

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50133040 (CHEMBL3634700) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Reversible-uncompetitive inhibition of sEH (unknown origin) by Lineweaver-Burk plot | Bioorg Med Chem Lett 25: 5097-101 (2015) Article DOI: 10.1016/j.bmcl.2015.10.014 BindingDB Entry DOI: 10.7270/Q2M61N3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM25737 (12-[(adamantan-1-ylcarbamoyl)amino]dodecanoic acid...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of sEH (unknown origin) assessed as 6-methoxy-2-naphthaldehyde formation by fluorometry assay using 40 uM cyano-(6-methoxy-naphthalen-2-yl... | Bioorg Med Chem Lett 25: 5097-101 (2015) Article DOI: 10.1016/j.bmcl.2015.10.014 BindingDB Entry DOI: 10.7270/Q2M61N3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50133040 (CHEMBL3634700) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of sEH (unknown origin) assessed as 6-methoxy-2-naphthaldehyde formation by fluorometry assay using 40 uM cyano-(6-methoxy-naphthalen-2-yl... | Bioorg Med Chem Lett 25: 5097-101 (2015) Article DOI: 10.1016/j.bmcl.2015.10.014 BindingDB Entry DOI: 10.7270/Q2M61N3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50133155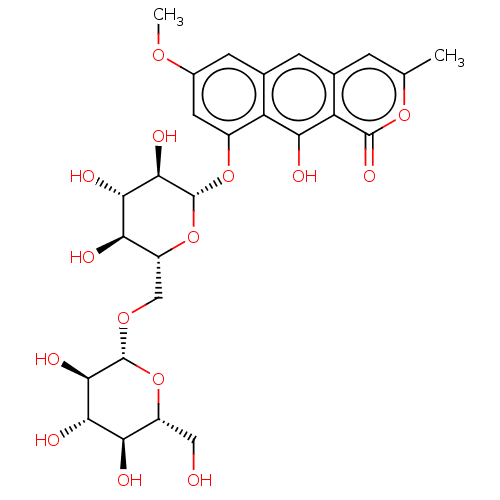 (CHEMBL3634701) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of sEH (unknown origin) assessed as 6-methoxy-2-naphthaldehyde formation by fluorometry assay using 40 uM cyano-(6-methoxy-naphthalen-2-yl... | Bioorg Med Chem Lett 25: 5097-101 (2015) Article DOI: 10.1016/j.bmcl.2015.10.014 BindingDB Entry DOI: 10.7270/Q2M61N3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50133129 (CHEBI:7716 | CHEMBL517625 | Gluco-Obtusifolin) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of sEH (unknown origin) assessed as 6-methoxy-2-naphthaldehyde formation by fluorometry assay using 40 uM cyano-(6-methoxy-naphthalen-2-yl... | Bioorg Med Chem Lett 25: 5097-101 (2015) Article DOI: 10.1016/j.bmcl.2015.10.014 BindingDB Entry DOI: 10.7270/Q2M61N3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50133044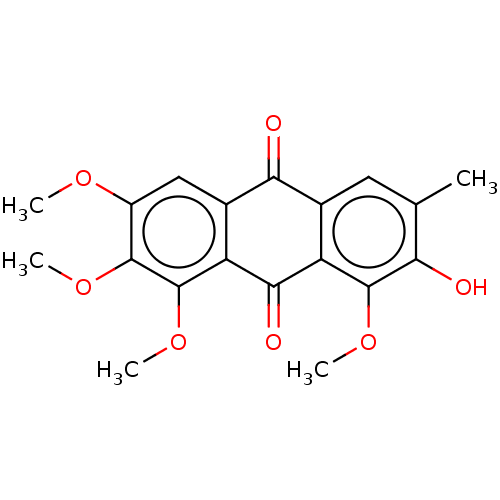 (CHEBI:81263 | Chrysoobtusin) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of sEH (unknown origin) assessed as 6-methoxy-2-naphthaldehyde formation by fluorometry assay using 40 uM cyano-(6-methoxy-naphthalen-2-yl... | Bioorg Med Chem Lett 25: 5097-101 (2015) Article DOI: 10.1016/j.bmcl.2015.10.014 BindingDB Entry DOI: 10.7270/Q2M61N3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50133046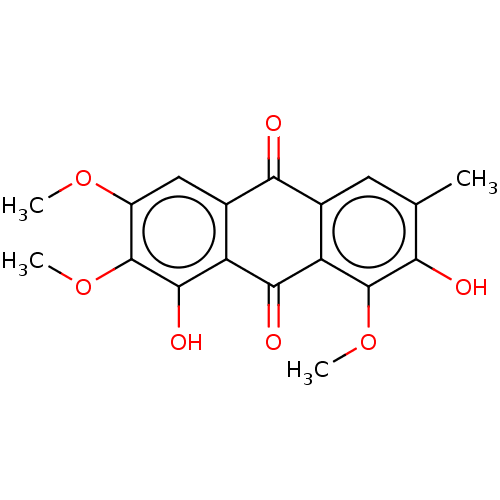 (CHEBI:80881 | CHEMBL511524) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of sEH (unknown origin) assessed as 6-methoxy-2-naphthaldehyde formation by fluorometry assay using 40 uM cyano-(6-methoxy-naphthalen-2-yl... | Bioorg Med Chem Lett 25: 5097-101 (2015) Article DOI: 10.1016/j.bmcl.2015.10.014 BindingDB Entry DOI: 10.7270/Q2M61N3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50133039 (CHEBI:28268 | CHEMBL464229 | Gluco-Aurantioobtusin) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of sEH (unknown origin) assessed as 6-methoxy-2-naphthaldehyde formation by fluorometry assay using 40 uM cyano-(6-methoxy-naphthalen-2-yl... | Bioorg Med Chem Lett 25: 5097-101 (2015) Article DOI: 10.1016/j.bmcl.2015.10.014 BindingDB Entry DOI: 10.7270/Q2M61N3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50133062 (CHEMBL3634697) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of sEH (unknown origin) assessed as 6-methoxy-2-naphthaldehyde formation by fluorometry assay using 40 uM cyano-(6-methoxy-naphthalen-2-yl... | Bioorg Med Chem Lett 25: 5097-101 (2015) Article DOI: 10.1016/j.bmcl.2015.10.014 BindingDB Entry DOI: 10.7270/Q2M61N3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM11318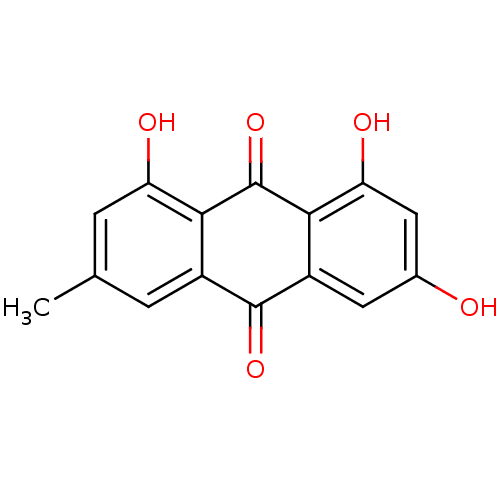 (1,3,8-trihydroxy-6-methyl-9,10-dihydroanthracene-9...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of sEH (unknown origin) assessed as 6-methoxy-2-naphthaldehyde formation by fluorometry assay using 40 uM cyano-(6-methoxy-naphthalen-2-yl... | Bioorg Med Chem Lett 25: 5097-101 (2015) Article DOI: 10.1016/j.bmcl.2015.10.014 BindingDB Entry DOI: 10.7270/Q2M61N3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50133113 (CHEMBL3634699) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of sEH (unknown origin) assessed as 6-methoxy-2-naphthaldehyde formation by fluorometry assay using 40 uM cyano-(6-methoxy-naphthalen-2-yl... | Bioorg Med Chem Lett 25: 5097-101 (2015) Article DOI: 10.1016/j.bmcl.2015.10.014 BindingDB Entry DOI: 10.7270/Q2M61N3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50133036 (CHEMBL3634702) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of sEH (unknown origin) assessed as 6-methoxy-2-naphthaldehyde formation by fluorometry assay using 40 uM cyano-(6-methoxy-naphthalen-2-yl... | Bioorg Med Chem Lett 25: 5097-101 (2015) Article DOI: 10.1016/j.bmcl.2015.10.014 BindingDB Entry DOI: 10.7270/Q2M61N3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||