

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Squalene synthase (Rattus norvegicus) | BDBM50291312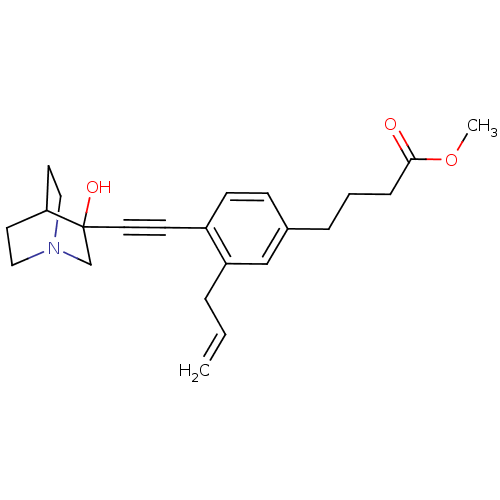 (4-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50075719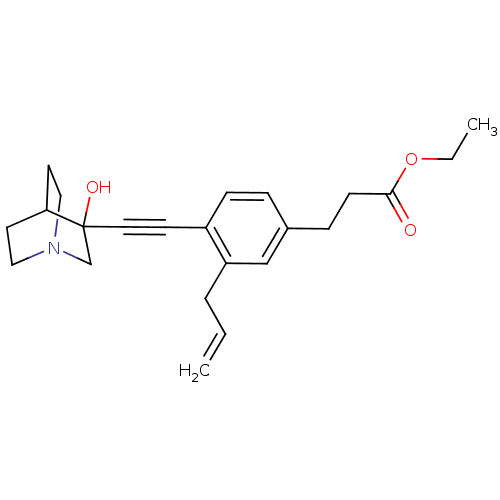 (3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50291315 (5-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50291311 (6-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50291316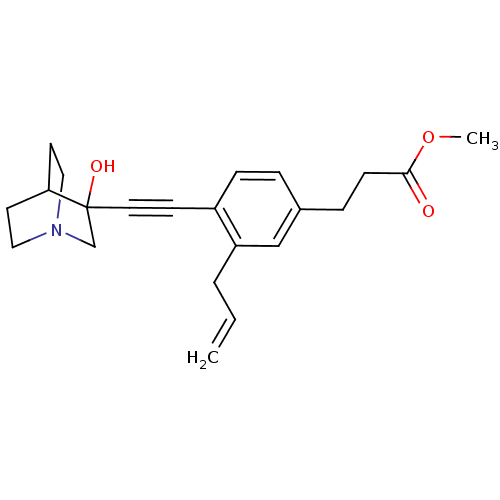 (3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50075719 (3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against human microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50291317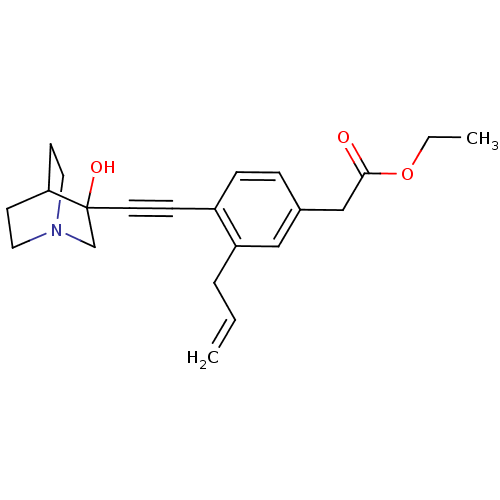 (CHEMBL154472 | [3-Allyl-4-(3-hydroxy-1-aza-bicyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50291318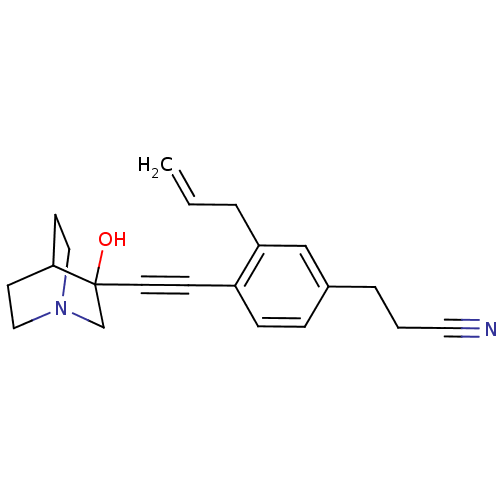 (3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | >250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50291314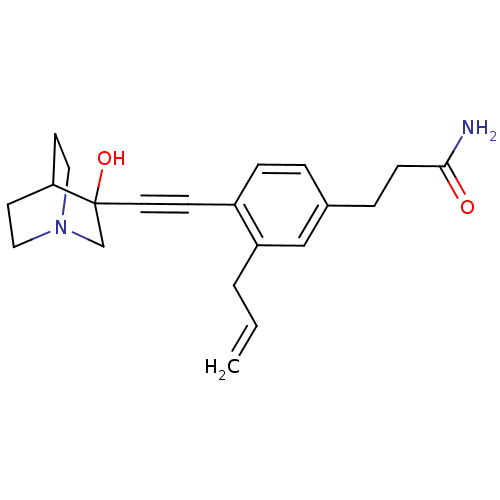 (3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | >250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50291313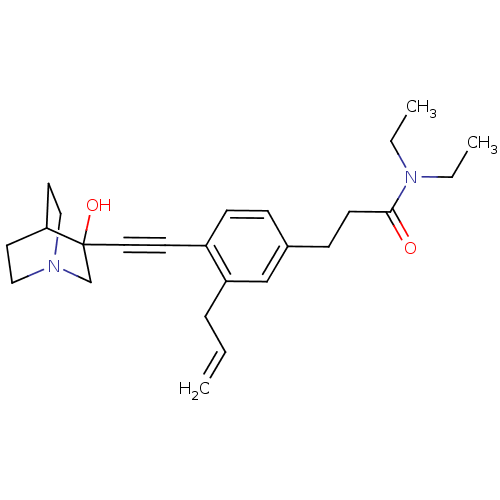 (3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | >250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50291319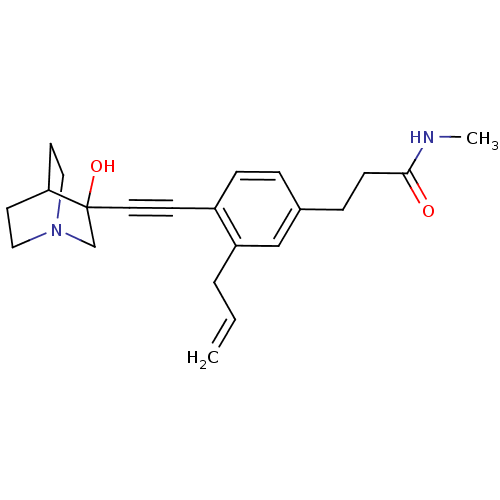 (3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | >250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50075719 (3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Compound was measured for the inhibition of rat microsomal squalene synthase enzyme | J Med Chem 42: 1306-11 (1999) Article DOI: 10.1021/jm990038q BindingDB Entry DOI: 10.7270/Q23X85TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052351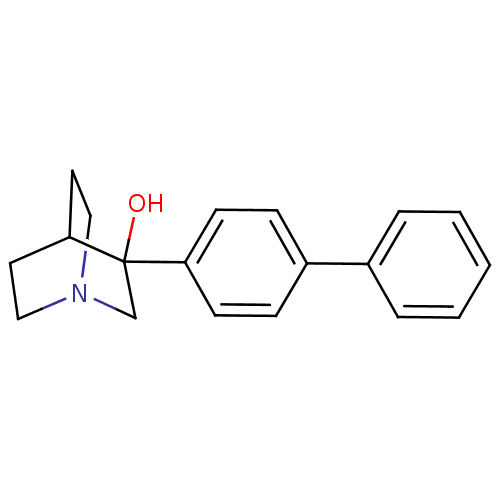 (3-Biphenyl-4-yl-1-aza-bicyclo[2.2.2]octan-3-ol | 3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Compound was measured for the inhibition of rat microsomal squalene synthase enzyme | J Med Chem 42: 1306-11 (1999) Article DOI: 10.1021/jm990038q BindingDB Entry DOI: 10.7270/Q23X85TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Homo sapiens (Human)) | BDBM50095601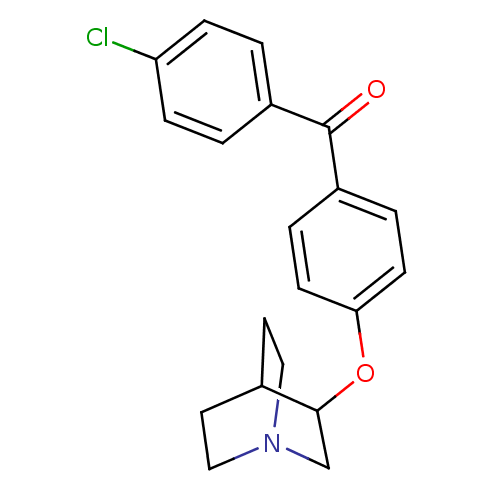 (CHEMBL25405 | [4-(1-Aza-bicyclo[2.2.2]oct-3-yloxy)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Compound was tested for its inhibition against human OSC enzyme | J Med Chem 43: 4964-72 (2001) BindingDB Entry DOI: 10.7270/Q2WW7GX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Homo sapiens (Human)) | BDBM50095598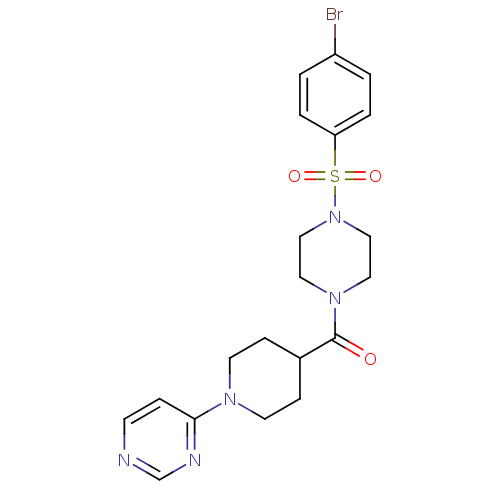 (CHEMBL70223 | [4-(4-Bromo-benzenesulfonyl)-piperaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Compound was tested for its inhibition against human OSC enzyme | J Med Chem 43: 4964-72 (2001) BindingDB Entry DOI: 10.7270/Q2WW7GX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Rattus norvegicus) | BDBM50095601 (CHEMBL25405 | [4-(1-Aza-bicyclo[2.2.2]oct-3-yloxy)...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description In vitro percent inhibitory activityof the compound against rat microsomal OSC enzyme at 1 microM concentration | J Med Chem 43: 4964-72 (2001) BindingDB Entry DOI: 10.7270/Q2WW7GX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Rattus norvegicus) | BDBM50095600 (CHEMBL148268 | [4-(4-Iodo-benzenesulfonyl)-piperaz...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description In vitro inhibitory activityof the compound against rat microsomal 2,3-Oxidosqualene cyclase-Lanosterol synthase (OSC) | J Med Chem 43: 4964-72 (2001) BindingDB Entry DOI: 10.7270/Q2WW7GX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Rattus norvegicus) | BDBM50095599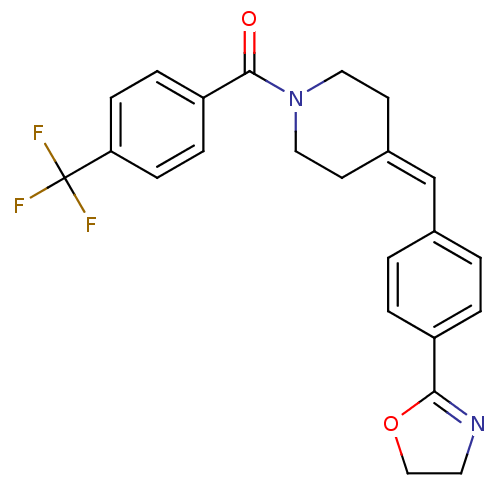 (CHEMBL25285 | {4-[4-(4,5-Dihydro-oxazol-2-yl)-benz...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description In vitro inhibitory activityof the compound against rat microsomal 2,3-Oxidosqualene lanosterol cyclase (OSC) | J Med Chem 43: 4964-72 (2001) BindingDB Entry DOI: 10.7270/Q2WW7GX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Rattus norvegicus) | BDBM50052351 (3-Biphenyl-4-yl-1-aza-bicyclo[2.2.2]octan-3-ol | 3...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of compound was measured on rat microsomal Oxidosqualene-lanosterol cyclase | J Med Chem 42: 1306-11 (1999) Article DOI: 10.1021/jm990038q BindingDB Entry DOI: 10.7270/Q23X85TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Rattus norvegicus) | BDBM50075719 (3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of compound was measured on rat microsomal Oxidosqualene-lanosterol cyclase | J Med Chem 42: 1306-11 (1999) Article DOI: 10.1021/jm990038q BindingDB Entry DOI: 10.7270/Q23X85TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Homo sapiens (Human)) | BDBM50095597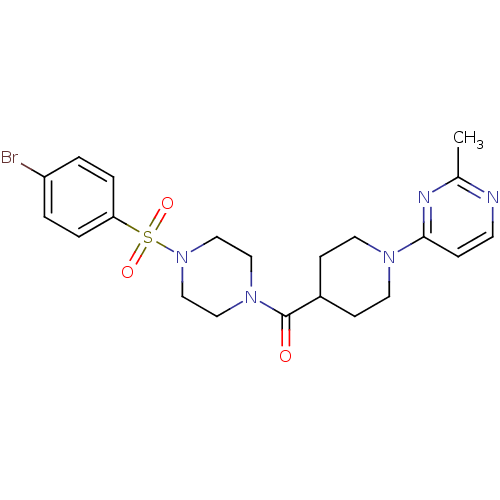 (CHEMBL359233 | [4-(4-Bromo-benzenesulfonyl)-pipera...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Compound was tested for its inhibition against human OSC enzyme | J Med Chem 43: 4964-72 (2001) BindingDB Entry DOI: 10.7270/Q2WW7GX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Rattus norvegicus) | BDBM50095598 (CHEMBL70223 | [4-(4-Bromo-benzenesulfonyl)-piperaz...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Compound was tested for its inhibition against rat OSC enzyme | J Med Chem 43: 4964-72 (2001) BindingDB Entry DOI: 10.7270/Q2WW7GX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Homo sapiens (Human)) | BDBM50095595 ((4-Benzenesulfonyl-piperazin-1-yl)-(3,4,5,6-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 143 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Compound was tested for its inhibition against human OSC enzyme | J Med Chem 43: 4964-72 (2001) BindingDB Entry DOI: 10.7270/Q2WW7GX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Homo sapiens (Human)) | BDBM50095596 (CHEMBL70222 | [4-(4-Bromo-benzenesulfonyl)-piperaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 161 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Compound was tested for its inhibition against human OSC enzyme | J Med Chem 43: 4964-72 (2001) BindingDB Entry DOI: 10.7270/Q2WW7GX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Rattus norvegicus) | BDBM50095596 (CHEMBL70222 | [4-(4-Bromo-benzenesulfonyl)-piperaz...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 228 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description In vitro inhibitory activityof the compound against rat microsomal 2,3-Oxidosqualene lanosterol cyclase (OSC) | J Med Chem 43: 4964-72 (2001) BindingDB Entry DOI: 10.7270/Q2WW7GX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Rattus norvegicus) | BDBM50095595 ((4-Benzenesulfonyl-piperazin-1-yl)-(3,4,5,6-tetrah...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 326 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description In vitro inhibitory activityof the compound against rat microsomal 2,3-Oxidosqualene lanosterol cyclase (OSC) | J Med Chem 43: 4964-72 (2001) BindingDB Entry DOI: 10.7270/Q2WW7GX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Rattus norvegicus) | BDBM50095597 (CHEMBL359233 | [4-(4-Bromo-benzenesulfonyl)-pipera...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Compound was tested for its inhibition against rat OSC enzyme | J Med Chem 43: 4964-72 (2001) BindingDB Entry DOI: 10.7270/Q2WW7GX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1/Tubulin beta-2B chain (Bos taurus) | BDBM50127066 (5-[2-(2,5-Dimethoxy-phenyl)-ethyl]-N-[2-(4-fluoro-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization was determined at 1.2 mg/mL concentration | J Med Chem 46: 1670-82 (2003) Article DOI: 10.1021/jm020292+ BindingDB Entry DOI: 10.7270/Q2HD7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1/Tubulin beta-2B chain (Bos taurus) | BDBM50009568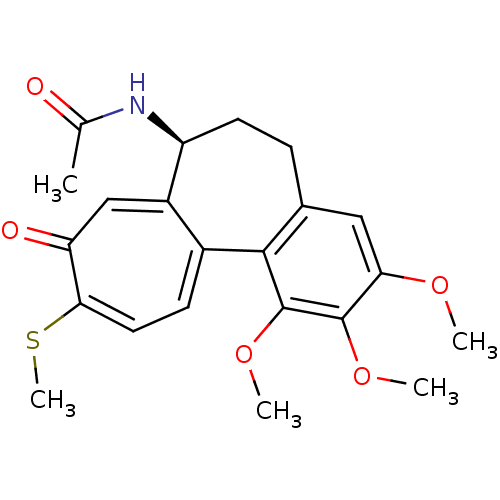 ((Thiocolchicine)N-(1,2,3-Trimethoxy-10-methylsulfa...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization was determined at 1.2 mg/mL concentration | J Med Chem 46: 1670-82 (2003) Article DOI: 10.1021/jm020292+ BindingDB Entry DOI: 10.7270/Q2HD7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1/Tubulin beta-2B chain (Bos taurus) | BDBM50127073 (5-[2-(2,5-Dimethoxy-phenyl)-acetyl]-N-[2-(4-fluoro...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization was determined at 1.2 mg/mL concentration | J Med Chem 46: 1670-82 (2003) Article DOI: 10.1021/jm020292+ BindingDB Entry DOI: 10.7270/Q2HD7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1/Tubulin beta-2B chain (Bos taurus) | BDBM50005480 ((-)-combretastatin | (Z)-3'-hydroxy-3,4,4',5-tetra...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization was determined at 1.2 mg/mL concentration | J Med Chem 46: 1670-82 (2003) Article DOI: 10.1021/jm020292+ BindingDB Entry DOI: 10.7270/Q2HD7V1V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Similar to alpha-tubulin isoform 1/Tubulin beta-2B chain (Bos taurus) | BDBM50035218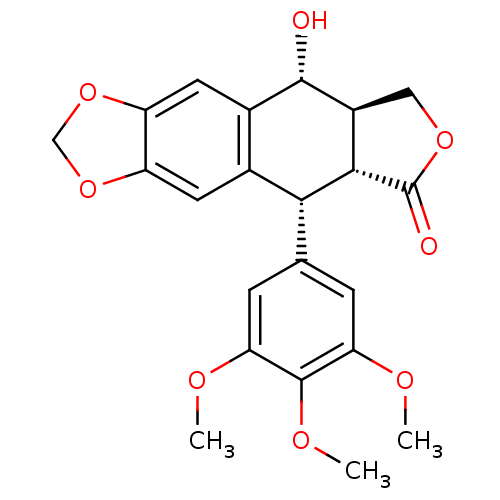 (CHEMBL61 | PODOFILOX | Podophyllinic acid lactone ...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization was determined at 1.2 mg/mL concentration | J Med Chem 46: 1670-82 (2003) Article DOI: 10.1021/jm020292+ BindingDB Entry DOI: 10.7270/Q2HD7V1V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Similar to alpha-tubulin isoform 1/Tubulin beta-2B chain (Bos taurus) | BDBM50127070 (5-[2-(2,5-Dihydroxy-phenyl)-vinyl]-N-[2-(4-fluoro-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization was determined at 1.2 mg/mL concentration | J Med Chem 46: 1670-82 (2003) Article DOI: 10.1021/jm020292+ BindingDB Entry DOI: 10.7270/Q2HD7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1/Tubulin beta-2B chain (Bos taurus) | BDBM50127061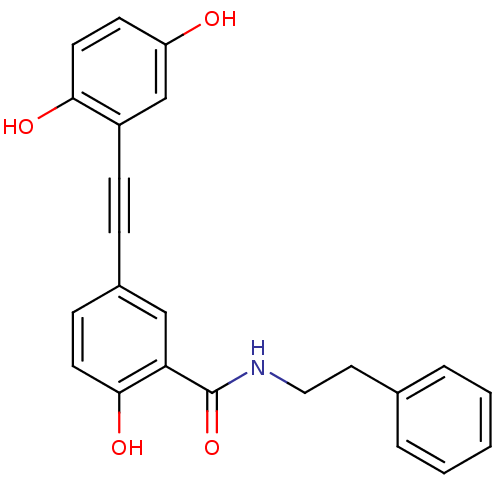 (5-(2,5-Dihydroxy-phenylethynyl)-2-hydroxy-N-phenet...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization was determined at 1.2 mg/mL concentration | J Med Chem 46: 1670-82 (2003) Article DOI: 10.1021/jm020292+ BindingDB Entry DOI: 10.7270/Q2HD7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1/Tubulin beta-2B chain (Bos taurus) | BDBM50127068 (5-[2-(2,5-Dihydroxy-phenyl)-vinyl]-2-hydroxy-N-phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization was determined at 1.2 mg/mL concentration | J Med Chem 46: 1670-82 (2003) Article DOI: 10.1021/jm020292+ BindingDB Entry DOI: 10.7270/Q2HD7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1/Tubulin beta-2B chain (Bos taurus) | BDBM50127064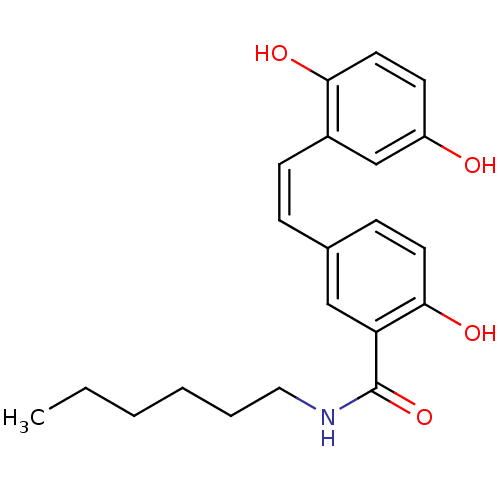 (5-[2-(2,5-Dihydroxy-phenyl)-vinyl]-N-hexyl-2-hydro...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization was determined at 1.2 mg/mL concentration | J Med Chem 46: 1670-82 (2003) Article DOI: 10.1021/jm020292+ BindingDB Entry DOI: 10.7270/Q2HD7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1/Tubulin beta-2B chain (Bos taurus) | BDBM50127077 (5-[2-(2,5-Dihydroxy-phenyl)-vinyl]-N-[2-(4-fluoro-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization was determined at 1.2 mg/mL concentration | J Med Chem 46: 1670-82 (2003) Article DOI: 10.1021/jm020292+ BindingDB Entry DOI: 10.7270/Q2HD7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1/Tubulin beta-2B chain (Bos taurus) | BDBM50127063 (5-[2-(2,5-Dihydroxy-phenyl)-vinyl]-N-hexyl-2-hydro...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization was determined at 1.2 mg/mL concentration | J Med Chem 46: 1670-82 (2003) Article DOI: 10.1021/jm020292+ BindingDB Entry DOI: 10.7270/Q2HD7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1/Tubulin beta-2B chain (Bos taurus) | BDBM50127078 (5-[2-(2,5-Dihydroxy-phenyl)-vinyl]-2-hydroxy-N-phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization was determined at 1.2 mg/mL concentration | J Med Chem 46: 1670-82 (2003) Article DOI: 10.1021/jm020292+ BindingDB Entry DOI: 10.7270/Q2HD7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1/Tubulin beta-2B chain (Bos taurus) | BDBM50127076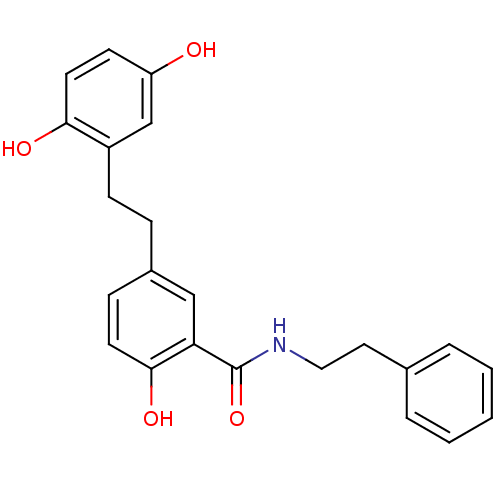 (5-[2-(2,5-Dihydroxy-phenyl)-ethyl]-2-hydroxy-N-phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization was determined at 1.2 mg/mL concentration | J Med Chem 46: 1670-82 (2003) Article DOI: 10.1021/jm020292+ BindingDB Entry DOI: 10.7270/Q2HD7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1/Tubulin beta-2B chain (Bos taurus) | BDBM50127065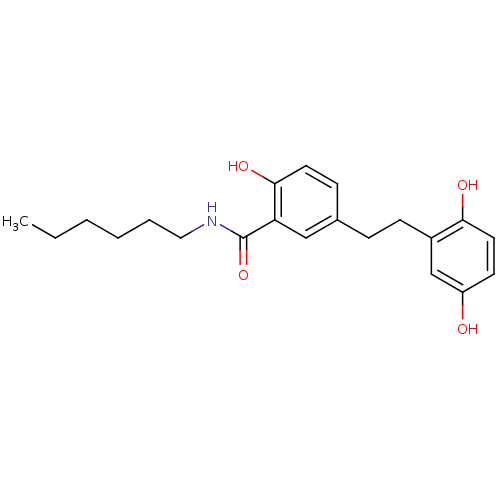 (5-[2-(2,5-Dihydroxy-phenyl)-ethyl]-N-hexyl-2-hydro...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization was determined at 1.2 mg/mL concentration | J Med Chem 46: 1670-82 (2003) Article DOI: 10.1021/jm020292+ BindingDB Entry DOI: 10.7270/Q2HD7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1/Tubulin beta-2B chain (Bos taurus) | BDBM50102416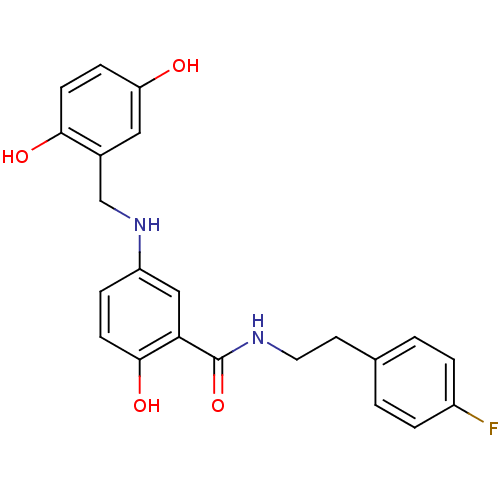 (5-(2,5-Dihydroxy-benzylamino)-N-[2-(4-fluoro-pheny...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization was determined at 1.2 mg/mL concentration | J Med Chem 46: 1670-82 (2003) Article DOI: 10.1021/jm020292+ BindingDB Entry DOI: 10.7270/Q2HD7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin alpha-1A chain (Sus scrofa (Pig)) | BDBM50119653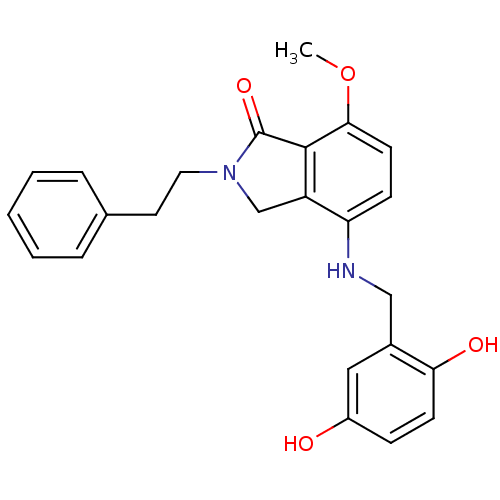 (4-(2,5-Dihydroxy-benzylamino)-7-methoxy-2-phenethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of tubulin polymerizationat concentration 10 microM | J Med Chem 45: 4774-85 (2002) BindingDB Entry DOI: 10.7270/Q2V40TJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1/Tubulin beta-2B chain (Bos taurus) | BDBM50127071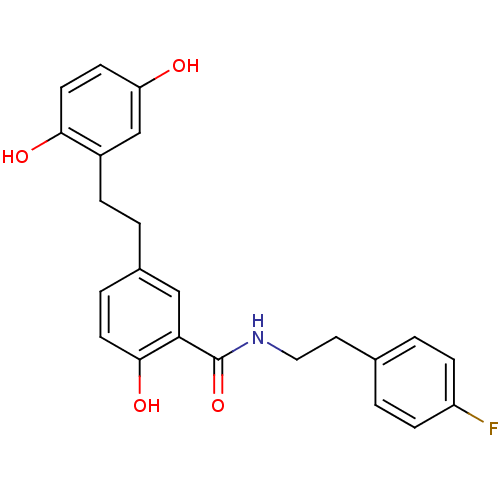 (5-[2-(2,5-Dihydroxy-phenyl)-ethyl]-N-[2-(4-fluoro-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization was determined at 1.2 mg/mL concentration | J Med Chem 46: 1670-82 (2003) Article DOI: 10.1021/jm020292+ BindingDB Entry DOI: 10.7270/Q2HD7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1/Tubulin beta-2B chain (Bos taurus) | BDBM50127067 (5-(2,5-Dimethoxy-phenylethynyl)-2-methoxy-benzoic ...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization was determined at 1.2 mg/mL concentration | J Med Chem 46: 1670-82 (2003) Article DOI: 10.1021/jm020292+ BindingDB Entry DOI: 10.7270/Q2HD7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1/Tubulin beta-2B chain (Bos taurus) | BDBM50127062 (5-(2,5-Dimethoxy-phenylethynyl)-2-methoxy-benzoic ...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization was determined at 1.2 mg/mL concentration | J Med Chem 46: 1670-82 (2003) Article DOI: 10.1021/jm020292+ BindingDB Entry DOI: 10.7270/Q2HD7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1/Tubulin beta-2B chain (Bos taurus) | BDBM50127069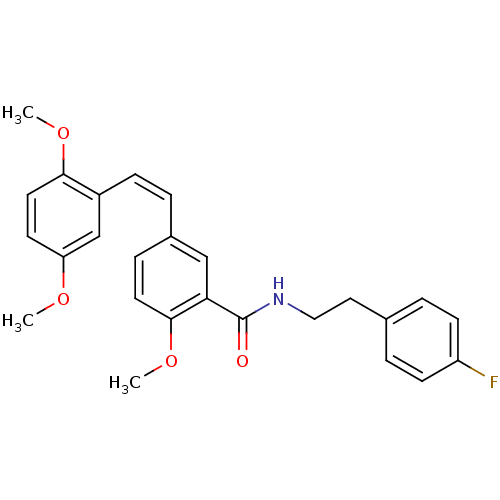 (5-[2-(2,5-Dimethoxy-phenyl)-vinyl]-N-[2-(4-fluoro-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization was determined at 1.2 mg/mL concentration | J Med Chem 46: 1670-82 (2003) Article DOI: 10.1021/jm020292+ BindingDB Entry DOI: 10.7270/Q2HD7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1/Tubulin beta-2B chain (Bos taurus) | BDBM50127072 (5-(2,5-Dimethoxy-phenylethynyl)-N-[2-(4-fluoro-phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization was determined at 1.2 mg/mL concentration | J Med Chem 46: 1670-82 (2003) Article DOI: 10.1021/jm020292+ BindingDB Entry DOI: 10.7270/Q2HD7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1/Tubulin beta-2B chain (Bos taurus) | BDBM50127060 (5-(2,5-Dimethoxy-phenylethynyl)-N-hexyl-2-methoxy-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization was determined at 1.2 mg/mL concentration | J Med Chem 46: 1670-82 (2003) Article DOI: 10.1021/jm020292+ BindingDB Entry DOI: 10.7270/Q2HD7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1/Tubulin beta-2B chain (Bos taurus) | BDBM50127074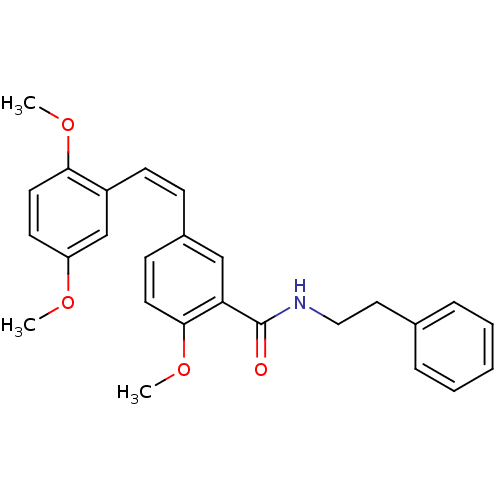 (5-[2-(2,5-Dimethoxy-phenyl)-vinyl]-2-methoxy-N-phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization was determined at 1.2 mg/mL concentration | J Med Chem 46: 1670-82 (2003) Article DOI: 10.1021/jm020292+ BindingDB Entry DOI: 10.7270/Q2HD7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 53 total ) | Next | Last >> |