 Found 21 hits with Last Name = 'lee' and Initial = 'kr'
Found 21 hits with Last Name = 'lee' and Initial = 'kr' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50022815

((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C |r| Show InChI InChI=1S/C62H111N11O12/c1-25-27-28-40(15)52(75)51-56(79)65-43(26-2)58(81)67(18)33-48(74)68(19)44(29-34(3)4)55(78)66-49(38(11)12)61(84)69(20)45(30-35(5)6)54(77)63-41(16)53(76)64-42(17)57(80)70(21)46(31-36(7)8)59(82)71(22)47(32-37(9)10)60(83)72(23)50(39(13)14)62(85)73(51)24/h25,27,34-47,49-52,75H,26,28-33H2,1-24H3,(H,63,77)(H,64,76)(H,65,79)(H,66,78)/b27-25+/t40-,41+,42-,43+,44+,45+,46+,47+,49+,50+,51+,52-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00211
BindingDB Entry DOI: 10.7270/Q2RF603J |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50601549

(CHEMBL5183632)Show SMILES CCC[C@@H]1NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00211
BindingDB Entry DOI: 10.7270/Q2RF603J |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50601547
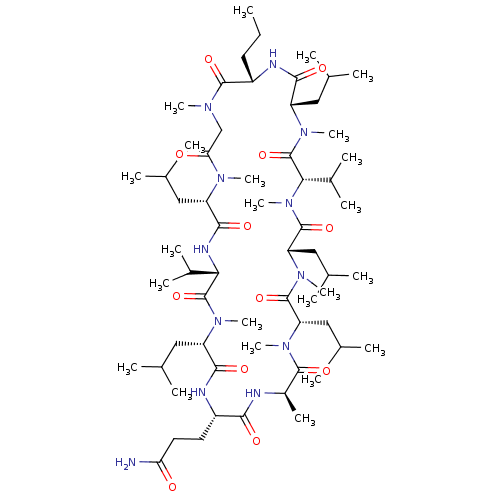
(CHEMBL5185739)Show SMILES CCC[C@@H]1NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00211
BindingDB Entry DOI: 10.7270/Q2RF603J |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50601550

(CHEMBL5183270)Show SMILES CCC[C@@H]1NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00211
BindingDB Entry DOI: 10.7270/Q2RF603J |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50601548

(CHEMBL5181883)Show SMILES CCC[C@@H]1NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00211
BindingDB Entry DOI: 10.7270/Q2RF603J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50554673
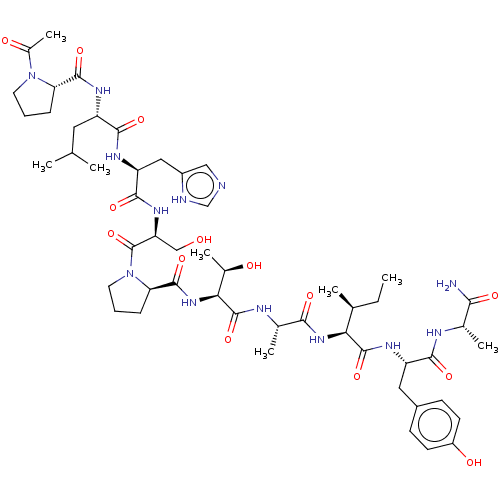
(CHEMBL4760204)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H]1CCCN1C(=O)[C@H](CO)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H]1CCCN1C(C)=O)[C@@H](C)O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of 5-carboxyfluorescein-labeled PBD-binding peptide from PLk1 PBD (unknown origin) expressed in bacterial expression system incubated fo... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01451
BindingDB Entry DOI: 10.7270/Q2W38105 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50163307
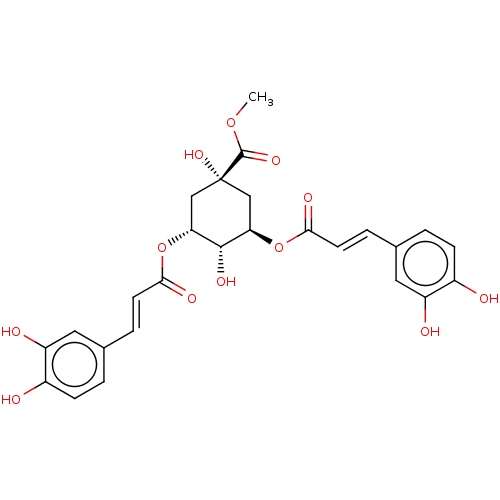
(3,5-Dicaffeoylquinic Acid Methyl Ester | CHEBI:667...)Show SMILES COC(=O)[C@@]1(O)C[C@@H](OC(=O)\C=C\c2ccc(O)c(O)c2)[C@H](O)[C@@H](C1)OC(=O)\C=C\c1ccc(O)c(O)c1 |r,wU:4.3,7.7,wD:21.22,23.26,(22.67,-40.58,;22.68,-42.11,;24.02,-42.88,;25.35,-42.1,;24.02,-44.42,;22.7,-45.2,;25.35,-43.64,;26.68,-44.42,;28.02,-43.65,;29.35,-44.43,;29.35,-45.97,;30.69,-43.66,;32.01,-44.44,;33.35,-43.67,;33.34,-42.14,;34.67,-41.37,;36.01,-42.14,;37.35,-41.38,;36.01,-43.68,;37.34,-44.46,;34.67,-44.45,;26.68,-45.96,;28.02,-46.73,;25.35,-46.72,;24.02,-45.96,;25.35,-48.26,;24.02,-49.03,;24.02,-50.57,;22.69,-48.26,;21.36,-49.03,;20.02,-48.26,;20.02,-46.71,;18.69,-45.95,;17.36,-46.72,;16.02,-45.95,;17.37,-48.26,;16.04,-49.04,;18.69,-49.03,)| Show InChI InChI=1S/C26H26O12/c1-36-25(34)26(35)12-20(37-22(31)8-4-14-2-6-16(27)18(29)10-14)24(33)21(13-26)38-23(32)9-5-15-3-7-17(28)19(30)11-15/h2-11,20-21,24,27-30,33,35H,12-13H2,1H3/b8-4+,9-5+/t20-,21-,24-,26+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa nuclear extract using fluor de lys as substrate after 10 to 15 mins by spectrofluorometry |
Bioorg Med Chem Lett 26: 2365-9 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.010
BindingDB Entry DOI: 10.7270/Q2KH0Q6F |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM19410

(CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...)Show InChI InChI=1S/C21H20N4O3/c22-18-5-1-2-6-19(18)25-20(26)17-9-7-15(8-10-17)13-24-21(27)28-14-16-4-3-11-23-12-16/h1-12H,13-14,22H2,(H,24,27)(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa nuclear extract using fluor de lys as substrate after 10 to 15 mins by spectrofluorometry |
Bioorg Med Chem Lett 26: 2365-9 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.010
BindingDB Entry DOI: 10.7270/Q2KH0Q6F |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50163307

(3,5-Dicaffeoylquinic Acid Methyl Ester | CHEBI:667...)Show SMILES COC(=O)[C@@]1(O)C[C@@H](OC(=O)\C=C\c2ccc(O)c(O)c2)[C@H](O)[C@@H](C1)OC(=O)\C=C\c1ccc(O)c(O)c1 |r,wU:4.3,7.7,wD:21.22,23.26,(22.67,-40.58,;22.68,-42.11,;24.02,-42.88,;25.35,-42.1,;24.02,-44.42,;22.7,-45.2,;25.35,-43.64,;26.68,-44.42,;28.02,-43.65,;29.35,-44.43,;29.35,-45.97,;30.69,-43.66,;32.01,-44.44,;33.35,-43.67,;33.34,-42.14,;34.67,-41.37,;36.01,-42.14,;37.35,-41.38,;36.01,-43.68,;37.34,-44.46,;34.67,-44.45,;26.68,-45.96,;28.02,-46.73,;25.35,-46.72,;24.02,-45.96,;25.35,-48.26,;24.02,-49.03,;24.02,-50.57,;22.69,-48.26,;21.36,-49.03,;20.02,-48.26,;20.02,-46.71,;18.69,-45.95,;17.36,-46.72,;16.02,-45.95,;17.37,-48.26,;16.04,-49.04,;18.69,-49.03,)| Show InChI InChI=1S/C26H26O12/c1-36-25(34)26(35)12-20(37-22(31)8-4-14-2-6-16(27)18(29)10-14)24(33)21(13-26)38-23(32)9-5-15-3-7-17(28)19(30)11-15/h2-11,20-21,24,27-30,33,35H,12-13H2,1H3/b8-4+,9-5+/t20-,21-,24-,26+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 7.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cells using Boc-Lys(AC)-AMC as substrate after 24 to 48 hrs by spectrofluorometry |
Bioorg Med Chem Lett 26: 2365-9 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.010
BindingDB Entry DOI: 10.7270/Q2KH0Q6F |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM19410

(CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...)Show InChI InChI=1S/C21H20N4O3/c22-18-5-1-2-6-19(18)25-20(26)17-9-7-15(8-10-17)13-24-21(27)28-14-16-4-3-11-23-12-16/h1-12H,13-14,22H2,(H,24,27)(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cells using Boc-Lys(AC)-AMC as substrate after 24 to 48 hrs by spectrofluorometry |
Bioorg Med Chem Lett 26: 2365-9 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.010
BindingDB Entry DOI: 10.7270/Q2KH0Q6F |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50554676
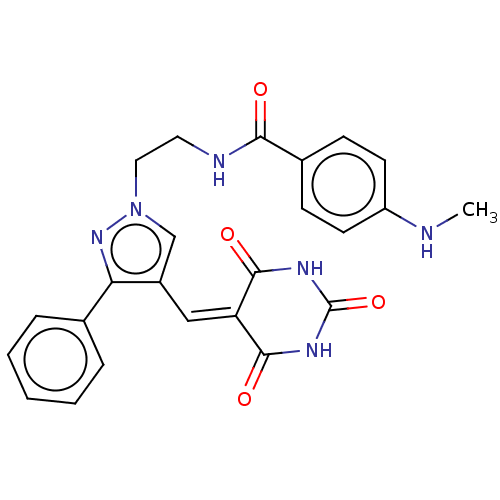
(CHEMBL4788427)Show SMILES [#6]-[#7]-c1ccc(cc1)-[#6](=O)-[#7]-[#6]-[#6]-n1cc(\[#6]=[#6]-2\[#6](=O)-[#7]-[#6](=O)-[#7]-[#6]-2=O)c(n1)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of 5-carboxyfluorescein-labeled PBD-binding peptide from PLk1 PBD (unknown origin) expressed in bacterial expression system incubated fo... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01451
BindingDB Entry DOI: 10.7270/Q2W38105 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50554672
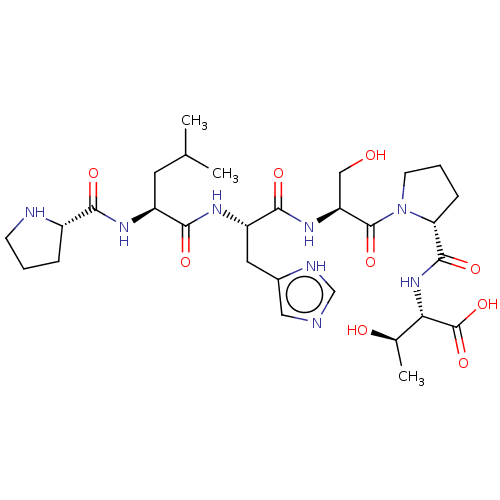
(CHEMBL4758139)Show SMILES CC(C)C[C@H](NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CO)C(=O)N1CCC[C@@H]1C(=O)N[C@@H]([C@@H](C)O)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of 5-carboxyfluorescein-labeled PBD-binding peptide from PLk1 PBD (unknown origin) expressed in bacterial expression system incubated fo... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01451
BindingDB Entry DOI: 10.7270/Q2W38105 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50554674
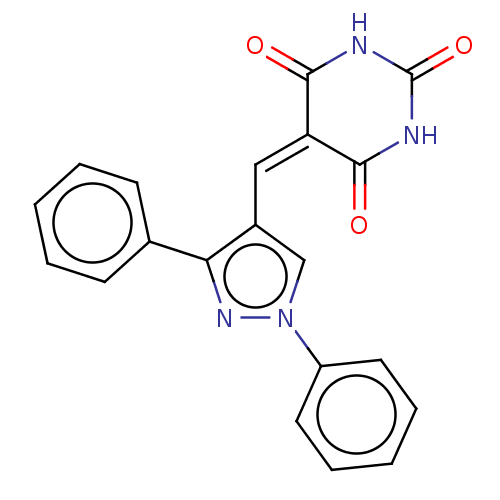
(CHEMBL4777323)Show SMILES O=[#6]-1-[#7]-[#6](=O)\[#6](=[#6]/c2cn(nc2-c2ccccc2)-c2ccccc2)-[#6](=O)-[#7]-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of 5-carboxyfluorescein-labeled PBD-binding peptide from PLk1 PBD (unknown origin) expressed in bacterial expression system incubated fo... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01451
BindingDB Entry DOI: 10.7270/Q2W38105 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50554675
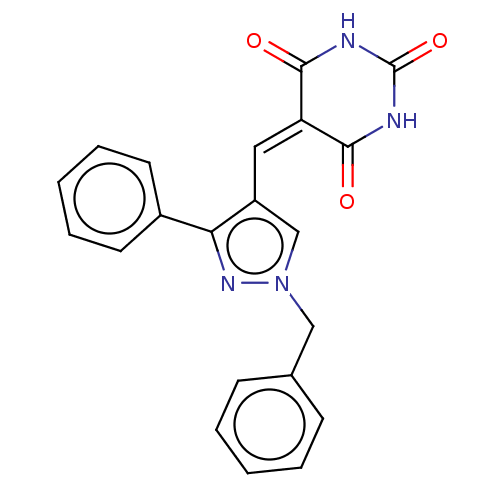
(CHEMBL4792348)Show SMILES O=[#6]-1-[#7]-[#6](=O)\[#6](=[#6]/c2cn(-[#6]-c3ccccc3)nc2-c2ccccc2)-[#6](=O)-[#7]-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of 5-carboxyfluorescein-labeled PBD-binding peptide from PLk1 PBD (unknown origin) expressed in bacterial expression system incubated fo... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01451
BindingDB Entry DOI: 10.7270/Q2W38105 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50554677
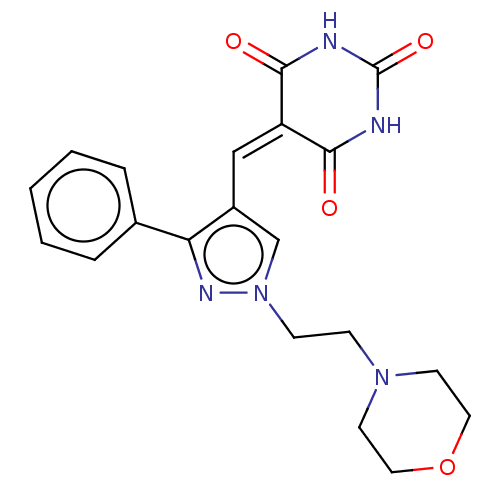
(CHEMBL4765111)Show SMILES O=[#6]-1-[#7]-[#6](=O)\[#6](=[#6]\c2cn(-[#6]-[#6]-[#7]-3-[#6]-[#6]-[#8]-[#6]-[#6]-3)nc2-c2ccccc2)-[#6](=O)-[#7]-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of 5-carboxyfluorescein-labeled PBD-binding peptide from PLk1 PBD (unknown origin) expressed in bacterial expression system incubated fo... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01451
BindingDB Entry DOI: 10.7270/Q2W38105 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50362839
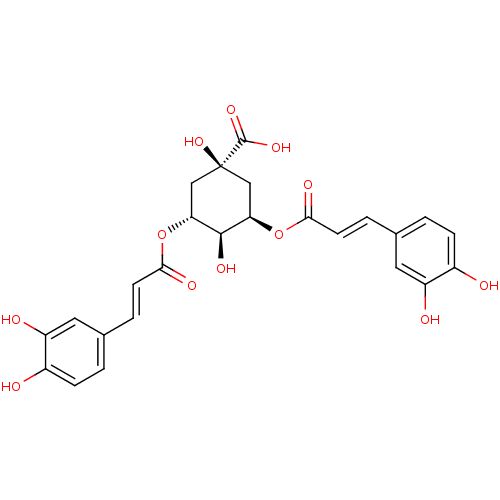
(CHEMBL249447)Show SMILES O[C@H]1[C@@H](C[C@@](O)(C[C@H]1OC(=O)\C=C\c1ccc(O)c(O)c1)C(O)=O)OC(=O)\C=C\c1ccc(O)c(O)c1 |r,wU:2.25,4.22,wD:1.0,7.8,4.4,(-1.06,-23.53,;-1.06,-21.99,;.29,-21.22,;.29,-19.66,;-1.06,-18.89,;.22,-18.03,;-2.39,-19.66,;-2.39,-21.22,;-3.73,-21.99,;-3.73,-23.53,;-2.39,-24.3,;-5.06,-24.3,;-5.06,-25.84,;-6.39,-26.61,;-7.73,-25.84,;-9.06,-26.61,;-9.06,-28.15,;-10.39,-28.92,;-7.72,-28.92,;-7.71,-30.46,;-6.39,-28.15,;-1.91,-17.6,;-.95,-16.4,;-3.45,-17.53,;1.62,-21.99,;1.61,-23.53,;.28,-24.3,;2.95,-24.3,;2.94,-25.84,;4.27,-26.62,;4.26,-28.16,;5.59,-28.93,;6.93,-28.16,;8.26,-28.93,;6.93,-26.61,;8.26,-25.84,;5.6,-25.85,)| Show InChI InChI=1S/C25H24O12/c26-15-5-1-13(9-17(15)28)3-7-21(30)36-19-11-25(35,24(33)34)12-20(23(19)32)37-22(31)8-4-14-2-6-16(27)18(29)10-14/h1-10,19-20,23,26-29,32,35H,11-12H2,(H,33,34)/b7-3+,8-4+/t19-,20-,23-,25+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.43E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa nuclear extract using fluor de lys as substrate after 10 to 15 mins by spectrofluorometry |
Bioorg Med Chem Lett 26: 2365-9 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.010
BindingDB Entry DOI: 10.7270/Q2KH0Q6F |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50163308
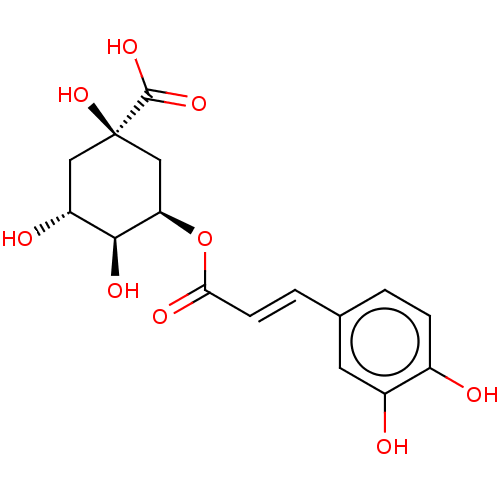
(3-O-Caffeoylquinic Acid | CHEBI:16384 | CHEMBL2494...)Show SMILES O[C@@H]1C[C@@](O)(C[C@@H](OC(=O)\C=C\c2ccc(O)c(O)c2)[C@H]1O)C(O)=O |r| Show InChI InChI=1S/C16H18O9/c17-9-3-1-8(5-10(9)18)2-4-13(20)25-12-7-16(24,15(22)23)6-11(19)14(12)21/h1-5,11-12,14,17-19,21,24H,6-7H2,(H,22,23)/b4-2+/t11-,12-,14+,16-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa nuclear extract using fluor de lys as substrate after 10 to 15 mins by spectrofluorometry |
Bioorg Med Chem Lett 26: 2365-9 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.010
BindingDB Entry DOI: 10.7270/Q2KH0Q6F |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50327036

((1R,3S,4S,5S)-3-[(E)-3-(3,4-Dihydroxy-phenyl)-acry...)Show SMILES O[C@@H]1C[C@](O)(C[C@@H](OC(=O)\C=C\c2ccc(O)c(O)c2)[C@@H]1O)C(O)=O |r| Show InChI InChI=1S/C16H18O9/c17-9-3-1-8(5-10(9)18)2-4-13(20)25-12-7-16(24,15(22)23)6-11(19)14(12)21/h1-5,11-12,14,17-19,21,24H,6-7H2,(H,22,23)/b4-2+/t11-,12-,14-,16+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa nuclear extract using fluor de lys as substrate after 10 to 15 mins by spectrofluorometry |
Bioorg Med Chem Lett 26: 2365-9 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.010
BindingDB Entry DOI: 10.7270/Q2KH0Q6F |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50327036

((1R,3S,4S,5S)-3-[(E)-3-(3,4-Dihydroxy-phenyl)-acry...)Show SMILES O[C@@H]1C[C@](O)(C[C@@H](OC(=O)\C=C\c2ccc(O)c(O)c2)[C@@H]1O)C(O)=O |r| Show InChI InChI=1S/C16H18O9/c17-9-3-1-8(5-10(9)18)2-4-13(20)25-12-7-16(24,15(22)23)6-11(19)14(12)21/h1-5,11-12,14,17-19,21,24H,6-7H2,(H,22,23)/b4-2+/t11-,12-,14-,16+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cells using Boc-Lys(AC)-AMC as substrate after 24 to 48 hrs by spectrofluorometry |
Bioorg Med Chem Lett 26: 2365-9 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.010
BindingDB Entry DOI: 10.7270/Q2KH0Q6F |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50163308

(3-O-Caffeoylquinic Acid | CHEBI:16384 | CHEMBL2494...)Show SMILES O[C@@H]1C[C@@](O)(C[C@@H](OC(=O)\C=C\c2ccc(O)c(O)c2)[C@H]1O)C(O)=O |r| Show InChI InChI=1S/C16H18O9/c17-9-3-1-8(5-10(9)18)2-4-13(20)25-12-7-16(24,15(22)23)6-11(19)14(12)21/h1-5,11-12,14,17-19,21,24H,6-7H2,(H,22,23)/b4-2+/t11-,12-,14+,16-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cells using Boc-Lys(AC)-AMC as substrate after 24 to 48 hrs by spectrofluorometry |
Bioorg Med Chem Lett 26: 2365-9 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.010
BindingDB Entry DOI: 10.7270/Q2KH0Q6F |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50362839

(CHEMBL249447)Show SMILES O[C@H]1[C@@H](C[C@@](O)(C[C@H]1OC(=O)\C=C\c1ccc(O)c(O)c1)C(O)=O)OC(=O)\C=C\c1ccc(O)c(O)c1 |r,wU:2.25,4.22,wD:1.0,7.8,4.4,(-1.06,-23.53,;-1.06,-21.99,;.29,-21.22,;.29,-19.66,;-1.06,-18.89,;.22,-18.03,;-2.39,-19.66,;-2.39,-21.22,;-3.73,-21.99,;-3.73,-23.53,;-2.39,-24.3,;-5.06,-24.3,;-5.06,-25.84,;-6.39,-26.61,;-7.73,-25.84,;-9.06,-26.61,;-9.06,-28.15,;-10.39,-28.92,;-7.72,-28.92,;-7.71,-30.46,;-6.39,-28.15,;-1.91,-17.6,;-.95,-16.4,;-3.45,-17.53,;1.62,-21.99,;1.61,-23.53,;.28,-24.3,;2.95,-24.3,;2.94,-25.84,;4.27,-26.62,;4.26,-28.16,;5.59,-28.93,;6.93,-28.16,;8.26,-28.93,;6.93,-26.61,;8.26,-25.84,;5.6,-25.85,)| Show InChI InChI=1S/C25H24O12/c26-15-5-1-13(9-17(15)28)3-7-21(30)36-19-11-25(35,24(33)34)12-20(23(19)32)37-22(31)8-4-14-2-6-16(27)18(29)10-14/h1-10,19-20,23,26-29,32,35H,11-12H2,(H,33,34)/b7-3+,8-4+/t19-,20-,23-,25+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cells using Boc-Lys(AC)-AMC as substrate after 24 to 48 hrs by spectrofluorometry |
Bioorg Med Chem Lett 26: 2365-9 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.010
BindingDB Entry DOI: 10.7270/Q2KH0Q6F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data