 Found 2382 hits with Last Name = 'lee' and Initial = 'hs'
Found 2382 hits with Last Name = 'lee' and Initial = 'hs' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM162702

(US9056847, Fluorophore 1-cyclophellitol)Show SMILES CC1=CC(C)=[N+]2C1=C(CCCCc1cn(C[C@H]3[C@H]4O[C@H]4[C@H](O)[C@@H](O)[C@@H]3O)nn1)c1c(C)cc(C)n1[B-]2(F)F |r,c:4,7,t:1| Show InChI InChI=1S/C26H34BF2N5O4/c1-13-9-15(3)33-20(13)18(21-14(2)10-16(4)34(21)27(33,28)29)8-6-5-7-17-11-32(31-30-17)12-19-22(35)23(36)24(37)26-25(19)38-26/h9-11,19,22-26,35-37H,5-8,12H2,1-4H3/t19-,22-,23+,24-,25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.950 | n/a | 2 | n/a | n/a | n/a | n/a | 5.2 | n/a |
ACADEMISCH MEDISCH CENTRUM BIJ UNIVERSITEIT VAN AMSTERDAM
US Patent
| Assay Description
Activity of GBA was measured at 37° C. with 4-methylumbelliferyl β-D-glucopyranoside as substrate as reported previously. To determine the IC50 ... |
US Patent US9056847 (2015)
BindingDB Entry DOI: 10.7270/Q2CV4GG7 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM60418
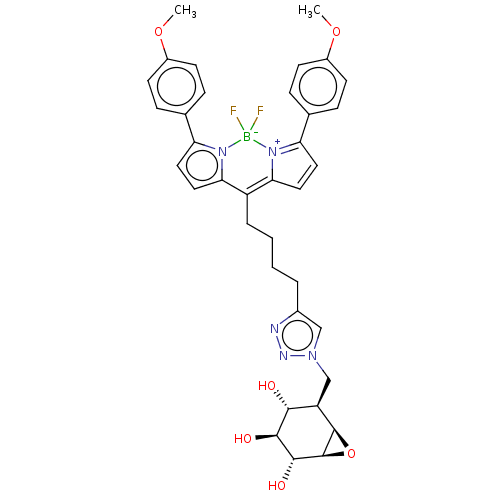
(US9056847, Fluorophore 2-cyclophellitol)Show SMILES COc1ccc(cc1)C1=[N+]2C(C=C1)=C(CCCCc1cn(C[C@H]3[C@H]4O[C@H]4[C@H](O)[C@@H](O)[C@@H]3O)nn1)c1ccc(-c3ccc(OC)cc3)n1[B-]2(F)F |r,c:12,t:9,14| Show InChI InChI=1S/C36H38BF2N5O6/c1-48-24-11-7-21(8-12-24)28-15-17-30-26(31-18-16-29(44(31)37(38,39)43(28)30)22-9-13-25(49-2)14-10-22)6-4-3-5-23-19-42(41-40-23)20-27-32(45)33(46)34(47)36-35(27)50-36/h7-19,27,32-36,45-47H,3-6,20H2,1-2H3/t27-,32-,33+,34-,35-,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.56 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
ACADEMISCH MEDISCH CENTRUM BIJ UNIVERSITEIT VAN AMSTERDAM
US Patent
| Assay Description
Activity of GBA was measured at 37° C. with 4-methylumbelliferyl β-D-glucopyranoside as substrate as reported previously. To determine the IC50 ... |
US Patent US9056847 (2015)
BindingDB Entry DOI: 10.7270/Q2CV4GG7 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM36514

(CID46912122 | MDW933, 5)Show SMILES CC1=CC(C)=[N+]2C1=C(CCCCc1cn(C[C@H]3[C@H]4O[C@H]4[C@H](O)[C@@H](O)[C@@H]3O)nn1)c1c(C)cc(C)n1[B-]2(F)F |c:4,7,t:1| Show InChI InChI=1S/C26H34BF2N5O4/c1-13-9-15(3)33-20(13)18(21-14(2)10-16(4)34(21)27(33,28)29)8-6-5-7-17-11-32(31-30-17)12-19-22(35)23(36)24(37)26-25(19)38-26/h9-11,19,22-26,35-37H,5-8,12H2,1-4H3/t19-,22-,23+,24-,25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | -48.4 | 1.24 | n/a | n/a | n/a | n/a | 5.2 | 37 |
Leiden University
| Assay Description
Enzymatic activity assay using fluorescent activity-based labeling method. |
Nat Chem Biol 6: 907-13 (2010)
Article DOI: 10.1038/nchembio.466
BindingDB Entry DOI: 10.7270/Q2FX77ST |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM36515
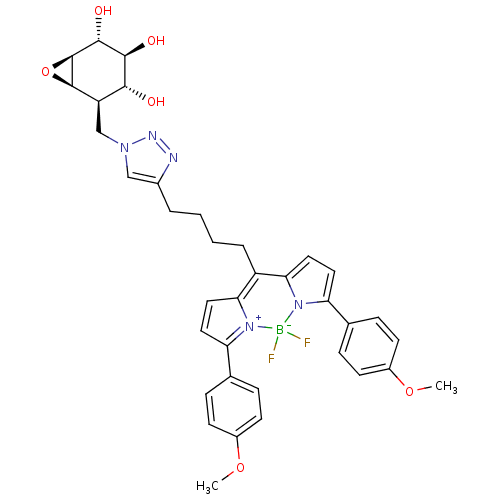
(CID46912120 | MDW941, 6)Show SMILES COc1ccc(cc1)C1=[N+]2C(C=C1)=C(CCCCc1cn(C[C@H]3[C@H]4O[C@H]4[C@H](O)[C@@H](O)[C@@H]3O)nn1)c1ccc(-c3ccc(OC)cc3)n1[B-]2(F)F |c:12,t:9,14| Show InChI InChI=1S/C36H38BF2N5O6/c1-48-24-11-7-21(8-12-24)28-15-17-30-26(31-18-16-29(44(31)37(38,39)43(28)30)22-9-13-25(49-2)14-10-22)6-4-3-5-23-19-42(41-40-23)20-27-32(45)33(46)34(47)36-35(27)50-36/h7-19,27,32-36,45-47H,3-6,20H2,1-2H3/t27-,32-,33+,34-,35-,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | -48.1 | 1.94 | n/a | n/a | n/a | n/a | 5.2 | 37 |
Leiden University
| Assay Description
Enzymatic activity assay using fluorescent activity-based labeling method. |
Nat Chem Biol 6: 907-13 (2010)
Article DOI: 10.1038/nchembio.466
BindingDB Entry DOI: 10.7270/Q2FX77ST |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50398211
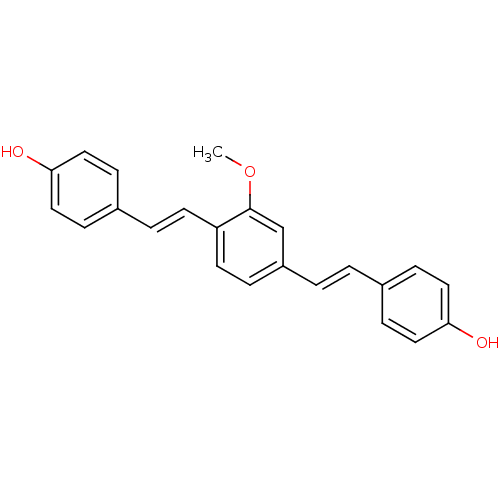
(CHEMBL2181036)Show SMILES COc1cc(\C=C\c2ccc(O)cc2)ccc1\C=C\c1ccc(O)cc1 Show InChI InChI=1S/C23H20O3/c1-26-23-16-19(3-2-17-6-12-21(24)13-7-17)5-11-20(23)10-4-18-8-14-22(25)15-9-18/h2-16,24-25H,1H3/b3-2+,10-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]chrysamine G from fibrillar amyloid beta (1 to 40) (unknown origin) after 1 hr by liquid scintillation spectrophotometric analysi... |
Bioorg Med Chem 22: 2469-81 (2014)
Article DOI: 10.1016/j.bmc.2014.02.054
BindingDB Entry DOI: 10.7270/Q2DB83C0 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50002834
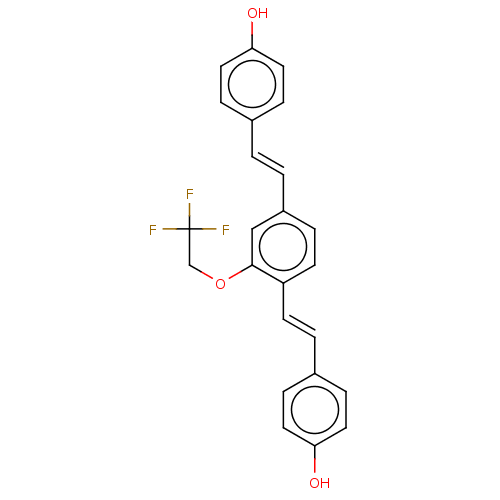
(CHEMBL3233658)Show SMILES Oc1ccc(\C=C\c2ccc(\C=C\c3ccc(O)cc3)c(OCC(F)(F)F)c2)cc1 Show InChI InChI=1S/C24H19F3O3/c25-24(26,27)16-30-23-15-19(2-1-17-5-11-21(28)12-6-17)4-10-20(23)9-3-18-7-13-22(29)14-8-18/h1-15,28-29H,16H2/b2-1+,9-3+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]chrysamine G from fibrillar amyloid beta (1 to 40) (unknown origin) after 1 hr by liquid scintillation spectrophotometric analysi... |
Bioorg Med Chem 22: 2469-81 (2014)
Article DOI: 10.1016/j.bmc.2014.02.054
BindingDB Entry DOI: 10.7270/Q2DB83C0 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM36513
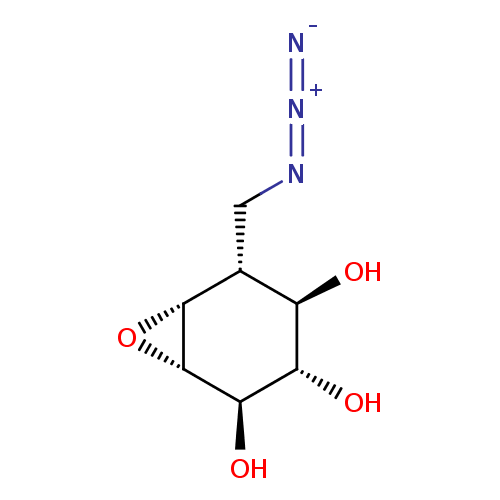
(CID46912128 | KY170, 4 | US9056847, Azido-cyclophe...)Show SMILES O[C@H]1[C@@H]2O[C@@H]2[C@H](CN=[N+]=[N-])[C@@H](O)[C@@H]1O Show InChI InChI=1S/C7H11N3O4/c8-10-9-1-2-3(11)4(12)5(13)7-6(2)14-7/h2-7,11-13H,1H2/t2-,3-,4+,5-,6-,7+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 44 | -43.7 | 120 | n/a | n/a | n/a | n/a | 5.2 | 37 |
Leiden University
| Assay Description
Enzymatic activity assay using fluorescent activity-based labeling method. |
Nat Chem Biol 6: 907-13 (2010)
Article DOI: 10.1038/nchembio.466
BindingDB Entry DOI: 10.7270/Q2FX77ST |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50002835
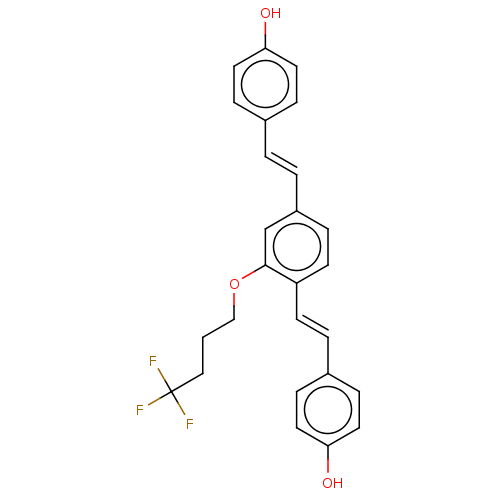
(CHEMBL3233659)Show SMILES Oc1ccc(\C=C\c2ccc(\C=C\c3ccc(O)cc3)c(OCCCC(F)(F)F)c2)cc1 Show InChI InChI=1S/C26H23F3O3/c27-26(28,29)16-1-17-32-25-18-21(3-2-19-6-12-23(30)13-7-19)5-11-22(25)10-4-20-8-14-24(31)15-9-20/h2-15,18,30-31H,1,16-17H2/b3-2+,10-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]chrysamine G from fibrillar amyloid beta (1 to 40) (unknown origin) after 1 hr by liquid scintillation spectrophotometric analysi... |
Bioorg Med Chem 22: 2469-81 (2014)
Article DOI: 10.1016/j.bmc.2014.02.054
BindingDB Entry DOI: 10.7270/Q2DB83C0 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM36513

(CID46912128 | KY170, 4 | US9056847, Azido-cyclophe...)Show SMILES O[C@H]1[C@@H]2O[C@@H]2[C@H](CN=[N+]=[N-])[C@@H](O)[C@@H]1O Show InChI InChI=1S/C7H11N3O4/c8-10-9-1-2-3(11)4(12)5(13)7-6(2)14-7/h2-7,11-13H,1H2/t2-,3-,4+,5-,6-,7+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 83 | n/a | 150 | n/a | n/a | n/a | n/a | 5.2 | n/a |
ACADEMISCH MEDISCH CENTRUM BIJ UNIVERSITEIT VAN AMSTERDAM
US Patent
| Assay Description
Activity of GBA was measured at 37° C. with 4-methylumbelliferyl β-D-glucopyranoside as substrate as reported previously. To determine the IC50 ... |
US Patent US9056847 (2015)
BindingDB Entry DOI: 10.7270/Q2CV4GG7 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50002836

(CHEMBL3233660)Show SMILES Oc1ccc(\C=C\c2ccc(\C=C\c3ccc(O)cc3)c(OC(C(F)(F)F)(C(F)(F)F)C(F)(F)F)c2)cc1 Show InChI InChI=1S/C26H17F9O3/c27-24(28,29)23(25(30,31)32,26(33,34)35)38-22-15-18(2-1-16-5-11-20(36)12-6-16)4-10-19(22)9-3-17-7-13-21(37)14-8-17/h1-15,36-37H/b2-1+,9-3+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]chrysamine G from fibrillar amyloid beta (1 to 40) (unknown origin) after 1 hr by liquid scintillation spectrophotometric analysi... |
Bioorg Med Chem 22: 2469-81 (2014)
Article DOI: 10.1016/j.bmc.2014.02.054
BindingDB Entry DOI: 10.7270/Q2DB83C0 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50376888
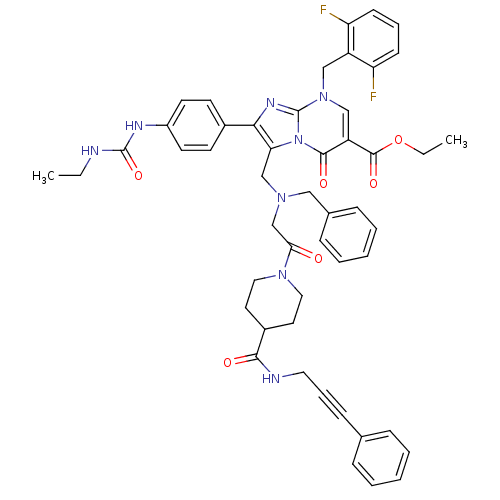
(CHEMBL415279)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2n(Cc3c(F)cccc3F)cc(C(=O)OCC)c(=O)n2c1CN(CC(=O)N1CCC(CC1)C(=O)NCC#Cc1ccccc1)Cc1ccccc1 Show InChI InChI=1S/C50H50F2N8O6/c1-3-53-49(65)55-38-22-20-36(21-23-38)45-43(60-47(63)40(48(64)66-4-2)31-59(50(60)56-45)30-39-41(51)18-11-19-42(39)52)32-57(29-35-15-9-6-10-16-35)33-44(61)58-27-24-37(25-28-58)46(62)54-26-12-17-34-13-7-5-8-14-34/h5-11,13-16,18-23,31,37H,3-4,24-30,32-33H2,1-2H3,(H,54,62)(H2,53,55,65) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [125I]triptorelin from human GnRHR expressed in CHO cells |
Bioorg Med Chem 16: 3744-58 (2008)
Article DOI: 10.1016/j.bmc.2008.01.054
BindingDB Entry DOI: 10.7270/Q2NC623R |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM36512
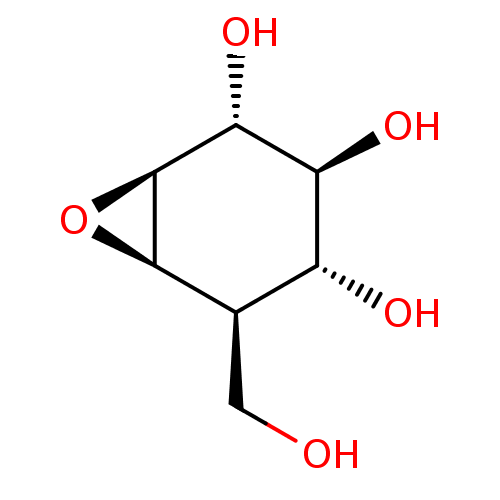
(CID164227 | Cylcophellitol, 3 | US11826435, Compou...)Show SMILES OC[C@H]1[C@H]2O[C@H]2[C@H](O)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C7H12O5/c8-1-2-3(9)4(10)5(11)7-6(2)12-7/h2-11H,1H2/t2-,3-,4+,5-,6-,7+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 152 | -40.5 | 150 | n/a | n/a | n/a | n/a | 5.2 | 37 |
Leiden University
| Assay Description
Enzymatic activity assay using fluorescent activity-based labeling method. |
Nat Chem Biol 6: 907-13 (2010)
Article DOI: 10.1038/nchembio.466
BindingDB Entry DOI: 10.7270/Q2FX77ST |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50376892

(CHEMBL406958)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2n(Cc3c(F)cccc3F)cc(C(=O)OCC)c(=O)n2c1CN(CC(=O)NCC(=O)NCC#Cc1ccccc1)Cc1ccccc1 Show InChI InChI=1S/C46H44F2N8O6/c1-3-49-45(61)52-34-22-20-33(21-23-34)42-39(56-43(59)36(44(60)62-4-2)28-55(46(56)53-42)27-35-37(47)18-11-19-38(35)48)29-54(26-32-15-9-6-10-16-32)30-41(58)51-25-40(57)50-24-12-17-31-13-7-5-8-14-31/h5-11,13-16,18-23,28H,3-4,24-27,29-30H2,1-2H3,(H,50,57)(H,51,58)(H2,49,52,61) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [125I]triptorelin from human GnRHR expressed in CHO cells |
Bioorg Med Chem 16: 3744-58 (2008)
Article DOI: 10.1016/j.bmc.2008.01.054
BindingDB Entry DOI: 10.7270/Q2NC623R |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50002833

(CHEMBL3233657)Show SMILES Oc1ccc(\C=C\c2ccc(\C=C\c3ccc(O)cc3)c(c2)C(F)(F)F)cc1 Show InChI InChI=1S/C23H17F3O2/c24-23(25,26)22-15-18(2-1-16-5-11-20(27)12-6-16)4-10-19(22)9-3-17-7-13-21(28)14-8-17/h1-15,27-28H/b2-1+,9-3+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 244 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]chrysamine G from fibrillar amyloid beta (1 to 40) (unknown origin) after 1 hr by liquid scintillation spectrophotometric analysi... |
Bioorg Med Chem 22: 2469-81 (2014)
Article DOI: 10.1016/j.bmc.2014.02.054
BindingDB Entry DOI: 10.7270/Q2DB83C0 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50376889
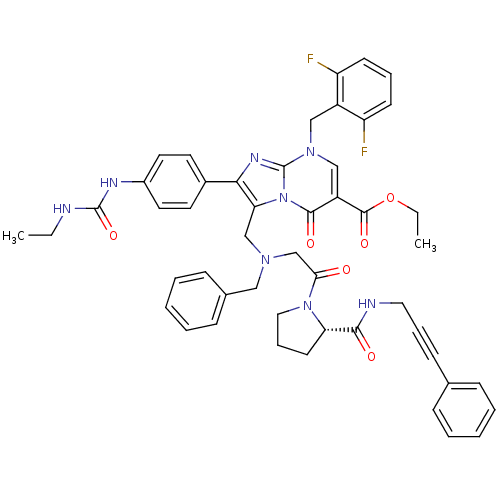
(CHEMBL260647)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2n(Cc3c(F)cccc3F)cc(C(=O)OCC)c(=O)n2c1CN(CC(=O)N1CCC[C@H]1C(=O)NCC#Cc1ccccc1)Cc1ccccc1 Show InChI InChI=1S/C49H48F2N8O6/c1-3-52-48(64)54-36-24-22-35(23-25-36)44-42(59-46(62)38(47(63)65-4-2)30-57(49(59)55-44)29-37-39(50)19-11-20-40(37)51)31-56(28-34-16-9-6-10-17-34)32-43(60)58-27-13-21-41(58)45(61)53-26-12-18-33-14-7-5-8-15-33/h5-11,14-17,19-20,22-25,30,41H,3-4,13,21,26-29,31-32H2,1-2H3,(H,53,61)(H2,52,54,64)/t41-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [125I]triptorelin from human GnRHR expressed in CHO cells |
Bioorg Med Chem 16: 3744-58 (2008)
Article DOI: 10.1016/j.bmc.2008.01.054
BindingDB Entry DOI: 10.7270/Q2NC623R |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50376893
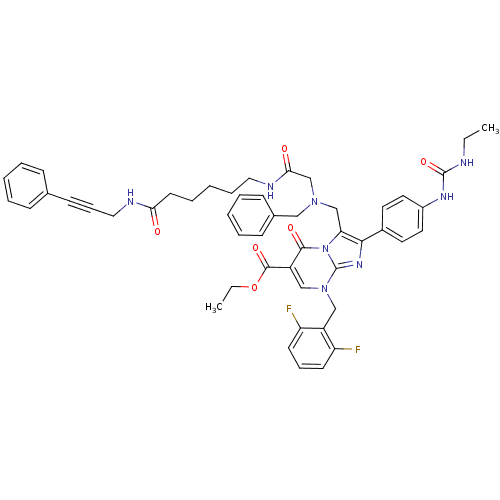
(CHEMBL262334)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2n(Cc3c(F)cccc3F)cc(C(=O)OCC)c(=O)n2c1CN(CC(=O)NCCCCCC(=O)NCC#Cc1ccccc1)Cc1ccccc1 Show InChI InChI=1S/C50H52F2N8O6/c1-3-53-49(65)56-38-26-24-37(25-27-38)46-43(60-47(63)40(48(64)66-4-2)32-59(50(60)57-46)31-39-41(51)21-14-22-42(39)52)33-58(30-36-18-10-6-11-19-36)34-45(62)55-28-13-7-12-23-44(61)54-29-15-20-35-16-8-5-9-17-35/h5-6,8-11,14,16-19,21-22,24-27,32H,3-4,7,12-13,23,28-31,33-34H2,1-2H3,(H,54,61)(H,55,62)(H2,53,56,65) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [125I]triptorelin from human GnRHR expressed in CHO cells |
Bioorg Med Chem 16: 3744-58 (2008)
Article DOI: 10.1016/j.bmc.2008.01.054
BindingDB Entry DOI: 10.7270/Q2NC623R |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50376878

(CHEMBL436906)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2n(Cc3c(F)cccc3F)cc(C(=O)OCC)c(=O)n2c1CN(CC(=O)N1CCC(CC1)C(=O)NCC#Cc1cccc(c1)C#CCNC(=O)C1CCN(CC1)C(=O)CN(Cc1c(nc2n(Cc3c(F)cccc3F)cc(C(=O)OCC)c(=O)n12)-c1ccc(NC(=O)NCC)cc1)Cc1ccccc1)Cc1ccccc1 Show InChI InChI=1S/C94H94F4N16O12/c1-5-99-91(123)103-69-36-32-65(33-37-69)83-79(113-87(119)73(89(121)125-7-3)55-111(93(113)105-83)53-71-75(95)28-16-29-76(71)96)57-107(51-63-20-11-9-12-21-63)59-81(115)109-46-40-67(41-47-109)85(117)101-44-18-26-61-24-15-25-62(50-61)27-19-45-102-86(118)68-42-48-110(49-43-68)82(116)60-108(52-64-22-13-10-14-23-64)58-80-84(66-34-38-70(39-35-66)104-92(124)100-6-2)106-94-112(54-72-77(97)30-17-31-78(72)98)56-74(88(120)114(80)94)90(122)126-8-4/h9-17,20-25,28-39,50,55-56,67-68H,5-8,40-49,51-54,57-60H2,1-4H3,(H,101,117)(H,102,118)(H2,99,103,123)(H2,100,104,124) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [125I]triptorelin from human GnRHR expressed in CHO cells |
Bioorg Med Chem 16: 3744-58 (2008)
Article DOI: 10.1016/j.bmc.2008.01.054
BindingDB Entry DOI: 10.7270/Q2NC623R |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50376877
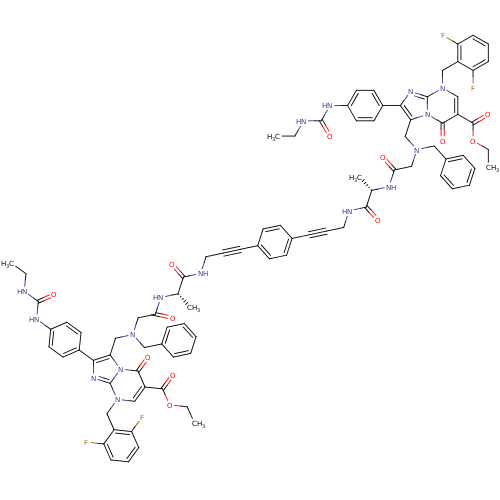
(CHEMBL259878)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2n(Cc3c(F)cccc3F)cc(C(=O)OCC)c(=O)n2c1CN(CC(=O)N[C@@H](C)C(=O)NCC#Cc1ccc(cc1)C#CCNC(=O)[C@H](C)NC(=O)CN(Cc1c(nc2n(Cc3c(F)cccc3F)cc(C(=O)OCC)c(=O)n12)-c1ccc(NC(=O)NCC)cc1)Cc1ccccc1)Cc1ccccc1 Show InChI InChI=1S/C88H86F4N16O12/c1-7-93-85(117)99-63-39-35-61(36-40-63)77-73(107-81(113)67(83(115)119-9-3)49-105(87(107)101-77)47-65-69(89)27-17-28-70(65)90)51-103(45-59-21-13-11-14-22-59)53-75(109)97-55(5)79(111)95-43-19-25-57-31-33-58(34-32-57)26-20-44-96-80(112)56(6)98-76(110)54-104(46-60-23-15-12-16-24-60)52-74-78(62-37-41-64(42-38-62)100-86(118)94-8-2)102-88-106(48-66-71(91)29-18-30-72(66)92)50-68(82(114)108(74)88)84(116)120-10-4/h11-18,21-24,27-42,49-50,55-56H,7-10,43-48,51-54H2,1-6H3,(H,95,111)(H,96,112)(H,97,109)(H,98,110)(H2,93,99,117)(H2,94,100,118)/t55-,56-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [125I]triptorelin from human GnRHR expressed in CHO cells |
Bioorg Med Chem 16: 3744-58 (2008)
Article DOI: 10.1016/j.bmc.2008.01.054
BindingDB Entry DOI: 10.7270/Q2NC623R |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50376883

(CHEMBL404611)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2n(Cc3c(F)cccc3F)ccc(=O)n2c1CN(CC(=O)NCC(=O)N1CCC(CC1)C(=O)NCC#Cc1ccccc1C#CCNC(=O)C1CCN(CC1)C(=O)CN(Cc1c(nc2n(Cc3c(F)cccc3F)cc(C(=O)OCC)c(=O)n12)-c1ccc(NC(=O)NCC)cc1)Cc1ccccc1)Cc1ccccc1 Show InChI InChI=1S/C93H93F4N17O11/c1-4-98-90(123)103-69-35-31-65(32-36-69)84-78(113-81(116)43-50-111(92(113)105-84)54-71-74(94)27-15-28-75(71)95)57-107(52-61-19-9-7-10-20-61)59-80(115)102-51-82(117)109-46-39-67(40-47-109)86(119)100-44-17-25-63-23-13-14-24-64(63)26-18-45-101-87(120)68-41-48-110(49-42-68)83(118)60-108(53-62-21-11-8-12-22-62)58-79-85(66-33-37-70(38-34-66)104-91(124)99-5-2)106-93-112(55-72-76(96)29-16-30-77(72)97)56-73(88(121)114(79)93)89(122)125-6-3/h7-16,19-24,27-38,43,50,56,67-68H,4-6,39-42,44-49,51-55,57-60H2,1-3H3,(H,100,119)(H,101,120)(H,102,115)(H2,98,103,123)(H2,99,104,124) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [125I]triptorelin from human GnRHR expressed in CHO cells |
Bioorg Med Chem 16: 3744-58 (2008)
Article DOI: 10.1016/j.bmc.2008.01.054
BindingDB Entry DOI: 10.7270/Q2NC623R |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50376873

(CHEMBL438168)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2n(Cc3c(F)cccc3F)cc(C(=O)OCC)c(=O)n2c1CN(CC(=O)N1CCC(CC1)C(=O)NCC#Cc1ccc(cc1)C#CCNC(=O)C1CCN(CC1)C(=O)CN(Cc1c(nc2n(Cc3c(F)cccc3F)cc(C(=O)OCC)c(=O)n12)-c1ccc(NC(=O)NCC)cc1)Cc1ccccc1)Cc1ccccc1 Show InChI InChI=1S/C94H94F4N16O12/c1-5-99-91(123)103-69-37-33-65(34-38-69)83-79(113-87(119)73(89(121)125-7-3)55-111(93(113)105-83)53-71-75(95)25-15-26-76(71)96)57-107(51-63-19-11-9-12-20-63)59-81(115)109-47-41-67(42-48-109)85(117)101-45-17-23-61-29-31-62(32-30-61)24-18-46-102-86(118)68-43-49-110(50-44-68)82(116)60-108(52-64-21-13-10-14-22-64)58-80-84(66-35-39-70(40-36-66)104-92(124)100-6-2)106-94-112(54-72-77(97)27-16-28-78(72)98)56-74(88(120)114(80)94)90(122)126-8-4/h9-16,19-22,25-40,55-56,67-68H,5-8,41-54,57-60H2,1-4H3,(H,101,117)(H,102,118)(H2,99,103,123)(H2,100,104,124) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [125I]triptorelin from human GnRHR expressed in CHO cells |
Bioorg Med Chem 16: 3744-58 (2008)
Article DOI: 10.1016/j.bmc.2008.01.054
BindingDB Entry DOI: 10.7270/Q2NC623R |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50376896

(CHEMBL262599)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2n(Cc3c(F)cccc3F)cc(C(=O)OCC)c(=O)n2c1CN(CC(=O)NCC(=O)NCC#Cc1ccc(cc1)C#CCNC(=O)CNC(=O)CN(Cc1c(nc2n(Cc3c(F)cccc3F)cc(C(=O)OCC)c(=O)n12)-c1ccc(NC(=O)NCC)cc1)Cc1ccccc1)Cc1ccccc1 Show InChI InChI=1S/C86H82F4N16O12/c1-5-91-83(115)97-61-37-33-59(34-38-61)77-71(105-79(111)65(81(113)117-7-3)49-103(85(105)99-77)47-63-67(87)25-15-26-68(63)88)51-101(45-57-19-11-9-12-20-57)53-75(109)95-43-73(107)93-41-17-23-55-29-31-56(32-30-55)24-18-42-94-74(108)44-96-76(110)54-102(46-58-21-13-10-14-22-58)52-72-78(60-35-39-62(40-36-60)98-84(116)92-6-2)100-86-104(48-64-69(89)27-16-28-70(64)90)50-66(80(112)106(72)86)82(114)118-8-4/h9-16,19-22,25-40,49-50H,5-8,41-48,51-54H2,1-4H3,(H,93,107)(H,94,108)(H,95,109)(H,96,110)(H2,91,97,115)(H2,92,98,116) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [125I]triptorelin from human GnRHR expressed in CHO cells |
Bioorg Med Chem 16: 3744-58 (2008)
Article DOI: 10.1016/j.bmc.2008.01.054
BindingDB Entry DOI: 10.7270/Q2NC623R |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50376890

(CHEMBL261649)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2n(Cc3c(F)cccc3F)cc(C(=O)OCC)c(=O)n2c1CN(CC(=O)NCCCC(=O)NCC#Cc1ccccc1)Cc1ccccc1 Show InChI InChI=1S/C48H48F2N8O6/c1-3-51-47(63)54-36-24-22-35(23-25-36)44-41(58-45(61)38(46(62)64-4-2)30-57(48(58)55-44)29-37-39(49)19-11-20-40(37)50)31-56(28-34-16-9-6-10-17-34)32-43(60)53-27-13-21-42(59)52-26-12-18-33-14-7-5-8-15-33/h5-11,14-17,19-20,22-25,30H,3-4,13,21,26-29,31-32H2,1-2H3,(H,52,59)(H,53,60)(H2,51,54,63) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [125I]triptorelin from human GnRHR expressed in CHO cells |
Bioorg Med Chem 16: 3744-58 (2008)
Article DOI: 10.1016/j.bmc.2008.01.054
BindingDB Entry DOI: 10.7270/Q2NC623R |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50376895
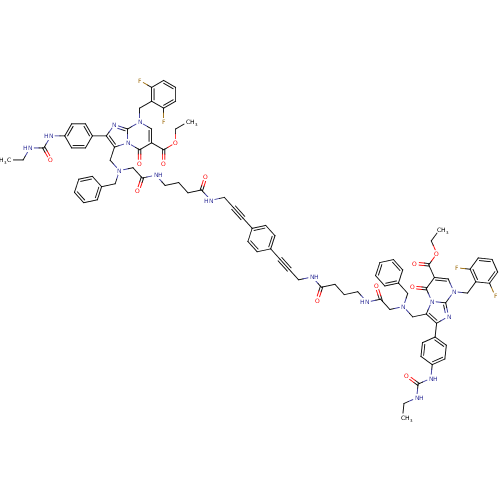
(CHEMBL406960)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2n(Cc3c(F)cccc3F)cc(C(=O)OCC)c(=O)n2c1CN(CC(=O)NCCCC(=O)NCC#Cc1ccc(cc1)C#CCNC(=O)CCCNC(=O)CN(Cc1c(nc2n(Cc3c(F)cccc3F)cc(C(=O)OCC)c(=O)n12)-c1ccc(NC(=O)NCC)cc1)Cc1ccccc1)Cc1ccccc1 Show InChI InChI=1S/C90H90F4N16O12/c1-5-95-87(119)101-65-41-37-63(38-42-65)81-75(109-83(115)69(85(117)121-7-3)53-107(89(109)103-81)51-67-71(91)27-15-28-72(67)92)55-105(49-61-21-11-9-12-22-61)57-79(113)99-47-19-31-77(111)97-45-17-25-59-33-35-60(36-34-59)26-18-46-98-78(112)32-20-48-100-80(114)58-106(50-62-23-13-10-14-24-62)56-76-82(64-39-43-66(44-40-64)102-88(120)96-6-2)104-90-108(52-68-73(93)29-16-30-74(68)94)54-70(84(116)110(76)90)86(118)122-8-4/h9-16,21-24,27-30,33-44,53-54H,5-8,19-20,31-32,45-52,55-58H2,1-4H3,(H,97,111)(H,98,112)(H,99,113)(H,100,114)(H2,95,101,119)(H2,96,102,120) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [125I]triptorelin from human GnRHR expressed in CHO cells |
Bioorg Med Chem 16: 3744-58 (2008)
Article DOI: 10.1016/j.bmc.2008.01.054
BindingDB Entry DOI: 10.7270/Q2NC623R |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50376875
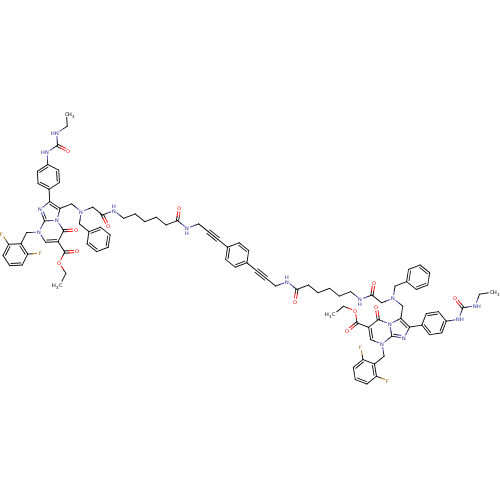
(CHEMBL260812)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2n(Cc3c(F)cccc3F)cc(C(=O)OCC)c(=O)n2c1CN(CC(=O)NCCCCCC(=O)NCC#Cc1ccc(cc1)C#CCNC(=O)CCCCCNC(=O)CN(Cc1c(nc2n(Cc3c(F)cccc3F)cc(C(=O)OCC)c(=O)n12)-c1ccc(NC(=O)NCC)cc1)Cc1ccccc1)Cc1ccccc1 Show InChI InChI=1S/C94H98F4N16O12/c1-5-99-91(123)105-69-45-41-67(42-46-69)85-79(113-87(119)73(89(121)125-7-3)57-111(93(113)107-85)55-71-75(95)31-21-32-76(71)96)59-109(53-65-25-13-9-14-26-65)61-83(117)103-49-19-11-17-35-81(115)101-51-23-29-63-37-39-64(40-38-63)30-24-52-102-82(116)36-18-12-20-50-104-84(118)62-110(54-66-27-15-10-16-28-66)60-80-86(68-43-47-70(48-44-68)106-92(124)100-6-2)108-94-112(56-72-77(97)33-22-34-78(72)98)58-74(88(120)114(80)94)90(122)126-8-4/h9-10,13-16,21-22,25-28,31-34,37-48,57-58H,5-8,11-12,17-20,35-36,49-56,59-62H2,1-4H3,(H,101,115)(H,102,116)(H,103,117)(H,104,118)(H2,99,105,123)(H2,100,106,124) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [125I]triptorelin from human GnRHR expressed in CHO cells |
Bioorg Med Chem 16: 3744-58 (2008)
Article DOI: 10.1016/j.bmc.2008.01.054
BindingDB Entry DOI: 10.7270/Q2NC623R |
More data for this
Ligand-Target Pair | |
Diacylglycerol lipase-alpha
(Homo sapiens (Human)) | BDBM50048620
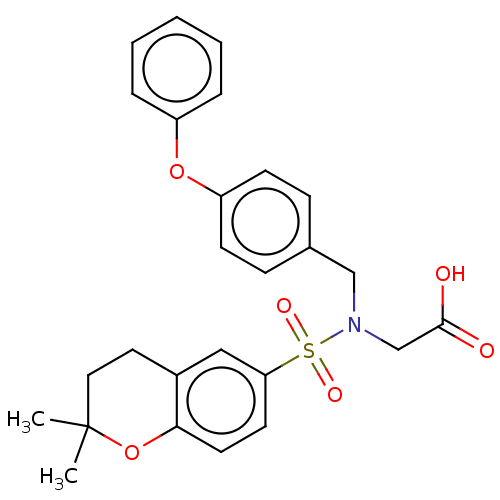
(CHEMBL3319620)Show SMILES CC1(C)CCc2cc(ccc2O1)S(=O)(=O)N(CC(O)=O)Cc1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C26H27NO6S/c1-26(2)15-14-20-16-23(12-13-24(20)33-26)34(30,31)27(18-25(28)29)17-19-8-10-22(11-9-19)32-21-6-4-3-5-7-21/h3-13,16H,14-15,17-18H2,1-2H3,(H,28,29) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of human DAGLalpha expressed in HEK293T cell membranes assessed as reduction in hydrolysis of inhibition of [14C]-sn-1-oleoyl-2-arachidono... |
J Med Chem 57: 6610-22 (2014)
Article DOI: 10.1021/jm500681z
BindingDB Entry DOI: 10.7270/Q2R49SD4 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50376899

(CHEMBL260318)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2n(Cc3c(F)cccc3F)ccc(=O)n2c1CN(CC(=O)NCCCCCC(=O)NCC#Cc1ccccc1C#CCNC(=O)CCCCCNC(=O)CN(Cc1c(nc2n(Cc3c(F)cccc3F)cc(C(=O)OCC)c(=O)n12)-c1ccc(NC(=O)NCC)cc1)Cc1ccccc1)Cc1ccccc1 Show InChI InChI=1S/C91H94F4N16O10/c1-4-96-88(119)102-68-43-39-66(40-44-68)84-77(110-83(116)47-52-108(90(110)104-84)55-70-73(92)33-21-34-74(70)93)58-106(53-62-25-11-7-12-26-62)60-81(114)100-48-19-9-15-37-79(112)98-50-23-31-64-29-17-18-30-65(64)32-24-51-99-80(113)38-16-10-20-49-101-82(115)61-107(54-63-27-13-8-14-28-63)59-78-85(67-41-45-69(46-42-67)103-89(120)97-5-2)105-91-109(56-71-75(94)35-22-36-76(71)95)57-72(86(117)111(78)91)87(118)121-6-3/h7-8,11-14,17-18,21-22,25-30,33-36,39-47,52,57H,4-6,9-10,15-16,19-20,37-38,48-51,53-56,58-61H2,1-3H3,(H,98,112)(H,99,113)(H,100,114)(H,101,115)(H2,96,102,119)(H2,97,103,120) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [125I]triptorelin from human GnRHR expressed in CHO cells |
Bioorg Med Chem 16: 3744-58 (2008)
Article DOI: 10.1016/j.bmc.2008.01.054
BindingDB Entry DOI: 10.7270/Q2NC623R |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50376876

(CHEMBL408760)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2n(Cc3c(F)cccc3F)cc(C(=O)OCC)c(=O)n2c1CN(CC(=O)N[C@@H](C(C)C)C(=O)NCC#Cc1ccc(cc1)C#CCNC(=O)[C@@H](NC(=O)CN(Cc1c(nc2n(Cc3c(F)cccc3F)cc(C(=O)OCC)c(=O)n12)-c1ccc(NC(=O)NCC)cc1)Cc1ccccc1)C(C)C)Cc1ccccc1 Show InChI InChI=1S/C92H94F4N16O12/c1-9-97-89(121)101-65-41-37-63(38-42-65)81-75(111-85(117)69(87(119)123-11-3)51-109(91(111)105-81)49-67-71(93)29-19-30-72(67)94)53-107(47-61-23-15-13-16-24-61)55-77(113)103-79(57(5)6)83(115)99-45-21-27-59-33-35-60(36-34-59)28-22-46-100-84(116)80(58(7)8)104-78(114)56-108(48-62-25-17-14-18-26-62)54-76-82(64-39-43-66(44-40-64)102-90(122)98-10-2)106-92-110(50-68-73(95)31-20-32-74(68)96)52-70(86(118)112(76)92)88(120)124-12-4/h13-20,23-26,29-44,51-52,57-58,79-80H,9-12,45-50,53-56H2,1-8H3,(H,99,115)(H,100,116)(H,103,113)(H,104,114)(H2,97,101,121)(H2,98,102,122)/t79-,80-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [125I]triptorelin from human GnRHR expressed in CHO cells |
Bioorg Med Chem 16: 3744-58 (2008)
Article DOI: 10.1016/j.bmc.2008.01.054
BindingDB Entry DOI: 10.7270/Q2NC623R |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50376874

(CHEMBL260750)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2n(Cc3c(F)cccc3F)cc(C(=O)OCC)c(=O)n2c1CN(CC(=O)N1CCC[C@H]1C(=O)NCC#Cc1ccc(cc1)C#CCNC(=O)[C@@H]1CCCN1C(=O)CN(Cc1c(nc2n(Cc3c(F)cccc3F)cc(C(=O)OCC)c(=O)n12)-c1ccc(NC(=O)NCC)cc1)Cc1ccccc1)Cc1ccccc1 Show InChI InChI=1S/C92H90F4N16O12/c1-5-97-89(121)101-65-41-37-63(38-42-65)81-77(111-85(117)69(87(119)123-7-3)53-107(91(111)103-81)51-67-71(93)27-15-28-72(67)94)55-105(49-61-21-11-9-12-22-61)57-79(113)109-47-19-31-75(109)83(115)99-45-17-25-59-33-35-60(36-34-59)26-18-46-100-84(116)76-32-20-48-110(76)80(114)58-106(50-62-23-13-10-14-24-62)56-78-82(64-39-43-66(44-40-64)102-90(122)98-6-2)104-92-108(52-68-73(95)29-16-30-74(68)96)54-70(86(118)112(78)92)88(120)124-8-4/h9-16,21-24,27-30,33-44,53-54,75-76H,5-8,19-20,31-32,45-52,55-58H2,1-4H3,(H,99,115)(H,100,116)(H2,97,101,121)(H2,98,102,122)/t75-,76-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [125I]triptorelin from human GnRHR expressed in CHO cells |
Bioorg Med Chem 16: 3744-58 (2008)
Article DOI: 10.1016/j.bmc.2008.01.054
BindingDB Entry DOI: 10.7270/Q2NC623R |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50376891

(CHEMBL439076)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2n(Cc3c(F)cccc3F)cc(C(=O)OCC)c(=O)n2c1CN(CC(=O)N[C@@H](C(C)C)C(=O)NCC#Cc1ccccc1)Cc1ccccc1 Show InChI InChI=1S/C49H50F2N8O6/c1-5-52-48(64)54-36-24-22-35(23-25-36)44-41(59-46(62)38(47(63)65-6-2)29-58(49(59)56-44)28-37-39(50)20-13-21-40(37)51)30-57(27-34-17-11-8-12-18-34)31-42(60)55-43(32(3)4)45(61)53-26-14-19-33-15-9-7-10-16-33/h7-13,15-18,20-25,29,32,43H,5-6,26-28,30-31H2,1-4H3,(H,53,61)(H,55,60)(H2,52,54,64)/t43-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [125I]triptorelin from human GnRHR expressed in CHO cells |
Bioorg Med Chem 16: 3744-58 (2008)
Article DOI: 10.1016/j.bmc.2008.01.054
BindingDB Entry DOI: 10.7270/Q2NC623R |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50044625
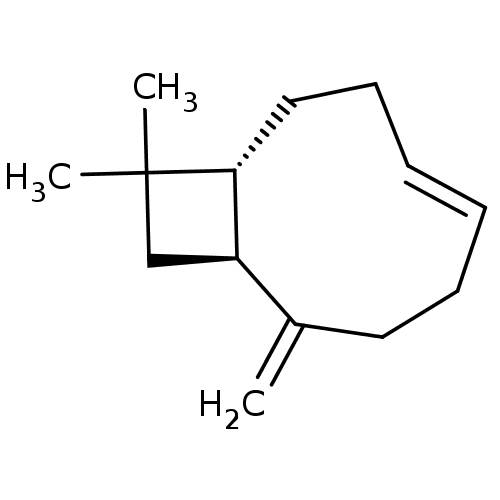
(CHEMBL3360190)Show SMILES [H][C@]12CC(C)(C)[C@]1([H])CC\C=C\CCC2=C |r,t:11| Show InChI InChI=1S/C14H22/c1-11-8-6-4-5-7-9-13-12(11)10-14(13,2)3/h4-5,12-13H,1,6-10H2,2-3H3/b5-4+/t12?,13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of CB2 receptor (unknown origin) |
Bioorg Med Chem Lett 24: 3168-74 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.112
BindingDB Entry DOI: 10.7270/Q2SN0BK2 |
More data for this
Ligand-Target Pair | |
Monoacylglycerol lipase ABHD6
(Homo sapiens (Human)) | BDBM50048620

(CHEMBL3319620)Show SMILES CC1(C)CCc2cc(ccc2O1)S(=O)(=O)N(CC(O)=O)Cc1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C26H27NO6S/c1-26(2)15-14-20-16-23(12-13-24(20)33-26)34(30,31)27(18-25(28)29)17-19-8-10-22(11-9-19)32-21-6-4-3-5-7-21/h3-13,16H,14-15,17-18H2,1-2H3,(H,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of human ABHD6 expressed in HEK293T cell membranes assessed as reduction in glycerol production using 2-arachidonoyl glycerol substrate |
J Med Chem 57: 6610-22 (2014)
Article DOI: 10.1021/jm500681z
BindingDB Entry DOI: 10.7270/Q2R49SD4 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50376880

(CHEMBL436862)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2n(Cc3c(F)cccc3F)cc(C(=O)OCC)c(=O)n2c1CN(CC(=O)NCCCC(=O)NCC#Cc1cccc(c1)C#CCNC(=O)CCCNC(=O)CN(Cc1c(nc2n(Cc3c(F)cccc3F)cc(C(=O)OCC)c(=O)n12)-c1ccc(NC(=O)NCC)cc1)Cc1ccccc1)Cc1ccccc1 Show InChI InChI=1S/C90H90F4N16O12/c1-5-95-87(119)101-65-40-36-63(37-41-65)81-75(109-83(115)69(85(117)121-7-3)53-107(89(109)103-81)51-67-71(91)30-16-31-72(67)92)55-105(49-61-22-11-9-12-23-61)57-79(113)99-46-20-34-77(111)97-44-18-28-59-26-15-27-60(48-59)29-19-45-98-78(112)35-21-47-100-80(114)58-106(50-62-24-13-10-14-25-62)56-76-82(64-38-42-66(43-39-64)102-88(120)96-6-2)104-90-108(52-68-73(93)32-17-33-74(68)94)54-70(84(116)110(76)90)86(118)122-8-4/h9-17,22-27,30-33,36-43,48,53-54H,5-8,20-21,34-35,44-47,49-52,55-58H2,1-4H3,(H,97,111)(H,98,112)(H,99,113)(H,100,114)(H2,95,101,119)(H2,96,102,120) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [125I]triptorelin from human GnRHR expressed in CHO cells |
Bioorg Med Chem 16: 3744-58 (2008)
Article DOI: 10.1016/j.bmc.2008.01.054
BindingDB Entry DOI: 10.7270/Q2NC623R |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50376885
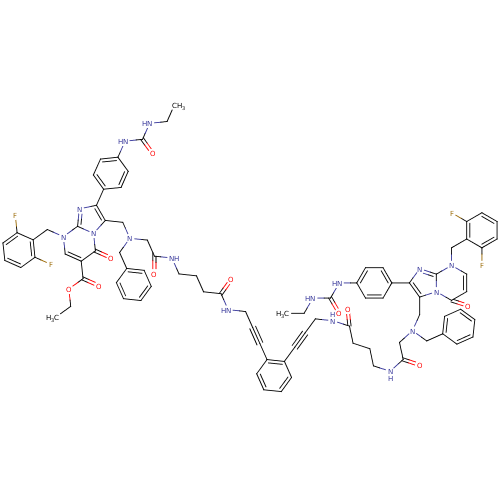
(CHEMBL262335)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2n(Cc3c(F)cccc3F)ccc(=O)n2c1CN(CC(=O)NCCCC(=O)NCC#Cc1ccccc1C#CCNC(=O)CCCNC(=O)CN(Cc1c(nc2n(Cc3c(F)cccc3F)cc(C(=O)OCC)c(=O)n12)-c1ccc(NC(=O)NCC)cc1)Cc1ccccc1)Cc1ccccc1 Show InChI InChI=1S/C87H86F4N16O10/c1-4-92-84(115)98-64-39-35-62(36-40-64)80-73(106-79(112)43-48-104(86(106)100-80)51-66-69(88)29-15-30-70(66)89)54-102(49-58-21-9-7-10-22-58)56-77(110)96-46-19-33-75(108)94-44-17-27-60-25-13-14-26-61(60)28-18-45-95-76(109)34-20-47-97-78(111)57-103(50-59-23-11-8-12-24-59)55-74-81(63-37-41-65(42-38-63)99-85(116)93-5-2)101-87-105(52-67-71(90)31-16-32-72(67)91)53-68(82(113)107(74)87)83(114)117-6-3/h7-16,21-26,29-32,35-43,48,53H,4-6,19-20,33-34,44-47,49-52,54-57H2,1-3H3,(H,94,108)(H,95,109)(H,96,110)(H,97,111)(H2,92,98,115)(H2,93,99,116) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [125I]triptorelin from human GnRHR expressed in CHO cells |
Bioorg Med Chem 16: 3744-58 (2008)
Article DOI: 10.1016/j.bmc.2008.01.054
BindingDB Entry DOI: 10.7270/Q2NC623R |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50376884

(CHEMBL429690)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2n(Cc3c(F)cccc3F)ccc(=O)n2c1CN(CC(=O)NCC(=O)N1CCC[C@H]1C(=O)NCC#Cc1ccccc1C#CCNC(=O)[C@@H]1CCCN1C(=O)CNC(=O)CN(Cc1c(nc2n(Cc3c(F)cccc3F)cc(C(=O)OCC)c(=O)n12)-c1ccc(NC(=O)NCC)cc1)Cc1ccccc1)Cc1ccccc1 Show InChI InChI=1S/C93H92F4N18O12/c1-4-98-90(125)104-66-39-35-64(36-40-66)84-77(114-81(118)43-48-110(92(114)106-84)53-68-71(94)29-15-30-72(68)95)56-108(51-60-21-9-7-10-22-60)58-79(116)102-49-82(119)112-46-19-33-75(112)86(121)100-44-17-27-62-25-13-14-26-63(62)28-18-45-101-87(122)76-34-20-47-113(76)83(120)50-103-80(117)59-109(52-61-23-11-8-12-24-61)57-78-85(65-37-41-67(42-38-65)105-91(126)99-5-2)107-93-111(54-69-73(96)31-16-32-74(69)97)55-70(88(123)115(78)93)89(124)127-6-3/h7-16,21-26,29-32,35-43,48,55,75-76H,4-6,19-20,33-34,44-47,49-54,56-59H2,1-3H3,(H,100,121)(H,101,122)(H,102,116)(H,103,117)(H2,98,104,125)(H2,99,105,126)/t75-,76-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [125I]triptorelin from human GnRHR expressed in CHO cells |
Bioorg Med Chem 16: 3744-58 (2008)
Article DOI: 10.1016/j.bmc.2008.01.054
BindingDB Entry DOI: 10.7270/Q2NC623R |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50376897

(CHEMBL406084)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2n(Cc3c(F)cccc3F)cc(C(=O)OCC)c(=O)n2c1CN(CC(=O)NCCCCCC(=O)NCC#Cc1cccc(c1)C#CCNC(=O)CCCCCNC(=O)CN(Cc1c(nc2n(Cc3c(F)cccc3F)cc(C(=O)OCC)c(=O)n12)-c1ccc(NC(=O)NCC)cc1)Cc1ccccc1)Cc1ccccc1 Show InChI InChI=1S/C94H98F4N16O12/c1-5-99-91(123)105-69-44-40-67(41-45-69)85-79(113-87(119)73(89(121)125-7-3)57-111(93(113)107-85)55-71-75(95)34-22-35-76(71)96)59-109(53-65-26-13-9-14-27-65)61-83(117)103-48-19-11-17-38-81(115)101-50-24-32-63-30-21-31-64(52-63)33-25-51-102-82(116)39-18-12-20-49-104-84(118)62-110(54-66-28-15-10-16-29-66)60-80-86(68-42-46-70(47-43-68)106-92(124)100-6-2)108-94-112(56-72-77(97)36-23-37-78(72)98)58-74(88(120)114(80)94)90(122)126-8-4/h9-10,13-16,21-23,26-31,34-37,40-47,52,57-58H,5-8,11-12,17-20,38-39,48-51,53-56,59-62H2,1-4H3,(H,101,115)(H,102,116)(H,103,117)(H,104,118)(H2,99,105,123)(H2,100,106,124) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [125I]triptorelin from human GnRHR expressed in CHO cells |
Bioorg Med Chem 16: 3744-58 (2008)
Article DOI: 10.1016/j.bmc.2008.01.054
BindingDB Entry DOI: 10.7270/Q2NC623R |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50376879

(CHEMBL266172)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2n(Cc3c(F)cccc3F)cc(C(=O)OCC)c(=O)n2c1CN(CC(=O)N1CCC[C@H]1C(=O)NCC#Cc1cccc(c1)C#CCNC(=O)[C@@H]1CCCN1C(=O)CN(Cc1c(nc2n(Cc3c(F)cccc3F)cc(C(=O)OCC)c(=O)n12)-c1ccc(NC(=O)NCC)cc1)Cc1ccccc1)Cc1ccccc1 Show InChI InChI=1S/C92H90F4N16O12/c1-5-97-89(121)101-65-40-36-63(37-41-65)81-77(111-85(117)69(87(119)123-7-3)53-107(91(111)103-81)51-67-71(93)30-16-31-72(67)94)55-105(49-61-22-11-9-12-23-61)57-79(113)109-46-20-34-75(109)83(115)99-44-18-28-59-26-15-27-60(48-59)29-19-45-100-84(116)76-35-21-47-110(76)80(114)58-106(50-62-24-13-10-14-25-62)56-78-82(64-38-42-66(43-39-64)102-90(122)98-6-2)104-92-108(52-68-73(95)32-17-33-74(68)96)54-70(86(118)112(78)92)88(120)124-8-4/h9-17,22-27,30-33,36-43,48,53-54,75-76H,5-8,20-21,34-35,44-47,49-52,55-58H2,1-4H3,(H,99,115)(H,100,116)(H2,97,101,121)(H2,98,102,122)/t75-,76-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [125I]triptorelin from human GnRHR expressed in CHO cells |
Bioorg Med Chem 16: 3744-58 (2008)
Article DOI: 10.1016/j.bmc.2008.01.054
BindingDB Entry DOI: 10.7270/Q2NC623R |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50376900

(CHEMBL258970)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2n(Cc3c(F)cccc3F)ccc(=O)n2c1CN(CC(=O)NCC(=O)NCC#Cc1ccccc1C#CCNC(=O)CNC(=O)CN(Cc1c(nc2n(Cc3c(F)cccc3F)cc(C(=O)OCC)c(=O)n12)-c1ccc(NC(=O)NCC)cc1)Cc1ccccc1)Cc1ccccc1 Show InChI InChI=1S/C83H78F4N16O10/c1-4-88-80(111)94-60-35-31-58(32-36-60)76-69(102-75(108)39-42-100(82(102)96-76)47-62-65(84)27-15-28-66(62)85)50-98(45-54-19-9-7-10-20-54)52-73(106)92-43-71(104)90-40-17-25-56-23-13-14-24-57(56)26-18-41-91-72(105)44-93-74(107)53-99(46-55-21-11-8-12-22-55)51-70-77(59-33-37-61(38-34-59)95-81(112)89-5-2)97-83-101(48-63-67(86)29-16-30-68(63)87)49-64(78(109)103(70)83)79(110)113-6-3/h7-16,19-24,27-39,42,49H,4-6,40-41,43-48,50-53H2,1-3H3,(H,90,104)(H,91,105)(H,92,106)(H,93,107)(H2,88,94,111)(H2,89,95,112) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [125I]triptorelin from human GnRHR expressed in CHO cells |
Bioorg Med Chem 16: 3744-58 (2008)
Article DOI: 10.1016/j.bmc.2008.01.054
BindingDB Entry DOI: 10.7270/Q2NC623R |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50376898
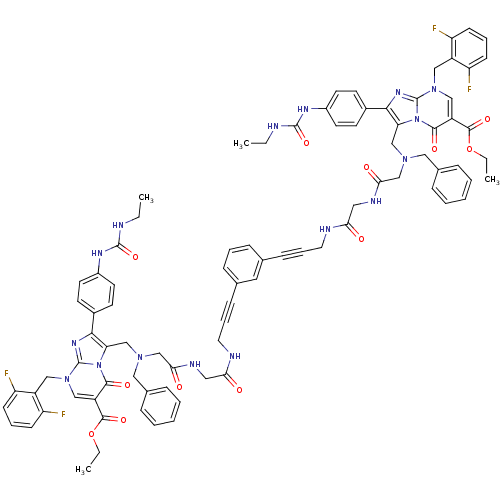
(CHEMBL408759)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2n(Cc3c(F)cccc3F)cc(C(=O)OCC)c(=O)n2c1CN(CC(=O)NCC(=O)NCC#Cc1cccc(c1)C#CCNC(=O)CNC(=O)CN(Cc1c(nc2n(Cc3c(F)cccc3F)cc(C(=O)OCC)c(=O)n12)-c1ccc(NC(=O)NCC)cc1)Cc1ccccc1)Cc1ccccc1 Show InChI InChI=1S/C86H82F4N16O12/c1-5-91-83(115)97-61-36-32-59(33-37-61)77-71(105-79(111)65(81(113)117-7-3)49-103(85(105)99-77)47-63-67(87)28-16-29-68(63)88)51-101(45-57-20-11-9-12-21-57)53-75(109)95-43-73(107)93-40-18-26-55-24-15-25-56(42-55)27-19-41-94-74(108)44-96-76(110)54-102(46-58-22-13-10-14-23-58)52-72-78(60-34-38-62(39-35-60)98-84(116)92-6-2)100-86-104(48-64-69(89)30-17-31-70(64)90)50-66(80(112)106(72)86)82(114)118-8-4/h9-17,20-25,28-39,42,49-50H,5-8,40-41,43-48,51-54H2,1-4H3,(H,93,107)(H,94,108)(H,95,109)(H,96,110)(H2,91,97,115)(H2,92,98,116) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [125I]triptorelin from human GnRHR expressed in CHO cells |
Bioorg Med Chem 16: 3744-58 (2008)
Article DOI: 10.1016/j.bmc.2008.01.054
BindingDB Entry DOI: 10.7270/Q2NC623R |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50376894

(CHEMBL259220)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2n(Cc3c(F)cccc3F)cc(C(=O)OCC)c(=O)n2c1CN(CC(=O)N[C@@H](C)C(=O)NCC#Cc1ccccc1)Cc1ccccc1 Show InChI InChI=1S/C47H46F2N8O6/c1-4-50-46(62)53-35-23-21-34(22-24-35)42-40(57-44(60)37(45(61)63-5-2)28-56(47(57)54-42)27-36-38(48)19-12-20-39(36)49)29-55(26-33-16-10-7-11-17-33)30-41(58)52-31(3)43(59)51-25-13-18-32-14-8-6-9-15-32/h6-12,14-17,19-24,28,31H,4-5,25-27,29-30H2,1-3H3,(H,51,59)(H,52,58)(H2,50,53,62)/t31-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [125I]triptorelin from human GnRHR expressed in CHO cells |
Bioorg Med Chem 16: 3744-58 (2008)
Article DOI: 10.1016/j.bmc.2008.01.054
BindingDB Entry DOI: 10.7270/Q2NC623R |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50376881
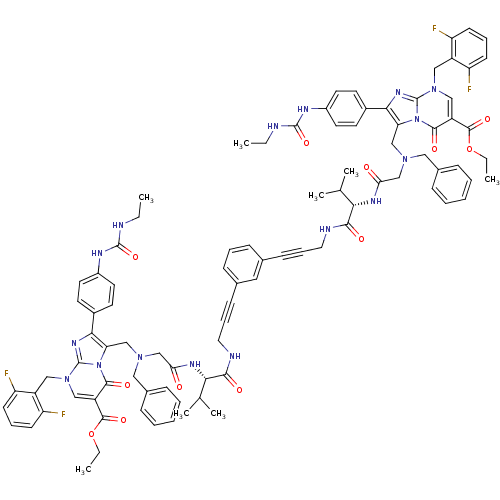
(CHEMBL406713)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2n(Cc3c(F)cccc3F)cc(C(=O)OCC)c(=O)n2c1CN(CC(=O)N[C@@H](C(C)C)C(=O)NCC#Cc1cccc(c1)C#CCNC(=O)[C@@H](NC(=O)CN(Cc1c(nc2n(Cc3c(F)cccc3F)cc(C(=O)OCC)c(=O)n12)-c1ccc(NC(=O)NCC)cc1)Cc1ccccc1)C(C)C)Cc1ccccc1 Show InChI InChI=1S/C92H94F4N16O12/c1-9-97-89(121)101-65-40-36-63(37-41-65)81-75(111-85(117)69(87(119)123-11-3)51-109(91(111)105-81)49-67-71(93)32-20-33-72(67)94)53-107(47-61-24-15-13-16-25-61)55-77(113)103-79(57(5)6)83(115)99-44-22-30-59-28-19-29-60(46-59)31-23-45-100-84(116)80(58(7)8)104-78(114)56-108(48-62-26-17-14-18-27-62)54-76-82(64-38-42-66(43-39-64)102-90(122)98-10-2)106-92-110(50-68-73(95)34-21-35-74(68)96)52-70(86(118)112(76)92)88(120)124-12-4/h13-21,24-29,32-43,46,51-52,57-58,79-80H,9-12,44-45,47-50,53-56H2,1-8H3,(H,99,115)(H,100,116)(H,103,113)(H,104,114)(H2,97,101,121)(H2,98,102,122)/t79-,80-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [125I]triptorelin from human GnRHR expressed in CHO cells |
Bioorg Med Chem 16: 3744-58 (2008)
Article DOI: 10.1016/j.bmc.2008.01.054
BindingDB Entry DOI: 10.7270/Q2NC623R |
More data for this
Ligand-Target Pair | |
Monoacylglycerol lipase ABHD6
(Homo sapiens (Human)) | BDBM50048618
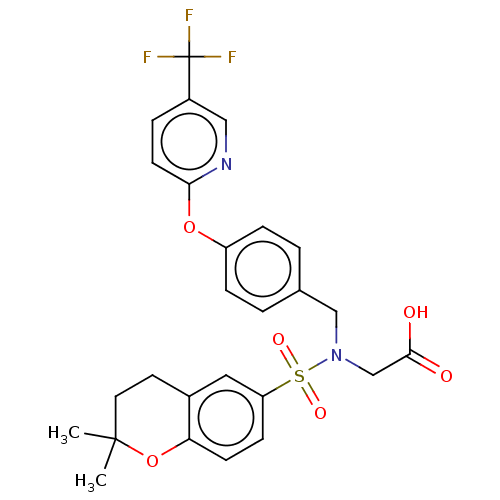
(CHEMBL3319622)Show SMILES CC1(C)CCc2cc(ccc2O1)S(=O)(=O)N(CC(O)=O)Cc1ccc(Oc2ccc(cn2)C(F)(F)F)cc1 Show InChI InChI=1S/C26H25F3N2O6S/c1-25(2)12-11-18-13-21(8-9-22(18)37-25)38(34,35)31(16-24(32)33)15-17-3-6-20(7-4-17)36-23-10-5-19(14-30-23)26(27,28)29/h3-10,13-14H,11-12,15-16H2,1-2H3,(H,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of human ABHD6 expressed in HEK293T cell membranes assessed as reduction in glycerol production using 2-arachidonoyl glycerol substrate |
J Med Chem 57: 6610-22 (2014)
Article DOI: 10.1021/jm500681z
BindingDB Entry DOI: 10.7270/Q2R49SD4 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50376887

(CHEMBL436878)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2n(Cc3c(F)cccc3F)ccc(=O)n2c1CN(CC(=O)N[C@@H](C)C(=O)NCC#Cc1ccccc1C#CCNC(=O)[C@H](C)NC(=O)CNC(=O)CN(Cc1c(nc2n(Cc3c(F)cccc3F)cc(C(=O)OCC)c(=O)n12)-c1ccc(NC(=O)NCC)cc1)Cc1ccccc1)Cc1ccccc1 Show InChI InChI=1S/C87H85F4N17O11/c1-6-92-84(117)99-63-37-33-61(34-38-63)78-72(107-77(112)41-44-105(86(107)101-78)48-65-68(88)29-17-30-69(65)89)51-104(47-58-23-13-10-14-24-58)54-76(111)98-56(5)81(114)95-43-20-28-60-26-16-15-25-59(60)27-19-42-94-80(113)55(4)97-74(109)45-96-75(110)53-103(46-57-21-11-9-12-22-57)52-73-79(62-35-39-64(40-36-62)100-85(118)93-7-2)102-87-106(49-66-70(90)31-18-32-71(66)91)50-67(82(115)108(73)87)83(116)119-8-3/h9-18,21-26,29-41,44,50,55-56H,6-8,42-43,45-49,51-54H2,1-5H3,(H,94,113)(H,95,114)(H,96,110)(H,97,109)(H,98,111)(H2,92,99,117)(H2,93,100,118)/t55-,56-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [125I]triptorelin from human GnRHR expressed in CHO cells |
Bioorg Med Chem 16: 3744-58 (2008)
Article DOI: 10.1016/j.bmc.2008.01.054
BindingDB Entry DOI: 10.7270/Q2NC623R |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50376886

(CHEMBL436697)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2n(Cc3c(F)cccc3F)ccc(=O)n2c1CN(CC(=O)N[C@@H](C(C)C)C(=O)NCC#Cc1ccccc1C#CCNC(=O)[C@@H](NC(=O)CNC(=O)CN(Cc1c(nc2n(Cc3c(F)cccc3F)cc(C(=O)OCC)c(=O)n12)-c1ccc(NC(=O)NCC)cc1)Cc1ccccc1)C(C)C)Cc1ccccc1 Show InChI InChI=1S/C91H93F4N17O11/c1-8-96-88(121)101-65-39-35-63(36-40-65)82-74(111-79(116)43-46-109(90(111)105-82)50-67-70(92)31-19-32-71(67)93)53-108(49-60-25-15-12-16-26-60)56-78(115)104-81(58(6)7)85(118)99-45-22-30-62-28-18-17-27-61(62)29-21-44-98-84(117)80(57(4)5)103-76(113)47-100-77(114)55-107(48-59-23-13-11-14-24-59)54-75-83(64-37-41-66(42-38-64)102-89(122)97-9-2)106-91-110(51-68-72(94)33-20-34-73(68)95)52-69(86(119)112(75)91)87(120)123-10-3/h11-20,23-28,31-43,46,52,57-58,80-81H,8-10,44-45,47-51,53-56H2,1-7H3,(H,98,117)(H,99,118)(H,100,114)(H,103,113)(H,104,115)(H2,96,101,121)(H2,97,102,122)/t80-,81-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [125I]triptorelin from human GnRHR expressed in CHO cells |
Bioorg Med Chem 16: 3744-58 (2008)
Article DOI: 10.1016/j.bmc.2008.01.054
BindingDB Entry DOI: 10.7270/Q2NC623R |
More data for this
Ligand-Target Pair | |
Acetylcholine-binding protein
(Lymnaea stagnalis) | BDBM50597662

(CHEMBL5178267)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@@H]1CSSC[C@H](NC(=O)CN)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](Cc3ccc(OS(O)(=O)=O)cc3)NC(=O)[C@H](CC(O)=O)NC2=O)C(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N2CCC[C@@]2([H])C(=O)N2CCC[C@@]2([H])C(=O)N1 |r| | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00182e
BindingDB Entry DOI: 10.7270/Q27W6H7C |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50376882

(CHEMBL437260)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2n(Cc3c(F)cccc3F)cc(C(=O)OCC)c(=O)n2c1CN(CC(=O)N[C@@H](C)C(=O)NCC#Cc1cccc(c1)C#CCNC(=O)[C@H](C)NC(=O)CN(Cc1c(nc2n(Cc3c(F)cccc3F)cc(C(=O)OCC)c(=O)n12)-c1ccc(NC(=O)NCC)cc1)Cc1ccccc1)Cc1ccccc1 Show InChI InChI=1S/C88H86F4N16O12/c1-7-93-85(117)99-63-38-34-61(35-39-63)77-73(107-81(113)67(83(115)119-9-3)49-105(87(107)101-77)47-65-69(89)30-18-31-70(65)90)51-103(45-59-22-13-11-14-23-59)53-75(109)97-55(5)79(111)95-42-20-28-57-26-17-27-58(44-57)29-21-43-96-80(112)56(6)98-76(110)54-104(46-60-24-15-12-16-25-60)52-74-78(62-36-40-64(41-37-62)100-86(118)94-8-2)102-88-106(48-66-71(91)32-19-33-72(66)92)50-68(82(114)108(74)88)84(116)120-10-4/h11-19,22-27,30-41,44,49-50,55-56H,7-10,42-43,45-48,51-54H2,1-6H3,(H,95,111)(H,96,112)(H,97,109)(H,98,110)(H2,93,99,117)(H2,94,100,118)/t55-,56-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [125I]triptorelin from human GnRHR expressed in CHO cells |
Bioorg Med Chem 16: 3744-58 (2008)
Article DOI: 10.1016/j.bmc.2008.01.054
BindingDB Entry DOI: 10.7270/Q2NC623R |
More data for this
Ligand-Target Pair | |
Acetylcholine-binding protein
(Lymnaea stagnalis) | BDBM50587085
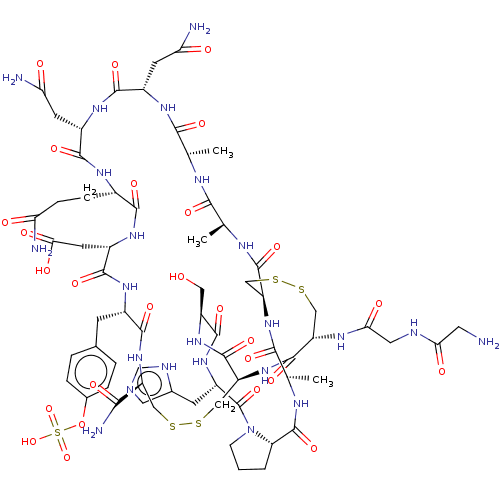
(CHEMBL5092179)Show SMILES C[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@H](CO)NC(=O)[C@@H]2CSSC[C@H](NC(=O)[C@H](Cc3ccc(OS(O)(=O)=O)cc3)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CSSC[C@H](NC(=O)CNC(=O)CN)C(=O)N2)NC1=O)C(N)=O |r| | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00182e
BindingDB Entry DOI: 10.7270/Q27W6H7C |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50078114

((1S,2R,3S,4S,5R,6R)-7-Oxa-bicyclo[4.1.0]heptane-2,...)Show SMILES O[C@H]1[C@H]2O[C@H]2[C@H](O)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C6H10O5/c7-1-2(8)4(10)6-5(11-6)3(1)9/h1-10H/t1-,2-,3+,4+,5-,6+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| 7.73E+3 | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | 5.2 | n/a |
ACADEMISCH MEDISCH CENTRUM BIJ UNIVERSITEIT VAN AMSTERDAM
US Patent
| Assay Description
Activity of GBA was measured at 37° C. with 4-methylumbelliferyl β-D-glucopyranoside as substrate as reported previously. To determine the IC50 ... |
US Patent US9056847 (2015)
BindingDB Entry DOI: 10.7270/Q2CV4GG7 |
More data for this
Ligand-Target Pair | |
Acetylcholine-binding protein
(Lymnaea stagnalis) | BDBM50516319
(CHEMBL4443205)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](CO)NC(=O)[C@@H]2CSSC[C@H](NC(=O)[C@H](Cc3ccc(O)cc3)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]3CCCN3C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CSSC[C@H](NC(=O)CN)C(=O)N2)NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C1=O)C(N)=O |r| Show InChI InChI=1S/C65H95N19O22S4/c1-29(2)18-37-63(104)84-17-7-10-46(84)65(106)83-16-6-9-45(83)62(103)81-42-27-110-108-26-41(72-49(89)23-66)59(100)80-43(60(101)78-39(24-85)57(98)76-37)28-109-107-25-40(51(69)92)79-54(95)34(19-32-11-13-33(86)14-12-32)74-56(97)36(22-50(90)91)75-61(102)44-8-5-15-82(44)64(105)38(21-48(68)88)77-55(96)35(20-47(67)87)73-53(94)31(4)70-52(93)30(3)71-58(42)99/h11-14,29-31,34-46,85-86H,5-10,15-28,66H2,1-4H3,(H2,67,87)(H2,68,88)(H2,69,92)(H,70,93)(H,71,99)(H,72,89)(H,73,94)(H,74,97)(H,75,102)(H,76,98)(H,77,96)(H,78,101)(H,79,95)(H,80,100)(H,81,103)(H,90,91)/t30-,31-,34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,44-,45-,46-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 9.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00182e
BindingDB Entry DOI: 10.7270/Q27W6H7C |
More data for this
Ligand-Target Pair | |
Monoacylglycerol lipase ABHD6
(Homo sapiens (Human)) | BDBM50048621
(CHEMBL3319619)Show SMILES CC1(C)CCc2cc(ccc2O1)S(=O)(=O)N(CC(O)=O)Cc1cccc(Oc2ccccc2)c1 Show InChI InChI=1S/C26H27NO6S/c1-26(2)14-13-20-16-23(11-12-24(20)33-26)34(30,31)27(18-25(28)29)17-19-7-6-10-22(15-19)32-21-8-4-3-5-9-21/h3-12,15-16H,13-14,17-18H2,1-2H3,(H,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of human ABHD6 expressed in HEK293T cell membranes assessed as reduction in glycerol production using 2-arachidonoyl glycerol substrate |
J Med Chem 57: 6610-22 (2014)
Article DOI: 10.1021/jm500681z
BindingDB Entry DOI: 10.7270/Q2R49SD4 |
More data for this
Ligand-Target Pair | |
Acetylcholine-binding protein
(Lymnaea stagnalis) | BDBM50597660

(CHEMBL5181935)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CO)NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](Cc3ccc(O)cc3)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CSSC[C@H](NC(=O)CNC(=O)CN)C(=O)N1)NC(=O)[C@H](C)NC2=O)C(N)=O |r| | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00182e
BindingDB Entry DOI: 10.7270/Q27W6H7C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data