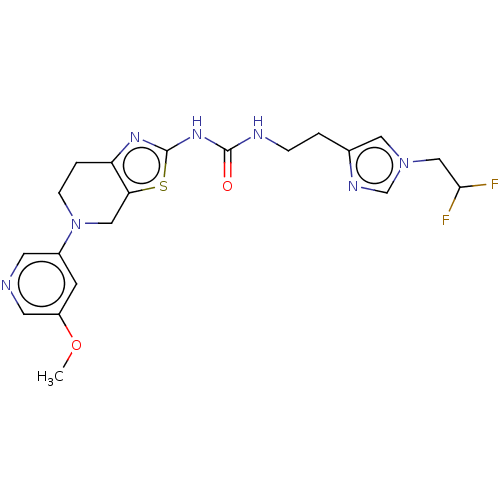
(CHEMBL3586677)Show SMILES COc1cncc(c1)N1CCc2nc(NC(=O)NCCc3cn(CC(F)F)cn3)sc2C1 Show InChI InChI=1S/C20H23F2N7O2S/c1-31-15-6-14(7-23-8-15)29-5-3-16-17(10-29)32-20(26-16)27-19(30)24-4-2-13-9-28(12-25-13)11-18(21)22/h6-9,12,18H,2-5,10-11H2,1H3,(H2,24,26,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50300196

(10-(4-hydroxyphenyl)-8,13,15-triazatetracyclo[9.6....)Show SMILES Oc1ccc(cc1)C1c2c[nH]c3nccc(-c4ccccc4NC1=O)c23 Show InChI InChI=1S/C21H15N3O2/c25-13-7-5-12(6-8-13)18-16-11-23-20-19(16)15(9-10-22-20)14-3-1-2-4-17(14)24-21(18)26/h1-11,18,25H,(H,22,23)(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JAK3 |
J Med Chem 52: 7938-41 (2009)
Checked by Author
Article DOI: 10.1021/jm901383u
BindingDB Entry DOI: 10.7270/Q2GF0TK0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50300196

(10-(4-hydroxyphenyl)-8,13,15-triazatetracyclo[9.6....)Show SMILES Oc1ccc(cc1)C1c2c[nH]c3nccc(-c4ccccc4NC1=O)c23 Show InChI InChI=1S/C21H15N3O2/c25-13-7-5-12(6-8-13)18-16-11-23-20-19(16)15(9-10-22-20)14-3-1-2-4-17(14)24-21(18)26/h1-11,18,25H,(H,22,23)(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JAK3 |
J Med Chem 52: 7938-41 (2009)
Checked by Author
Article DOI: 10.1021/jm901383u
BindingDB Entry DOI: 10.7270/Q2GF0TK0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093356
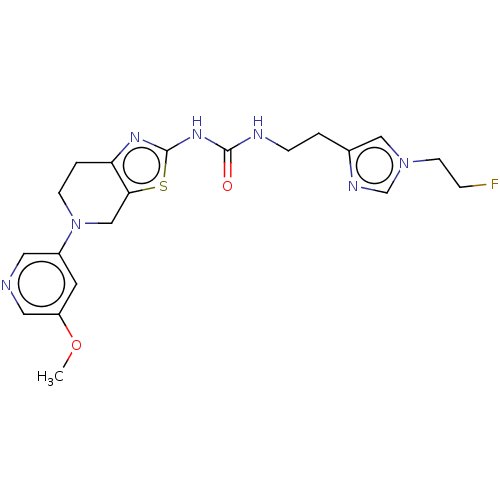
(CHEMBL3586676)Show SMILES COc1cncc(c1)N1CCc2nc(NC(=O)NCCc3cn(CCF)cn3)sc2C1 Show InChI InChI=1S/C20H24FN7O2S/c1-30-16-8-15(9-22-10-16)28-6-3-17-18(12-28)31-20(25-17)26-19(29)23-5-2-14-11-27(7-4-21)13-24-14/h8-11,13H,2-7,12H2,1H3,(H2,23,25,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093354

(CHEMBL3586679)Show SMILES COc1cncc(c1)N1CCc2nc(NC(=O)NCCc3cn(CC(F)(F)F)cn3)sc2C1 Show InChI InChI=1S/C20H22F3N7O2S/c1-32-15-6-14(7-24-8-15)30-5-3-16-17(10-30)33-19(27-16)28-18(31)25-4-2-13-9-29(12-26-13)11-20(21,22)23/h6-9,12H,2-5,10-11H2,1H3,(H2,25,27,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093395
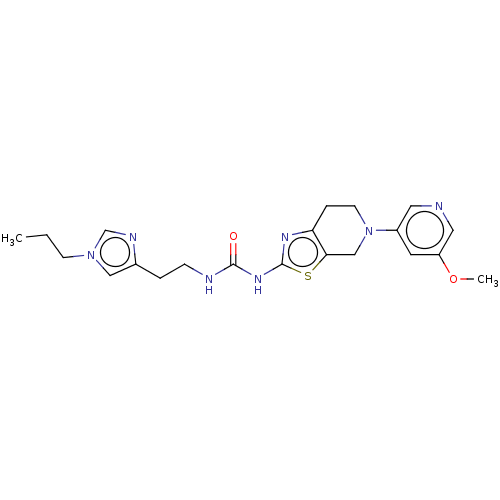
(CHEMBL3586674)Show SMILES CCCn1cnc(CCNC(=O)Nc2nc3CCN(Cc3s2)c2cncc(OC)c2)c1 Show InChI InChI=1S/C21H27N7O2S/c1-3-7-27-12-15(24-14-27)4-6-23-20(29)26-21-25-18-5-8-28(13-19(18)31-21)16-9-17(30-2)11-22-10-16/h9-12,14H,3-8,13H2,1-2H3,(H2,23,25,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093417
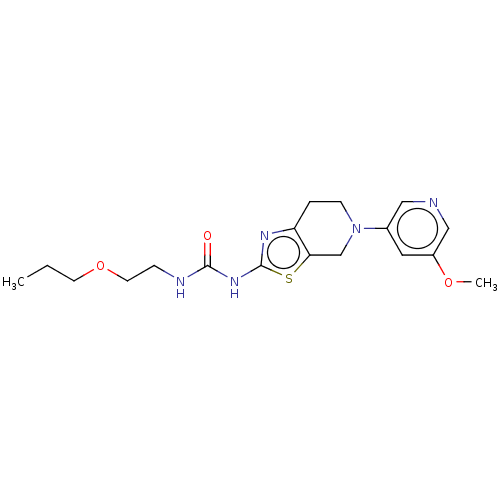
(CHEMBL3586672)Show InChI InChI=1S/C18H25N5O3S/c1-3-7-26-8-5-20-17(24)22-18-21-15-4-6-23(12-16(15)27-18)13-9-14(25-2)11-19-10-13/h9-11H,3-8,12H2,1-2H3,(H2,20,21,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093437
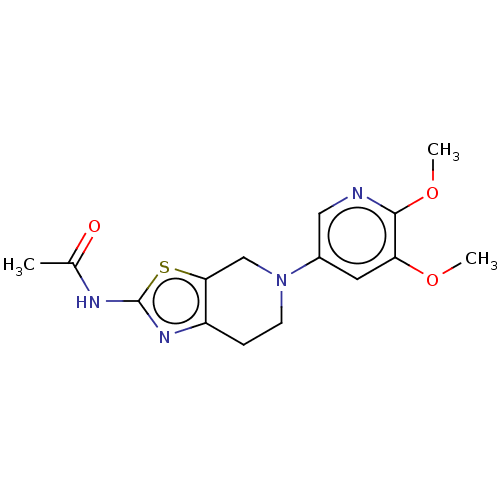
(CHEMBL3586668)Show InChI InChI=1S/C20H32O2/c1-19-7-5-14(21)10-13(19)3-4-15-16(19)6-8-20(2)17(15)9-12-11-22-18(12)20/h12-18,21H,3-11H2,1-2H3/t12-,13+,14-,15-,16+,17+,18+,19+,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093399
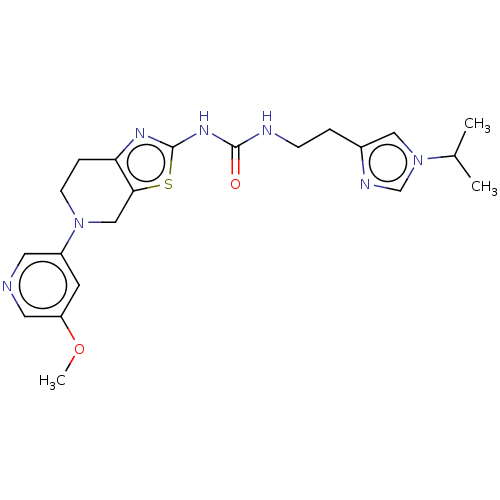
(CHEMBL3586673)Show SMILES COc1cncc(c1)N1CCc2nc(NC(=O)NCCc3cn(cn3)C(C)C)sc2C1 Show InChI InChI=1S/C21H27N7O2S/c1-14(2)28-11-15(24-13-28)4-6-23-20(29)26-21-25-18-5-7-27(12-19(18)31-21)16-8-17(30-3)10-22-9-16/h8-11,13-14H,4-7,12H2,1-3H3,(H2,23,25,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093434
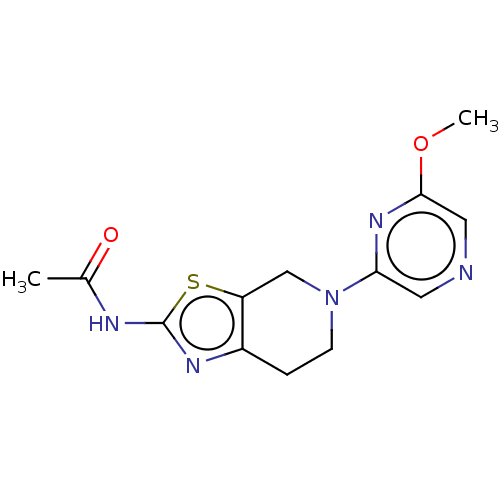
(CHEMBL3586670)Show InChI InChI=1S/C12H11NO8S2/c1-6(14)13-10-4-8(22(16,17)18)2-7-3-9(23(19,20)21)5-11(15)12(7)10/h2-5,15H,1H3,(H,13,14)(H,16,17,18)(H,19,20,21)/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093436
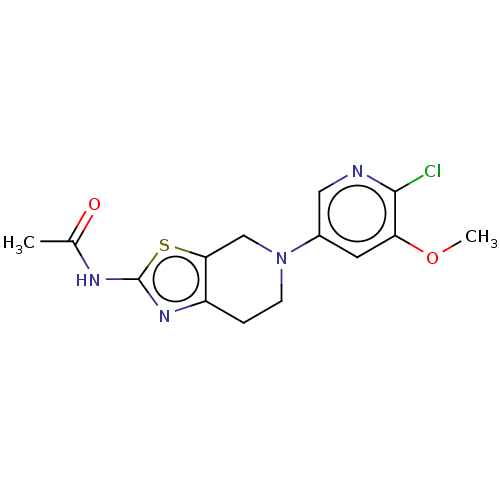
(CHEMBL3586669)Show InChI InChI=1S/C22H18O12/c23-13-5-1-11(9-15(13)25)3-7-17(27)33-19(21(29)30)20(22(31)32)34-18(28)8-4-12-2-6-14(24)16(26)10-12/h1-10,19-20,23-26H,(H,29,30)(H,31,32)/p-2/b7-3+,8-4+/t19-,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093353
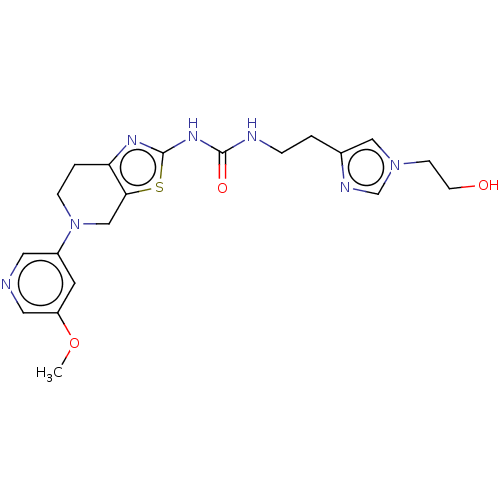
(CHEMBL3586680)Show SMILES COc1cncc(c1)N1CCc2nc(NC(=O)NCCc3cn(CCO)cn3)sc2C1 Show InChI InChI=1S/C20H25N7O3S/c1-30-16-8-15(9-21-10-16)27-5-3-17-18(12-27)31-20(24-17)25-19(29)22-4-2-14-11-26(6-7-28)13-23-14/h8-11,13,28H,2-7,12H2,1H3,(H2,22,24,25,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093391
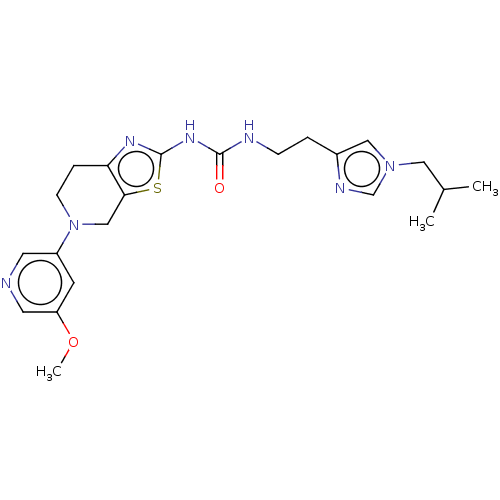
(CHEMBL3586675)Show SMILES COc1cncc(c1)N1CCc2nc(NC(=O)NCCc3cn(CC(C)C)cn3)sc2C1 Show InChI InChI=1S/C22H29N7O2S/c1-15(2)11-28-12-16(25-14-28)4-6-24-21(30)27-22-26-19-5-7-29(13-20(19)32-22)17-8-18(31-3)10-23-9-17/h8-10,12,14-15H,4-7,11,13H2,1-3H3,(H2,24,26,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093439
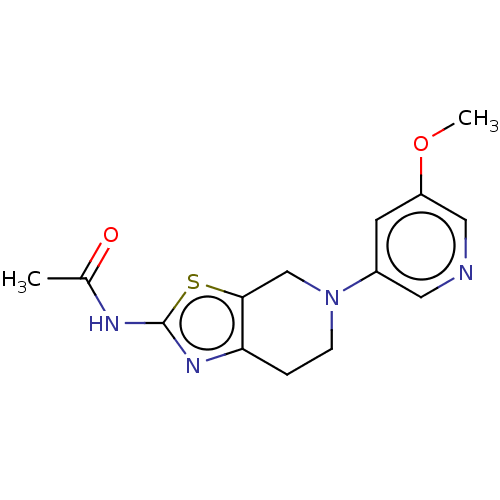
(CHEMBL3586666)Show InChI InChI=1S/C19H30O2/c1-18-7-5-12(20)9-11(18)3-4-13-14(18)6-8-19(2)15(13)10-16-17(19)21-16/h11-17,20H,3-10H2,1-2H3/t11-,12+,13+,14-,15-,16+,17+,18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093419
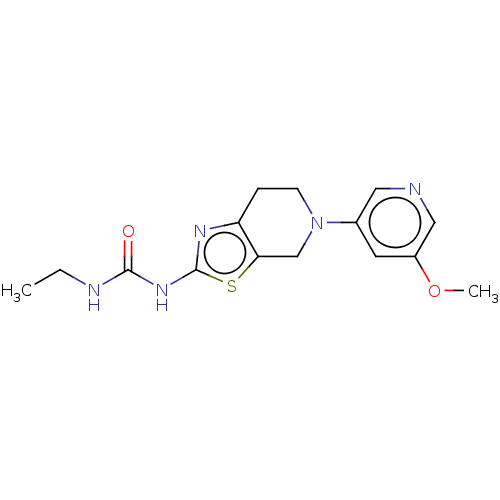
(CHEMBL3586671)Show InChI InChI=1S/C15H19N5O2S/c1-3-17-14(21)19-15-18-12-4-5-20(9-13(12)23-15)10-6-11(22-2)8-16-7-10/h6-8H,3-5,9H2,1-2H3,(H2,17,18,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093440
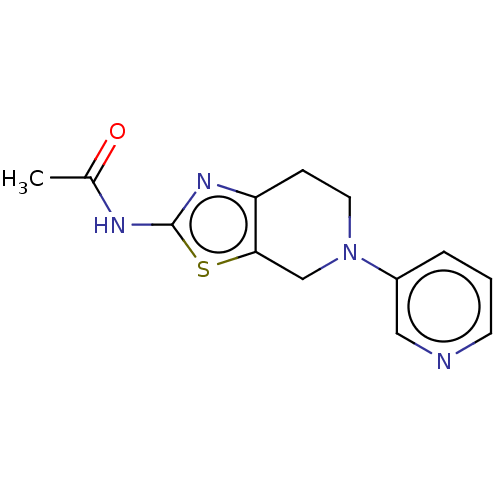
(CHEMBL3586665)Show InChI InChI=1S/C21H32O3/c1-12(22)16-6-7-17-15-5-4-13-10-14(23)8-9-20(13,2)19(15)18(24)11-21(16,17)3/h13-17,19,23H,4-11H2,1-3H3/t13?,14-,15?,16-,17?,19?,20+,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093438
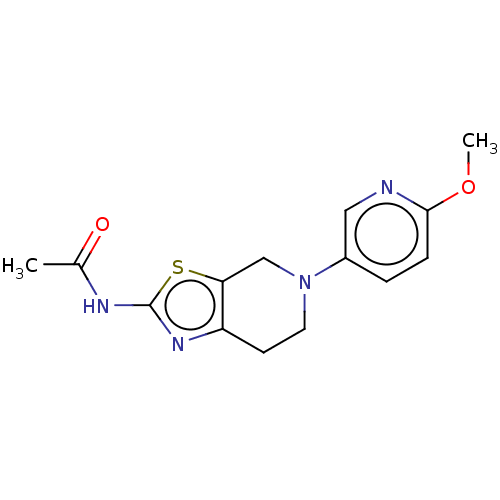
(CHEMBL3586667)Show InChI InChI=1S/C20H31NO/c1-19-9-7-15(22)11-13(19)3-5-16-17-6-4-14(12-21)20(17,2)10-8-18(16)19/h13-18,22H,3-11H2,1-2H3/t13-,14-,15+,16-,17-,18-,19-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50305149
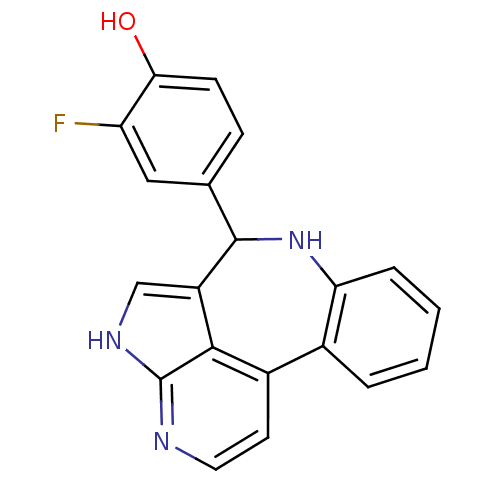
(2-fluoro-4-{8,12,14-triazatetracyclo[8.6.1.0^{2,7}...)Show InChI InChI=1S/C20H14FN3O/c21-15-9-11(5-6-17(15)25)19-14-10-23-20-18(14)13(7-8-22-20)12-3-1-2-4-16(12)24-19/h1-10,19,24-25H,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 20: 153-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.021
BindingDB Entry DOI: 10.7270/Q2PN95RQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50305150
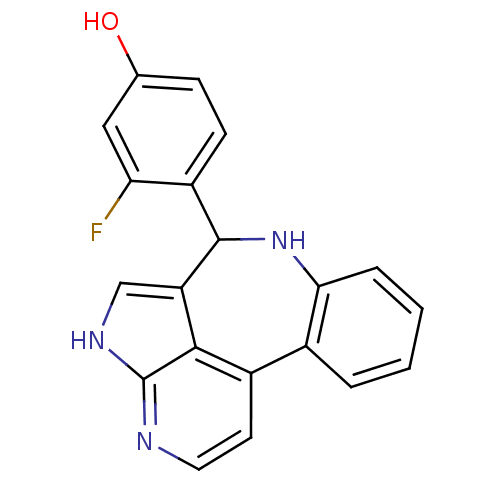
(3-fluoro-4-{8,12,14-triazatetracyclo[8.6.1.0^{2,7}...)Show SMILES Oc1ccc(C2Nc3ccccc3-c3ccnc4[nH]cc2c34)c(F)c1 Show InChI InChI=1S/C20H14FN3O/c21-16-9-11(25)5-6-14(16)19-15-10-23-20-18(15)13(7-8-22-20)12-3-1-2-4-17(12)24-19/h1-10,19,24-25H,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 20: 153-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.021
BindingDB Entry DOI: 10.7270/Q2PN95RQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50305157
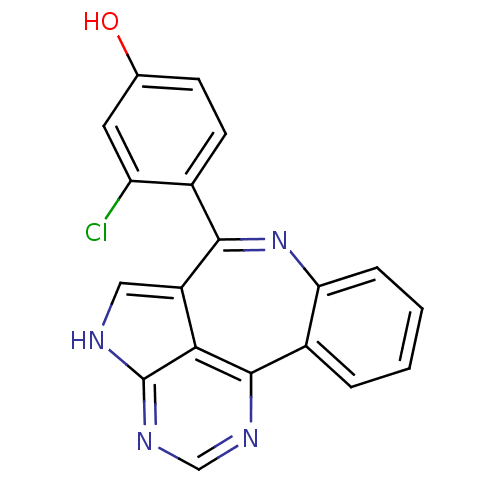
(3-chloro-4-{8,12,14,16-tetraazatetracyclo[8.6.1.0^...)Show SMILES Oc1ccc(c(Cl)c1)-c1nc2ccccc2c2ncnc3[nH]cc1c23 Show InChI InChI=1S/C19H11ClN4O/c20-14-7-10(25)5-6-11(14)17-13-8-21-19-16(13)18(22-9-23-19)12-3-1-2-4-15(12)24-17/h1-9,25H,(H,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 20: 153-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.021
BindingDB Entry DOI: 10.7270/Q2PN95RQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50305155
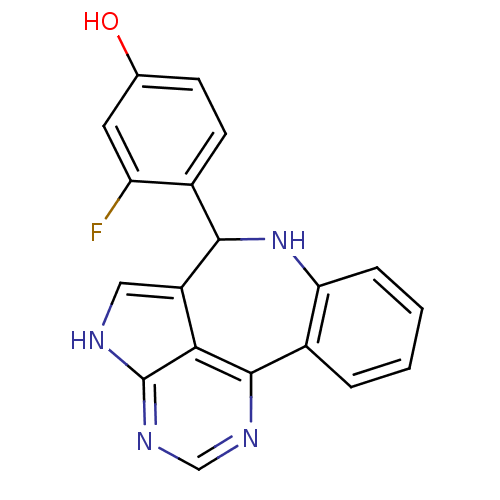
(3-fluoro-4-{8,12,14,16-tetraazatetracyclo[8.6.1.0^...)Show SMILES Oc1ccc(C2Nc3ccccc3-c3ncnc4[nH]cc2c34)c(F)c1 Show InChI InChI=1S/C19H13FN4O/c20-14-7-10(25)5-6-11(14)17-13-8-21-19-16(13)18(22-9-23-19)12-3-1-2-4-15(12)24-17/h1-9,17,24-25H,(H,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 20: 153-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.021
BindingDB Entry DOI: 10.7270/Q2PN95RQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50305156
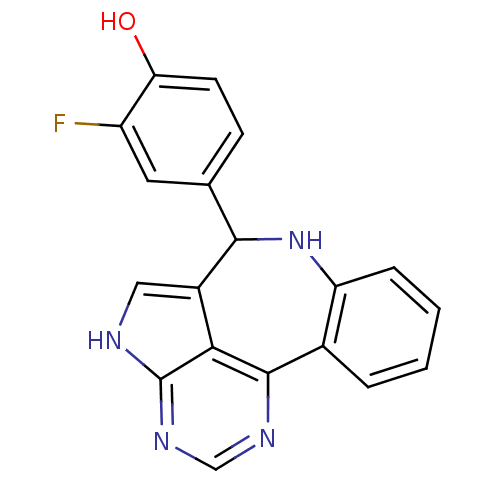
(2-fluoro-4-{8,12,14,16-tetraazatetracyclo[8.6.1.0^...)Show InChI InChI=1S/C19H13FN4O/c20-13-7-10(5-6-15(13)25)17-12-8-21-19-16(12)18(22-9-23-19)11-3-1-2-4-14(11)24-17/h1-9,17,24-25H,(H,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 20: 153-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.021
BindingDB Entry DOI: 10.7270/Q2PN95RQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50305148
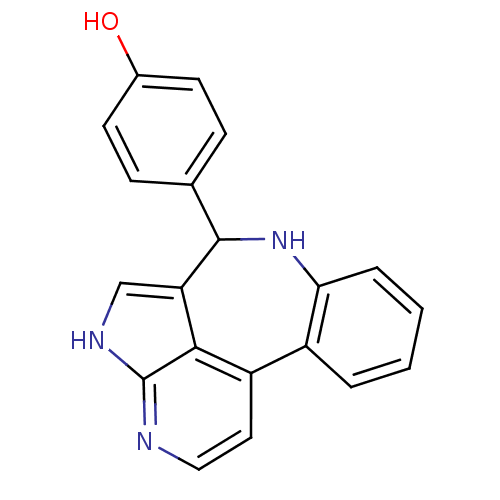
(4-{8,12,14-triazatetracyclo[8.6.1.0^{2,7}.0^{13,17...)Show InChI InChI=1S/C20H15N3O/c24-13-7-5-12(6-8-13)19-16-11-22-20-18(16)15(9-10-21-20)14-3-1-2-4-17(14)23-19/h1-11,19,23-24H,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 20: 153-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.021
BindingDB Entry DOI: 10.7270/Q2PN95RQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50305153
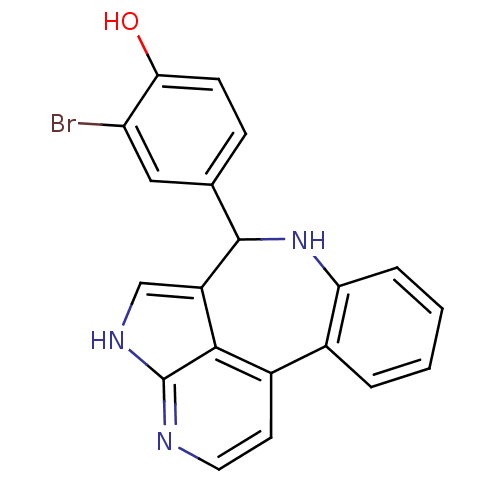
(2-bromo-4-{8,12,14-triazatetracyclo[8.6.1.0^{2,7}....)Show InChI InChI=1S/C20H14BrN3O/c21-15-9-11(5-6-17(15)25)19-14-10-23-20-18(14)13(7-8-22-20)12-3-1-2-4-16(12)24-19/h1-10,19,24-25H,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 20: 153-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.021
BindingDB Entry DOI: 10.7270/Q2PN95RQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50305154
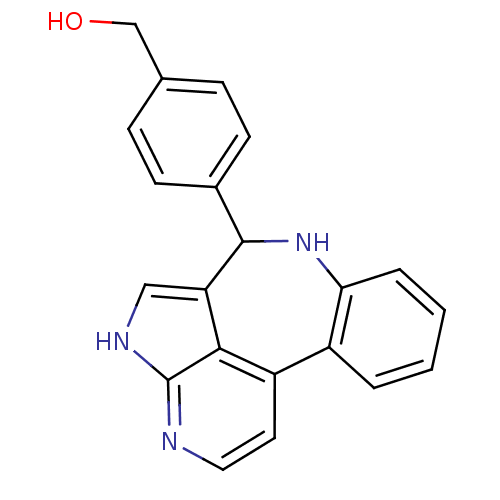
((4-{8,12,14-triazatetracyclo[8.6.1.0^{2,7}.0^{13,1...)Show InChI InChI=1S/C21H17N3O/c25-12-13-5-7-14(8-6-13)20-17-11-23-21-19(17)16(9-10-22-21)15-3-1-2-4-18(15)24-20/h1-11,20,24-25H,12H2,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 20: 153-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.021
BindingDB Entry DOI: 10.7270/Q2PN95RQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50305152
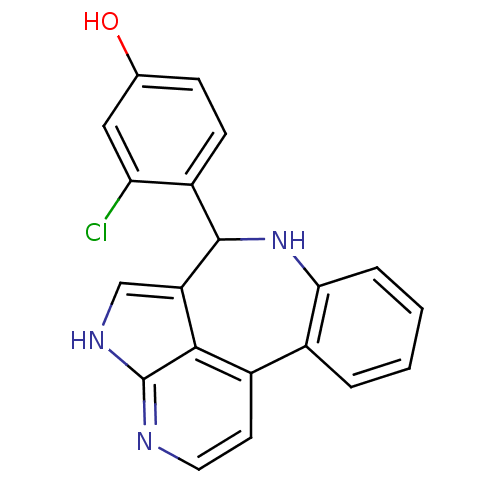
(3-chloro-4-{8,12,14-triazatetracyclo[8.6.1.0^{2,7}...)Show SMILES Oc1ccc(C2Nc3ccccc3-c3ccnc4[nH]cc2c34)c(Cl)c1 Show InChI InChI=1S/C20H14ClN3O/c21-16-9-11(25)5-6-14(16)19-15-10-23-20-18(15)13(7-8-22-20)12-3-1-2-4-17(12)24-19/h1-10,19,24-25H,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 20: 153-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.021
BindingDB Entry DOI: 10.7270/Q2PN95RQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50378535
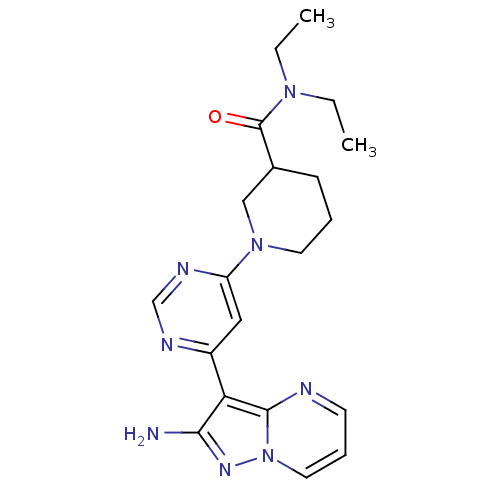
(CHEMBL1204012)Show SMILES CCN(CC)C(=O)C1CCCN(C1)c1cc(ncn1)-c1c(N)nn2cccnc12 Show InChI InChI=1S/C20H26N8O/c1-3-26(4-2)20(29)14-7-5-9-27(12-14)16-11-15(23-13-24-16)17-18(21)25-28-10-6-8-22-19(17)28/h6,8,10-11,13-14H,3-5,7,9,12H2,1-2H3,(H2,21,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 19: 6529-33 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.053
BindingDB Entry DOI: 10.7270/Q2SJ1MKQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50305151
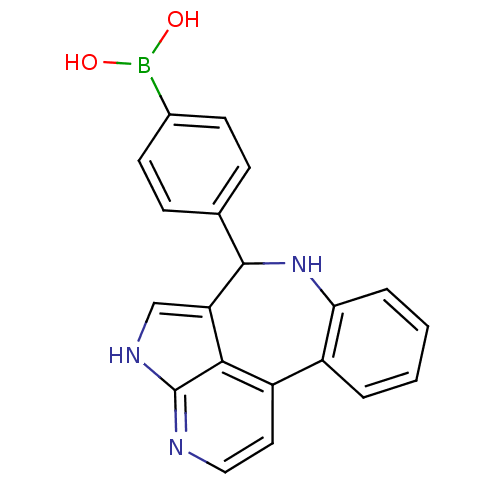
((4-{8,12,14-triazatetracyclo[8.6.1.0^{2,7}.0^{13,1...)Show SMILES OB(O)c1ccc(cc1)C1Nc2ccccc2-c2ccnc3[nH]cc1c23 Show InChI InChI=1S/C20H16BN3O2/c25-21(26)13-7-5-12(6-8-13)19-16-11-23-20-18(16)15(9-10-22-20)14-3-1-2-4-17(14)24-19/h1-11,19,24-26H,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 20: 153-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.021
BindingDB Entry DOI: 10.7270/Q2PN95RQ |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50300196

(10-(4-hydroxyphenyl)-8,13,15-triazatetracyclo[9.6....)Show SMILES Oc1ccc(cc1)C1c2c[nH]c3nccc(-c4ccccc4NC1=O)c23 Show InChI InChI=1S/C21H15N3O2/c25-13-7-5-12(6-8-13)18-16-11-23-20-19(16)15(9-10-22-20)14-3-1-2-4-17(14)24-21(18)26/h1-11,18,25H,(H,22,23)(H,24,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ALK |
J Med Chem 52: 7938-41 (2009)
Checked by Author
Article DOI: 10.1021/jm901383u
BindingDB Entry DOI: 10.7270/Q2GF0TK0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50067514
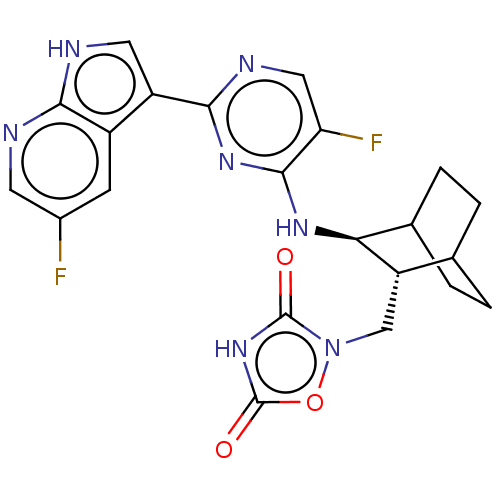
(CHEMBL3401978)Show SMILES Fc1cnc2[nH]cc(-c3ncc(F)c(N[C@H]4C5CCC(CC5)[C@@H]4Cn4oc(=O)[nH]c4=O)n3)c2c1 |r,wU:15.14,wD:22.24,(-5.47,-.22,;-5.12,-1.4,;-6.19,-2.51,;-5.76,-4.01,;-4.26,-4.36,;-3.53,-5.71,;-2.01,-5.43,;-1.83,-3.93,;-.48,-3.21,;.83,-4.02,;2.19,-3.3,;2.24,-1.76,;3.33,-1.18,;.93,-.95,;.98,.59,;-.33,1.41,;-1.7,.63,;-3.03,1.41,;-3.03,2.98,;-1.7,3.76,;-2.37,2.68,;-.96,1.7,;-.29,2.98,;1.05,3.73,;2.38,2.95,;2.5,1.43,;4,1.09,;4.49,-.04,;4.79,2.42,;3.77,3.57,;4.03,4.78,;-.42,-1.67,;-3.19,-3.25,;-3.61,-1.77,)| Show InChI InChI=1S/C22H21F2N7O3/c23-12-5-13-14(7-26-18(13)25-6-12)19-27-8-16(24)20(29-19)28-17-11-3-1-10(2-4-11)15(17)9-31-21(32)30-22(33)34-31/h5-8,10-11,15,17H,1-4,9H2,(H,25,26)(H,27,28,29)(H,30,32,33)/t10?,11?,15-,17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GSK3 (unknown origin) |
Bioorg Med Chem Lett 25: 1990-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.013
BindingDB Entry DOI: 10.7270/Q2V40WW5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50305193

(CHEMBL589387 | Methyl 9-(3-fluoro-4-hydroxyphenyl)...)Show SMILES COC(=O)C1(Nc2ccccc2-c2ccnc3[nH]cc1c23)c1ccc(O)c(F)c1 Show InChI InChI=1S/C22H16FN3O3/c1-29-21(28)22(12-6-7-18(27)16(23)10-12)15-11-25-20-19(15)14(8-9-24-20)13-4-2-3-5-17(13)26-22/h2-11,26-27H,1H3,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 20: 153-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.021
BindingDB Entry DOI: 10.7270/Q2PN95RQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50305196

(10-(2-fluoro-4-hydroxyphenyl)-9,13,15-triazatetrac...)Show SMILES Oc1ccc(C2NC(=O)c3ccccc3-c3ccnc4[nH]cc2c34)c(F)c1 Show InChI InChI=1S/C21H14FN3O2/c22-17-9-11(26)5-6-15(17)19-16-10-24-20-18(16)13(7-8-23-20)12-3-1-2-4-14(12)21(27)25-19/h1-10,19,26H,(H,23,24)(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 20: 153-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.021
BindingDB Entry DOI: 10.7270/Q2PN95RQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50305178
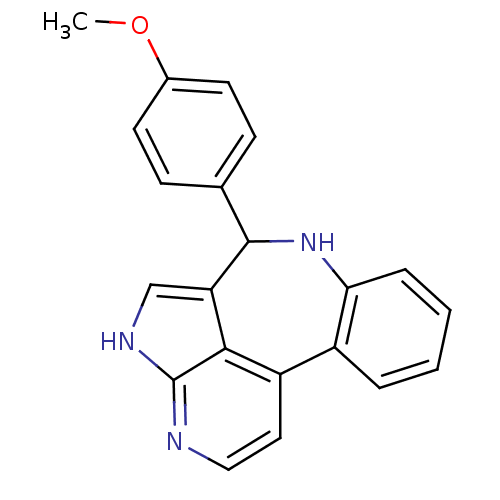
(9-(4-methoxyphenyl)-8,12,14-triazatetracyclo[8.6.1...)Show InChI InChI=1S/C21H17N3O/c1-25-14-8-6-13(7-9-14)20-17-12-23-21-19(17)16(10-11-22-21)15-4-2-3-5-18(15)24-20/h2-12,20,24H,1H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 20: 153-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.021
BindingDB Entry DOI: 10.7270/Q2PN95RQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
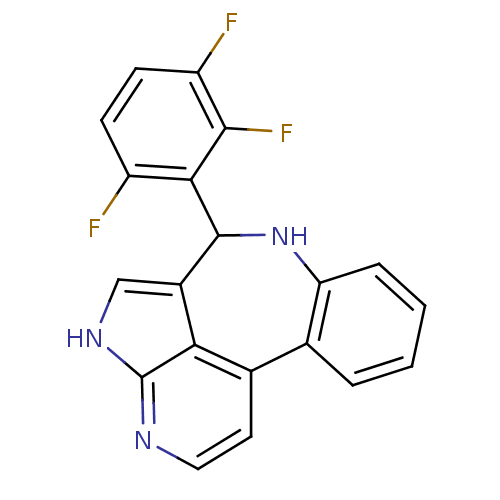
(Homo sapiens (Human)) | BDBM50305175
(9-(2,3,6-trifluorophenyl)-8,12,14-triazatetracyclo...)Show SMILES Fc1ccc(F)c(C2Nc3ccccc3-c3ccnc4[nH]cc2c34)c1F Show InChI InChI=1S/C20H12F3N3/c21-13-5-6-14(22)18(23)17(13)19-12-9-25-20-16(12)11(7-8-24-20)10-3-1-2-4-15(10)26-19/h1-9,19,26H,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 20: 153-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.021
BindingDB Entry DOI: 10.7270/Q2PN95RQ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50067515
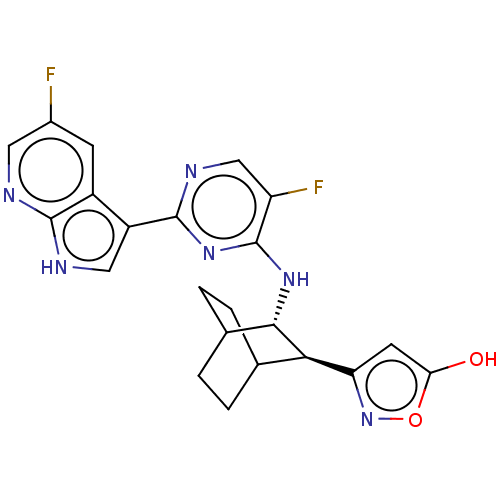
(CHEMBL3401979)Show SMILES Oc1cc(no1)[C@H]1C2CCC(CC2)[C@@H]1Nc1nc(ncc1F)-c1c[nH]c2ncc(F)cc12 |r,wU:13.16,wD:6.6,(4.75,4.05,;3.53,4.21,;2.47,3.09,;1.1,3.76,;1.27,5.28,;2.79,5.56,;-.29,2.98,;-1.7,3.76,;-3.04,2.98,;-3.04,1.41,;-1.7,.63,;-.96,1.7,;-2.37,2.68,;-.33,1.41,;.98,.59,;.93,-.95,;-.42,-1.67,;-.48,-3.21,;.83,-4.03,;2.19,-3.3,;2.24,-1.76,;3.33,-1.18,;-1.84,-3.94,;-2.01,-5.43,;-3.53,-5.71,;-4.26,-4.36,;-5.76,-4.01,;-6.19,-2.51,;-5.13,-1.4,;-5.47,-.22,;-3.61,-1.77,;-3.19,-3.25,)| Show InChI InChI=1S/C22H20F2N6O2/c23-12-5-13-14(8-26-20(13)25-7-12)21-27-9-15(24)22(29-21)28-19-11-3-1-10(2-4-11)18(19)16-6-17(31)32-30-16/h5-11,18-19,31H,1-4H2,(H,25,26)(H,27,28,29)/t10?,11?,18-,19+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GSK3 (unknown origin) |
Bioorg Med Chem Lett 25: 1990-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.013
BindingDB Entry DOI: 10.7270/Q2V40WW5 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50067537
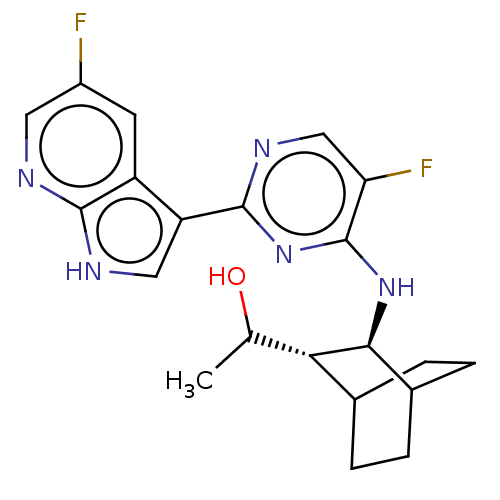
(CHEMBL3401987)Show SMILES [H][C@]1(C(C)O)C2CCC(CC2)[C@@H]1Nc1nc(ncc1F)-c1c[nH]c2ncc(F)cc12 |r,wD:11.13,1.0,TLB:2:1:7.6:9.10,THB:12:11:7.6:9.10,(-.64,1.54,;.13,.87,;1.58,1.51,;1.76,2.72,;2.54,.74,;1.38,,;2.86,.48,;2.23,-.94,;,-.67,;-.29,-2.25,;1.15,-1.65,;-1.3,.28,;-2.82,.5,;-3.77,-.71,;-3.2,-2.14,;-4.15,-3.35,;-5.67,-3.13,;-6.25,-1.7,;-5.29,-.49,;-5.75,.65,;-3.58,-4.78,;-4.4,-6.03,;-3.42,-7.22,;-2,-6.66,;-.62,-7.36,;.67,-6.49,;.57,-4.96,;1.59,-4.27,;-.83,-4.26,;-2.1,-5.13,)| Show InChI InChI=1S/C21H23F2N5O/c1-10(29)17-11-2-4-12(5-3-11)18(17)27-21-16(23)9-26-20(28-21)15-8-25-19-14(15)6-13(22)7-24-19/h6-12,17-18,29H,2-5H2,1H3,(H,24,25)(H,26,27,28)/t10?,11?,12?,17-,18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GSK3 (unknown origin) |
Bioorg Med Chem Lett 25: 1990-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.013
BindingDB Entry DOI: 10.7270/Q2V40WW5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50305179
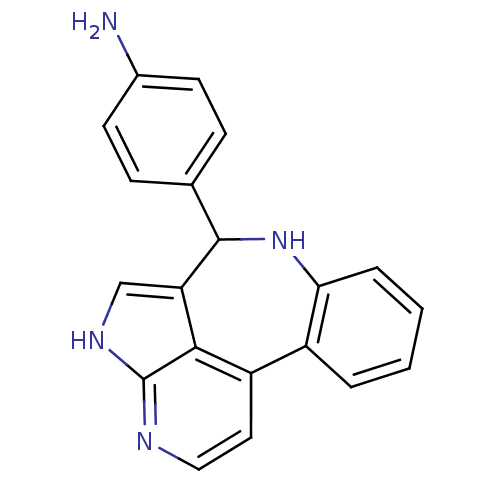
(4-{8,12,14-triazatetracyclo[8.6.1.0^{2,7}.0^{13,17...)Show InChI InChI=1S/C20H16N4/c21-13-7-5-12(6-8-13)19-16-11-23-20-18(16)15(9-10-22-20)14-3-1-2-4-17(14)24-19/h1-11,19,24H,21H2,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 20: 153-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.021
BindingDB Entry DOI: 10.7270/Q2PN95RQ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50067540
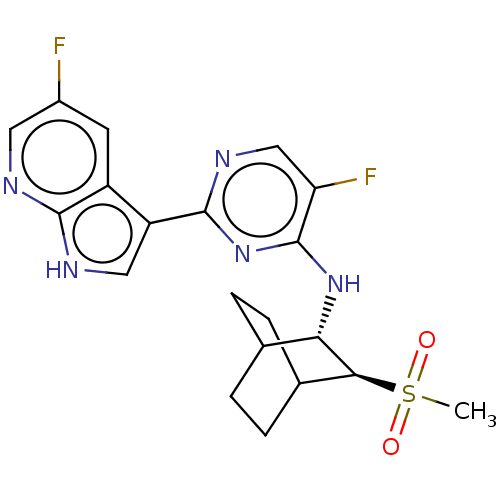
(CHEMBL3401990)Show SMILES CS(=O)(=O)[C@H]1C2CCC(CC2)[C@@H]1Nc1nc(ncc1F)-c1c[nH]c2ncc(F)cc12 |r,wU:11.13,wD:4.3,(1.06,4.97,;1.05,3.73,;1.93,3.21,;2.12,4.34,;-.29,2.98,;-1.7,3.76,;-3.03,2.98,;-3.03,1.41,;-1.7,.63,;-.96,1.7,;-2.37,2.68,;-.33,1.41,;.98,.59,;2.34,1.32,;2.39,2.86,;3.74,3.59,;5.05,2.78,;5.01,1.24,;3.65,.51,;3.61,-.72,;3.79,5.12,;5.05,5.94,;4.63,7.43,;3.1,7.48,;2.12,8.67,;.58,8.39,;.06,6.95,;-1.15,6.73,;1.06,5.75,;2.58,6.04,)| Show InChI InChI=1S/C20H21F2N5O2S/c1-30(28,29)17-11-4-2-10(3-5-11)16(17)26-20-15(22)9-25-19(27-20)14-8-24-18-13(14)6-12(21)7-23-18/h6-11,16-17H,2-5H2,1H3,(H,23,24)(H,25,26,27)/t10?,11?,16-,17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GSK3 (unknown origin) |
Bioorg Med Chem Lett 25: 1990-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.013
BindingDB Entry DOI: 10.7270/Q2V40WW5 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50067533
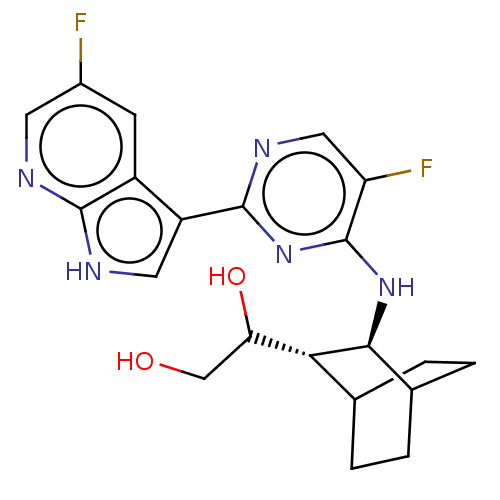
(CHEMBL3401984)Show SMILES [H][C@]1(C(O)CO)C2CCC(CC2)[C@@H]1Nc1nc(ncc1F)-c1c[nH]c2ncc(F)cc12 |r,wD:12.14,1.0,TLB:2:1:8.7:10.11,THB:13:12:8.7:10.11,(-.64,1.55,;.13,.87,;1.58,1.51,;2.6,.82,;1.69,3.04,;2.79,3.59,;1.39,,;2.86,.48,;2.23,-.94,;,-.67,;-.29,-2.25,;1.15,-1.65,;-1.3,.28,;-2.82,.5,;-3.77,-.71,;-3.2,-2.14,;-4.15,-3.35,;-5.68,-3.13,;-6.25,-1.7,;-5.3,-.49,;-5.76,.65,;-3.58,-4.78,;-4.4,-6.04,;-3.43,-7.23,;-2,-6.66,;-.62,-7.36,;.67,-6.49,;.57,-4.96,;1.59,-4.28,;-.83,-4.26,;-2.1,-5.13,)| Show InChI InChI=1S/C21H23F2N5O2/c22-12-5-13-14(7-25-19(13)24-6-12)20-26-8-15(23)21(28-20)27-18-11-3-1-10(2-4-11)17(18)16(30)9-29/h5-8,10-11,16-18,29-30H,1-4,9H2,(H,24,25)(H,26,27,28)/t10?,11?,16?,17-,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GSK3 (unknown origin) |
Bioorg Med Chem Lett 25: 1990-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.013
BindingDB Entry DOI: 10.7270/Q2V40WW5 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50067531
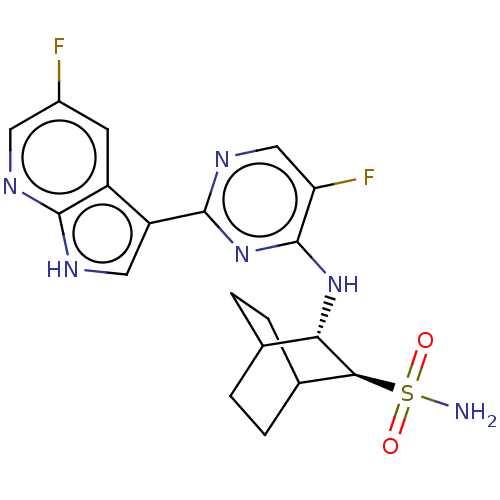
(CHEMBL3401983)Show SMILES NS(=O)(=O)[C@H]1C2CCC(CC2)[C@@H]1Nc1nc(ncc1F)-c1c[nH]c2ncc(F)cc12 |r,wU:11.13,wD:4.3,(1.06,4.97,;1.05,3.73,;1.93,3.21,;2.12,4.34,;-.29,2.98,;-1.7,3.76,;-3.03,2.98,;-3.03,1.41,;-1.7,.63,;-.96,1.7,;-2.37,2.68,;-.33,1.41,;.98,.59,;2.34,1.32,;2.39,2.86,;3.74,3.59,;5.05,2.78,;5.01,1.24,;3.65,.51,;3.61,-.72,;3.79,5.12,;5.05,5.94,;4.63,7.43,;3.1,7.48,;2.12,8.67,;.58,8.39,;.06,6.95,;-1.15,6.73,;1.06,5.75,;2.58,6.04,)| Show InChI InChI=1S/C19H20F2N6O2S/c20-11-5-12-13(7-24-17(12)23-6-11)18-25-8-14(21)19(27-18)26-15-9-1-3-10(4-2-9)16(15)30(22,28)29/h5-10,15-16H,1-4H2,(H,23,24)(H2,22,28,29)(H,25,26,27)/t9?,10?,15-,16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GSK3 (unknown origin) |
Bioorg Med Chem Lett 25: 1990-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.013
BindingDB Entry DOI: 10.7270/Q2V40WW5 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50067513
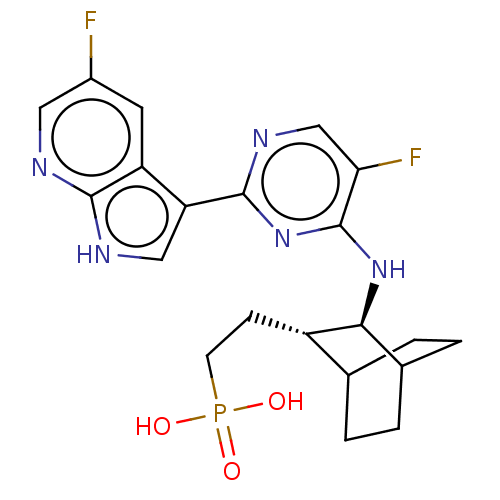
(CHEMBL3401977)Show SMILES OP(O)(=O)CC[C@H]1C2CCC(CC2)[C@@H]1Nc1nc(ncc1F)-c1c[nH]c2ncc(F)cc12 |r,wU:13.15,wD:6.5,(3.73,4.94,;3.72,3.71,;4.79,4.32,;4.78,3.08,;2.38,2.95,;1.05,3.73,;-.29,2.98,;-1.7,3.76,;-3.03,2.98,;-3.03,1.41,;-1.7,.63,;-.96,1.7,;-2.37,2.68,;-.33,1.41,;.98,.59,;.93,-.95,;-.42,-1.67,;-.48,-3.21,;.83,-4.02,;2.19,-3.3,;2.24,-1.76,;3.33,-1.18,;-1.83,-3.93,;-2.01,-5.43,;-3.53,-5.71,;-4.26,-4.36,;-5.76,-4.01,;-6.19,-2.51,;-5.12,-1.4,;-5.47,-.22,;-3.61,-1.77,;-3.19,-3.25,)| Show InChI InChI=1S/C21H24F2N5O3P/c22-13-7-15-16(9-25-19(15)24-8-13)20-26-10-17(23)21(28-20)27-18-12-3-1-11(2-4-12)14(18)5-6-32(29,30)31/h7-12,14,18H,1-6H2,(H,24,25)(H,26,27,28)(H2,29,30,31)/t11?,12?,14-,18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GSK3 (unknown origin) |
Bioorg Med Chem Lett 25: 1990-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.013
BindingDB Entry DOI: 10.7270/Q2V40WW5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50305173

(9-(2,6-difluorophenyl)-8,12,14-triazatetracyclo[8....)Show InChI InChI=1S/C20H13F2N3/c21-14-5-3-6-15(22)18(14)19-13-10-24-20-17(13)12(8-9-23-20)11-4-1-2-7-16(11)25-19/h1-10,19,25H,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 20: 153-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.021
BindingDB Entry DOI: 10.7270/Q2PN95RQ |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50067537

(CHEMBL3401987)Show SMILES [H][C@]1(C(C)O)C2CCC(CC2)[C@@H]1Nc1nc(ncc1F)-c1c[nH]c2ncc(F)cc12 |r,wD:11.13,1.0,TLB:2:1:7.6:9.10,THB:12:11:7.6:9.10,(-.64,1.54,;.13,.87,;1.58,1.51,;1.76,2.72,;2.54,.74,;1.38,,;2.86,.48,;2.23,-.94,;,-.67,;-.29,-2.25,;1.15,-1.65,;-1.3,.28,;-2.82,.5,;-3.77,-.71,;-3.2,-2.14,;-4.15,-3.35,;-5.67,-3.13,;-6.25,-1.7,;-5.29,-.49,;-5.75,.65,;-3.58,-4.78,;-4.4,-6.03,;-3.42,-7.22,;-2,-6.66,;-.62,-7.36,;.67,-6.49,;.57,-4.96,;1.59,-4.27,;-.83,-4.26,;-2.1,-5.13,)| Show InChI InChI=1S/C21H23F2N5O/c1-10(29)17-11-2-4-12(5-3-11)18(17)27-21-16(23)9-26-20(28-21)15-8-25-19-14(15)6-13(22)7-24-19/h6-12,17-18,29H,2-5H2,1H3,(H,24,25)(H,26,27,28)/t10?,11?,12?,17-,18-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) |
Bioorg Med Chem Lett 25: 1990-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.013
BindingDB Entry DOI: 10.7270/Q2V40WW5 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50067533

(CHEMBL3401984)Show SMILES [H][C@]1(C(O)CO)C2CCC(CC2)[C@@H]1Nc1nc(ncc1F)-c1c[nH]c2ncc(F)cc12 |r,wD:12.14,1.0,TLB:2:1:8.7:10.11,THB:13:12:8.7:10.11,(-.64,1.55,;.13,.87,;1.58,1.51,;2.6,.82,;1.69,3.04,;2.79,3.59,;1.39,,;2.86,.48,;2.23,-.94,;,-.67,;-.29,-2.25,;1.15,-1.65,;-1.3,.28,;-2.82,.5,;-3.77,-.71,;-3.2,-2.14,;-4.15,-3.35,;-5.68,-3.13,;-6.25,-1.7,;-5.3,-.49,;-5.76,.65,;-3.58,-4.78,;-4.4,-6.04,;-3.43,-7.23,;-2,-6.66,;-.62,-7.36,;.67,-6.49,;.57,-4.96,;1.59,-4.28,;-.83,-4.26,;-2.1,-5.13,)| Show InChI InChI=1S/C21H23F2N5O2/c22-12-5-13-14(7-25-19(13)24-6-12)20-26-8-15(23)21(28-20)27-18-11-3-1-10(2-4-11)17(18)16(30)9-29/h5-8,10-11,16-18,29-30H,1-4,9H2,(H,24,25)(H,26,27,28)/t10?,11?,16?,17-,18+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) |
Bioorg Med Chem Lett 25: 1990-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.013
BindingDB Entry DOI: 10.7270/Q2V40WW5 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50303076
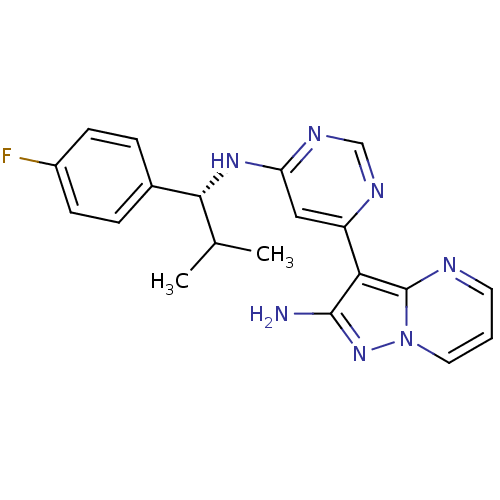
((R)-3-(6-(1-(4-fluorophenyl)-2-methylpropylamino)p...)Show SMILES CC(C)[C@@H](Nc1cc(ncn1)-c1c(N)nn2cccnc12)c1ccc(F)cc1 |r| Show InChI InChI=1S/C20H20FN7/c1-12(2)18(13-4-6-14(21)7-5-13)26-16-10-15(24-11-25-16)17-19(22)27-28-9-3-8-23-20(17)28/h3-12,18H,1-2H3,(H2,22,27)(H,24,25,26)/t18-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 |
Bioorg Med Chem Lett 19: 6529-33 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.053
BindingDB Entry DOI: 10.7270/Q2SJ1MKQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50067514

(CHEMBL3401978)Show SMILES Fc1cnc2[nH]cc(-c3ncc(F)c(N[C@H]4C5CCC(CC5)[C@@H]4Cn4oc(=O)[nH]c4=O)n3)c2c1 |r,wU:15.14,wD:22.24,(-5.47,-.22,;-5.12,-1.4,;-6.19,-2.51,;-5.76,-4.01,;-4.26,-4.36,;-3.53,-5.71,;-2.01,-5.43,;-1.83,-3.93,;-.48,-3.21,;.83,-4.02,;2.19,-3.3,;2.24,-1.76,;3.33,-1.18,;.93,-.95,;.98,.59,;-.33,1.41,;-1.7,.63,;-3.03,1.41,;-3.03,2.98,;-1.7,3.76,;-2.37,2.68,;-.96,1.7,;-.29,2.98,;1.05,3.73,;2.38,2.95,;2.5,1.43,;4,1.09,;4.49,-.04,;4.79,2.42,;3.77,3.57,;4.03,4.78,;-.42,-1.67,;-3.19,-3.25,;-3.61,-1.77,)| Show InChI InChI=1S/C22H21F2N7O3/c23-12-5-13-14(7-26-18(13)25-6-12)19-27-8-16(24)20(29-19)28-17-11-3-1-10(2-4-11)15(17)9-31-21(32)30-22(33)34-31/h5-8,10-11,15,17H,1-4,9H2,(H,25,26)(H,27,28,29)(H,30,32,33)/t10?,11?,15-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Bioorg Med Chem Lett 25: 1990-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.013
BindingDB Entry DOI: 10.7270/Q2V40WW5 |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50300196

(10-(4-hydroxyphenyl)-8,13,15-triazatetracyclo[9.6....)Show SMILES Oc1ccc(cc1)C1c2c[nH]c3nccc(-c4ccccc4NC1=O)c23 Show InChI InChI=1S/C21H15N3O2/c25-13-7-5-12(6-8-13)18-16-11-23-20-19(16)15(9-10-22-20)14-3-1-2-4-17(14)24-21(18)26/h1-11,18,25H,(H,22,23)(H,24,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cKit |
J Med Chem 52: 7938-41 (2009)
Checked by Author
Article DOI: 10.1021/jm901383u
BindingDB Entry DOI: 10.7270/Q2GF0TK0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data