

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50134709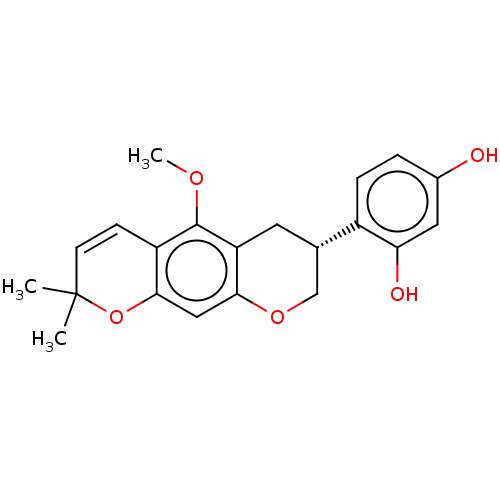 (CHEMBL3747057) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Time dependent inhibition of monophenolase activity of mushroom tyrosinase using L-tyrosine as substrate measured every 30 secs by Morrison and Walsh... | Bioorg Med Chem 24: 153-9 (2016) Article DOI: 10.1016/j.bmc.2015.11.040 BindingDB Entry DOI: 10.7270/Q2RN39PT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50134709 (CHEMBL3747057) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Time dependent inhibition of monophenolase activity of mushroom tyrosinase using L-tyrosine as substrate measured every 30 secs by Morrison and Walsh... | Bioorg Med Chem 24: 153-9 (2016) Article DOI: 10.1016/j.bmc.2015.11.040 BindingDB Entry DOI: 10.7270/Q2RN39PT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50134709 (CHEMBL3747057) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of monophenolase activity of mushroom tyrosinase using L-tyrosine as substrate preincubated for 5 to 60 mins by Lineweaver-Bur... | Bioorg Med Chem 24: 153-9 (2016) Article DOI: 10.1016/j.bmc.2015.11.040 BindingDB Entry DOI: 10.7270/Q2RN39PT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50134709 (CHEMBL3747057) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of monophenolase activity of mushroom tyrosinase using L-tyrosine as substrate preincubated for 5 to 60 mins by Lineweaver-Bur... | Bioorg Med Chem 24: 153-9 (2016) Article DOI: 10.1016/j.bmc.2015.11.040 BindingDB Entry DOI: 10.7270/Q2RN39PT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50134709 (CHEMBL3747057) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of diphenolase activity of mushroom tyrosinase using L-DOPA as substrate preincubated for 5 to 60 mins by Lineweaver-Burk and ... | Bioorg Med Chem 24: 153-9 (2016) Article DOI: 10.1016/j.bmc.2015.11.040 BindingDB Entry DOI: 10.7270/Q2RN39PT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50134709 (CHEMBL3747057) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of diphenolase activity of mushroom tyrosinase using L-DOPA as substrate preincubated for 5 to 60 mins by Lineweaver-Burk and ... | Bioorg Med Chem 24: 153-9 (2016) Article DOI: 10.1016/j.bmc.2015.11.040 BindingDB Entry DOI: 10.7270/Q2RN39PT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50134710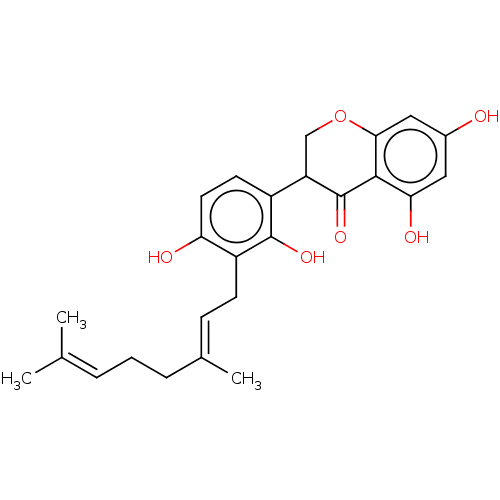 (CHEMBL3745886) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Time dependent inhibition of monophenolase activity of mushroom tyrosinase using L-tyrosine as substrate measured every 30 secs by Morrison and Walsh... | Bioorg Med Chem 24: 153-9 (2016) Article DOI: 10.1016/j.bmc.2015.11.040 BindingDB Entry DOI: 10.7270/Q2RN39PT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50134710 (CHEMBL3745886) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Time dependent inhibition of monophenolase activity of mushroom tyrosinase using L-tyrosine as substrate measured every 30 secs by Morrison and Walsh... | Bioorg Med Chem 24: 153-9 (2016) Article DOI: 10.1016/j.bmc.2015.11.040 BindingDB Entry DOI: 10.7270/Q2RN39PT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50366237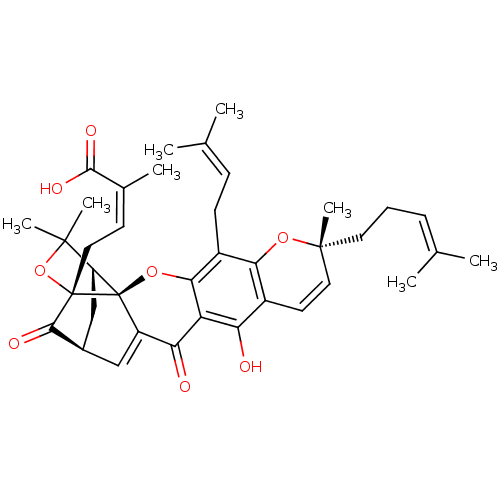 (GAMBOGIC ACID) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Applied Life Science (BK21 Plus), IALS, Gyeongsang National University, Jinju 52828, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate by Dixon plot analysis | Bioorg Med Chem 25: 2498-2506 (2017) Article DOI: 10.1016/j.bmc.2017.03.010 BindingDB Entry DOI: 10.7270/Q2Q81GHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50256793 (CHEMBL4095800) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Applied Life Science (BK21 Plus), IALS, Gyeongsang National University, Jinju 52828, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate by Dixon plot analysis | Bioorg Med Chem 25: 2498-2506 (2017) Article DOI: 10.1016/j.bmc.2017.03.010 BindingDB Entry DOI: 10.7270/Q2Q81GHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50256807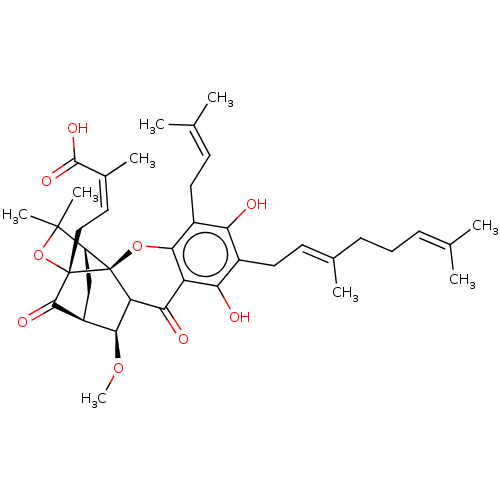 (CHEMBL4069859) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Applied Life Science (BK21 Plus), IALS, Gyeongsang National University, Jinju 52828, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 60 mins followed by ... | Bioorg Med Chem 25: 2498-2506 (2017) Article DOI: 10.1016/j.bmc.2017.03.010 BindingDB Entry DOI: 10.7270/Q2Q81GHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50366237 (GAMBOGIC ACID) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 499 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Applied Life Science (BK21 Plus), IALS, Gyeongsang National University, Jinju 52828, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 60 mins followed by ... | Bioorg Med Chem 25: 2498-2506 (2017) Article DOI: 10.1016/j.bmc.2017.03.010 BindingDB Entry DOI: 10.7270/Q2Q81GHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50256792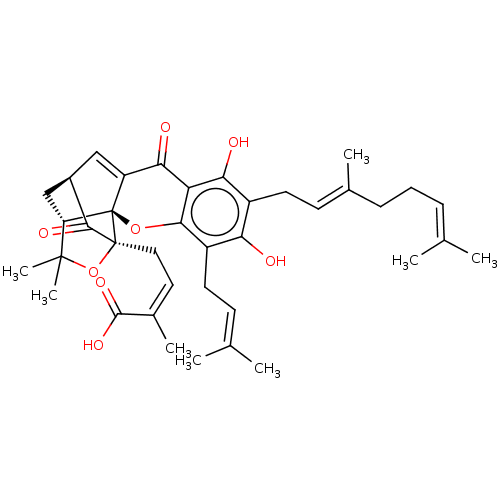 (Gambogenic Acid) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Applied Life Science (BK21 Plus), IALS, Gyeongsang National University, Jinju 52828, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 60 mins followed by ... | Bioorg Med Chem 25: 2498-2506 (2017) Article DOI: 10.1016/j.bmc.2017.03.010 BindingDB Entry DOI: 10.7270/Q2Q81GHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50256790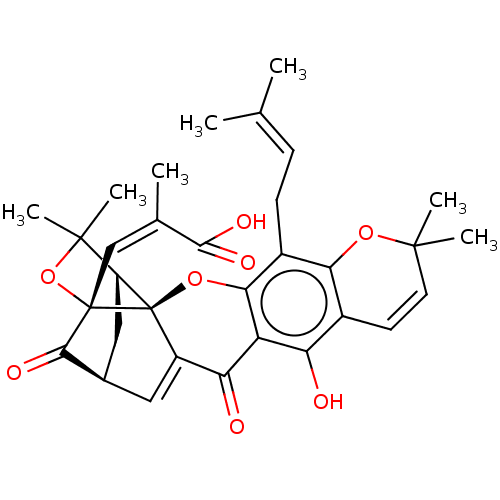 (CHEMBL4068566) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Applied Life Science (BK21 Plus), IALS, Gyeongsang National University, Jinju 52828, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 60 mins followed by ... | Bioorg Med Chem 25: 2498-2506 (2017) Article DOI: 10.1016/j.bmc.2017.03.010 BindingDB Entry DOI: 10.7270/Q2Q81GHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50256791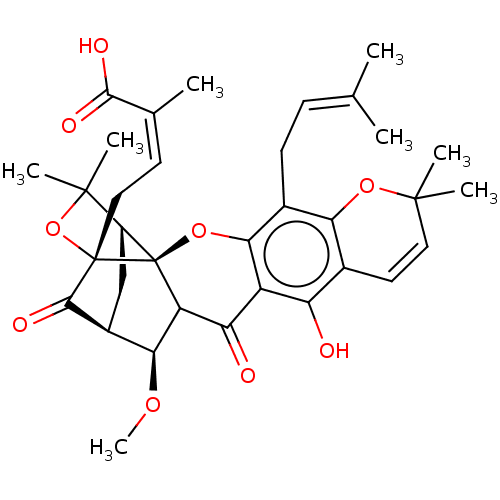 (CHEMBL4090412) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Applied Life Science (BK21 Plus), IALS, Gyeongsang National University, Jinju 52828, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate by Dixon plot analysis | Bioorg Med Chem 25: 2498-2506 (2017) Article DOI: 10.1016/j.bmc.2017.03.010 BindingDB Entry DOI: 10.7270/Q2Q81GHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50134710 (CHEMBL3745886) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of monophenolase activity of mushroom tyrosinase using L-tyrosine as substrate preincubated for 5 to 60 mins by Lineweaver-Bur... | Bioorg Med Chem 24: 153-9 (2016) Article DOI: 10.1016/j.bmc.2015.11.040 BindingDB Entry DOI: 10.7270/Q2RN39PT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50134710 (CHEMBL3745886) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of monophenolase activity of mushroom tyrosinase using L-tyrosine as substrate preincubated for 5 to 60 mins by Lineweaver-Bur... | Bioorg Med Chem 24: 153-9 (2016) Article DOI: 10.1016/j.bmc.2015.11.040 BindingDB Entry DOI: 10.7270/Q2RN39PT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM84968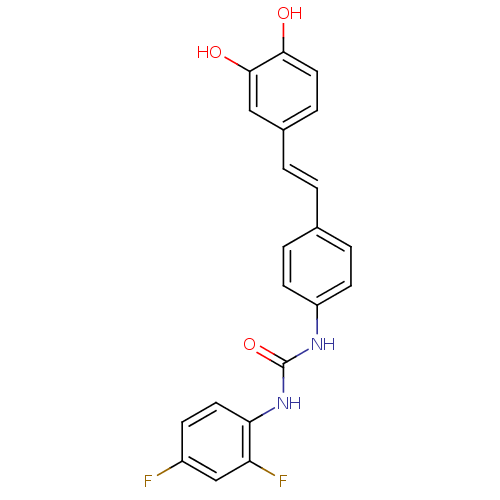 (Urea derivative, 12) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.20E+3 | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University | Assay Description All enzymatic activities were determined by using the appropriate substrate (p-nitrophenyl-alpha-D-glucopyranoside, p-nitrophenyl-beta-D-gulcopyranos... | Chembiochem 11: 2125-31 (2010) Article DOI: 10.1002/cbic.201000376 BindingDB Entry DOI: 10.7270/Q2542M33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50256794 (CHEMBL4059800) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 4.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Applied Life Science (BK21 Plus), IALS, Gyeongsang National University, Jinju 52828, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate by Dixon plot analysis | Bioorg Med Chem 25: 2498-2506 (2017) Article DOI: 10.1016/j.bmc.2017.03.010 BindingDB Entry DOI: 10.7270/Q2Q81GHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM84969 (Urea derivative, 13) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.60E+3 | n/a | 1.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University | Assay Description All enzymatic activities were determined by using the appropriate substrate (p-nitrophenyl-alpha-D-glucopyranoside, p-nitrophenyl-beta-D-gulcopyranos... | Chembiochem 11: 2125-31 (2010) Article DOI: 10.1002/cbic.201000376 BindingDB Entry DOI: 10.7270/Q2542M33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM84967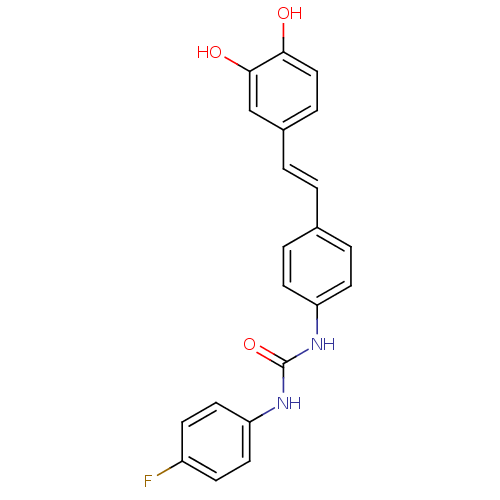 (Urea derivative, 11) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.20E+3 | n/a | 1.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University | Assay Description All enzymatic activities were determined by using the appropriate substrate (p-nitrophenyl-alpha-D-glucopyranoside, p-nitrophenyl-beta-D-gulcopyranos... | Chembiochem 11: 2125-31 (2010) Article DOI: 10.1002/cbic.201000376 BindingDB Entry DOI: 10.7270/Q2542M33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50134712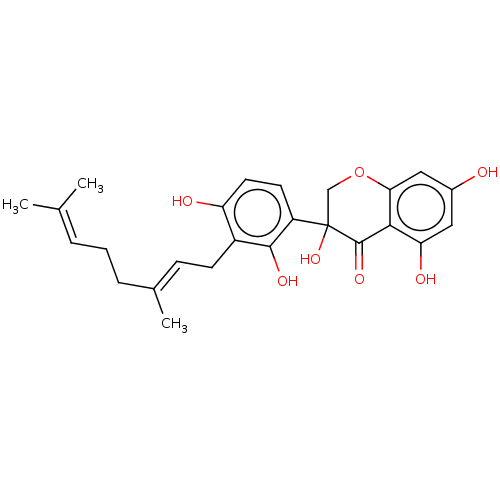 (CHEMBL3746218) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of monophenolase activity of mushroom tyrosinase using L-tyrosine as substrate preincubated for 5 to 60 mins by Lineweaver-Bur... | Bioorg Med Chem 24: 153-9 (2016) Article DOI: 10.1016/j.bmc.2015.11.040 BindingDB Entry DOI: 10.7270/Q2RN39PT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50134712 (CHEMBL3746218) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of monophenolase activity of mushroom tyrosinase using L-tyrosine as substrate preincubated for 5 to 60 mins by Lineweaver-Bur... | Bioorg Med Chem 24: 153-9 (2016) Article DOI: 10.1016/j.bmc.2015.11.040 BindingDB Entry DOI: 10.7270/Q2RN39PT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM84972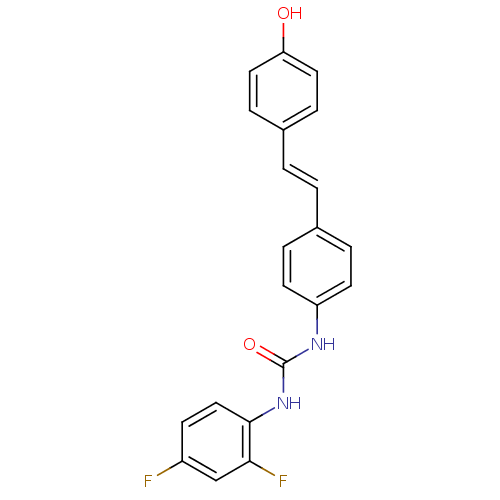 (Urea derivative, 16) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9.40E+3 | n/a | 1.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University | Assay Description All enzymatic activities were determined by using the appropriate substrate (p-nitrophenyl-alpha-D-glucopyranoside, p-nitrophenyl-beta-D-gulcopyranos... | Chembiochem 11: 2125-31 (2010) Article DOI: 10.1002/cbic.201000376 BindingDB Entry DOI: 10.7270/Q2542M33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM84963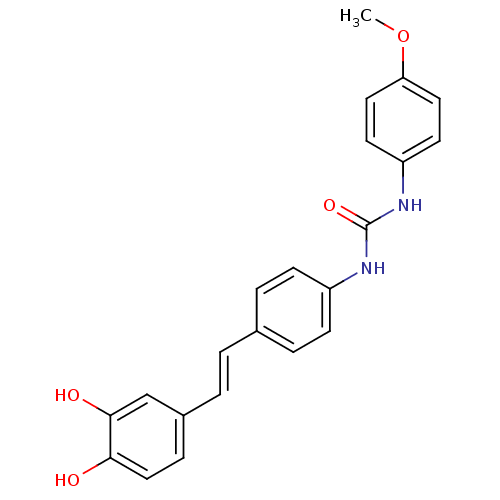 (Urea derivative, 7) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.05E+4 | n/a | 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University | Assay Description All enzymatic activities were determined by using the appropriate substrate (p-nitrophenyl-alpha-D-glucopyranoside, p-nitrophenyl-beta-D-gulcopyranos... | Chembiochem 11: 2125-31 (2010) Article DOI: 10.1002/cbic.201000376 BindingDB Entry DOI: 10.7270/Q2542M33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM84962 (Urea derivative, 6) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.06E+4 | n/a | 4.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University | Assay Description All enzymatic activities were determined by using the appropriate substrate (p-nitrophenyl-alpha-D-glucopyranoside, p-nitrophenyl-beta-D-gulcopyranos... | Chembiochem 11: 2125-31 (2010) Article DOI: 10.1002/cbic.201000376 BindingDB Entry DOI: 10.7270/Q2542M33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM84966 (Urea derivative, 10) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.21E+4 | n/a | 2.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University | Assay Description All enzymatic activities were determined by using the appropriate substrate (p-nitrophenyl-alpha-D-glucopyranoside, p-nitrophenyl-beta-D-gulcopyranos... | Chembiochem 11: 2125-31 (2010) Article DOI: 10.1002/cbic.201000376 BindingDB Entry DOI: 10.7270/Q2542M33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM84970 (Urea derivative, 14) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.70E+4 | n/a | 2.91E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University | Assay Description All enzymatic activities were determined by using the appropriate substrate (p-nitrophenyl-alpha-D-glucopyranoside, p-nitrophenyl-beta-D-gulcopyranos... | Chembiochem 11: 2125-31 (2010) Article DOI: 10.1002/cbic.201000376 BindingDB Entry DOI: 10.7270/Q2542M33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.80E+4 | n/a | 3.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University | Assay Description All enzymatic activities were determined by using the appropriate substrate (p-nitrophenyl-alpha-D-glucopyranoside, p-nitrophenyl-beta-D-gulcopyranos... | Chembiochem 11: 2125-31 (2010) Article DOI: 10.1002/cbic.201000376 BindingDB Entry DOI: 10.7270/Q2542M33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM23926 ((E)-resveratrol | 5-[(E)-2-(4-hydroxyphenyl)etheny...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.15E+4 | n/a | 2.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University | Assay Description All enzymatic activities were determined by using the appropriate substrate (p-nitrophenyl-alpha-D-glucopyranoside, p-nitrophenyl-beta-D-gulcopyranos... | Chembiochem 11: 2125-31 (2010) Article DOI: 10.1002/cbic.201000376 BindingDB Entry DOI: 10.7270/Q2542M33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50256806 (CHEMBL4068458) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Applied Life Science (BK21 Plus), IALS, Gyeongsang National University, Jinju 52828, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 60 mins followed by ... | Bioorg Med Chem 25: 2498-2506 (2017) Article DOI: 10.1016/j.bmc.2017.03.010 BindingDB Entry DOI: 10.7270/Q2Q81GHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM84959 (Stilbene derivative, 2) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.51E+4 | n/a | 4.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University | Assay Description All enzymatic activities were determined by using the appropriate substrate (p-nitrophenyl-alpha-D-glucopyranoside, p-nitrophenyl-beta-D-gulcopyranos... | Chembiochem 11: 2125-31 (2010) Article DOI: 10.1002/cbic.201000376 BindingDB Entry DOI: 10.7270/Q2542M33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50134713 (CHEMBL3746757) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of monophenolase activity of mushroom tyrosinase using L-tyrosine as substrate preincubated for 5 to 60 mins by Lineweaver-Bur... | Bioorg Med Chem 24: 153-9 (2016) Article DOI: 10.1016/j.bmc.2015.11.040 BindingDB Entry DOI: 10.7270/Q2RN39PT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50134713 (CHEMBL3746757) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of monophenolase activity of mushroom tyrosinase using L-tyrosine as substrate preincubated for 5 to 60 mins by Lineweaver-Bur... | Bioorg Med Chem 24: 153-9 (2016) Article DOI: 10.1016/j.bmc.2015.11.040 BindingDB Entry DOI: 10.7270/Q2RN39PT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50134711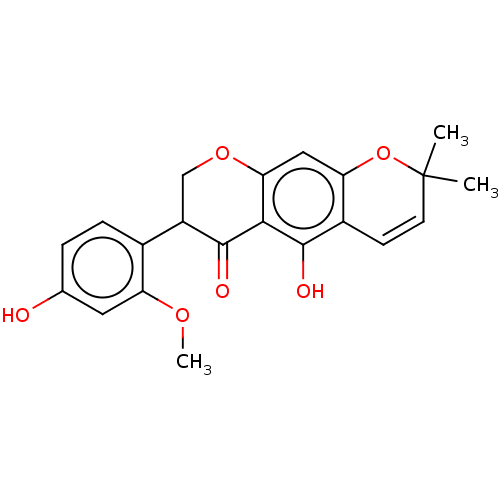 (CHEMBL3746307) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of diphenolase activity of mushroom tyrosinase using L-DOPA as substrate preincubated for 5 to 60 mins by Lineweaver-Burk and ... | Bioorg Med Chem 24: 153-9 (2016) Article DOI: 10.1016/j.bmc.2015.11.040 BindingDB Entry DOI: 10.7270/Q2RN39PT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50134711 (CHEMBL3746307) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of diphenolase activity of mushroom tyrosinase using L-DOPA as substrate preincubated for 5 to 60 mins by Lineweaver-Burk and ... | Bioorg Med Chem 24: 153-9 (2016) Article DOI: 10.1016/j.bmc.2015.11.040 BindingDB Entry DOI: 10.7270/Q2RN39PT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50134710 (CHEMBL3745886) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 7.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of diphenolase activity of mushroom tyrosinase using L-DOPA as substrate preincubated for 5 to 60 mins by Lineweaver-Burk and ... | Bioorg Med Chem 24: 153-9 (2016) Article DOI: 10.1016/j.bmc.2015.11.040 BindingDB Entry DOI: 10.7270/Q2RN39PT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50134710 (CHEMBL3745886) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 7.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of diphenolase activity of mushroom tyrosinase using L-DOPA as substrate preincubated for 5 to 60 mins by Lineweaver-Burk and ... | Bioorg Med Chem 24: 153-9 (2016) Article DOI: 10.1016/j.bmc.2015.11.040 BindingDB Entry DOI: 10.7270/Q2RN39PT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50134712 (CHEMBL3746218) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of diphenolase activity of mushroom tyrosinase using L-DOPA as substrate preincubated for 5 to 60 mins by Lineweaver-Burk and ... | Bioorg Med Chem 24: 153-9 (2016) Article DOI: 10.1016/j.bmc.2015.11.040 BindingDB Entry DOI: 10.7270/Q2RN39PT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50134712 (CHEMBL3746218) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of diphenolase activity of mushroom tyrosinase using L-DOPA as substrate preincubated for 5 to 60 mins by Lineweaver-Burk and ... | Bioorg Med Chem 24: 153-9 (2016) Article DOI: 10.1016/j.bmc.2015.11.040 BindingDB Entry DOI: 10.7270/Q2RN39PT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50330355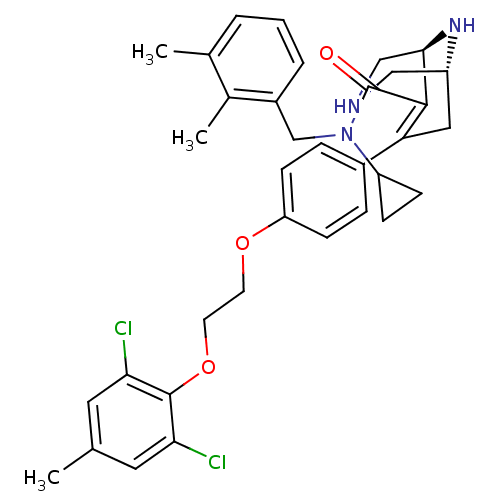 ((1R,5S)-N-cyclopropyl-7-(4-(2-(2,6-dichloro-4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU) Curated by ChEMBL | Assay Description Inhibition of human renin | Eur J Med Chem 46: 2469-76 (2011) Article DOI: 10.1016/j.ejmech.2011.03.035 BindingDB Entry DOI: 10.7270/Q2GM87NM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17949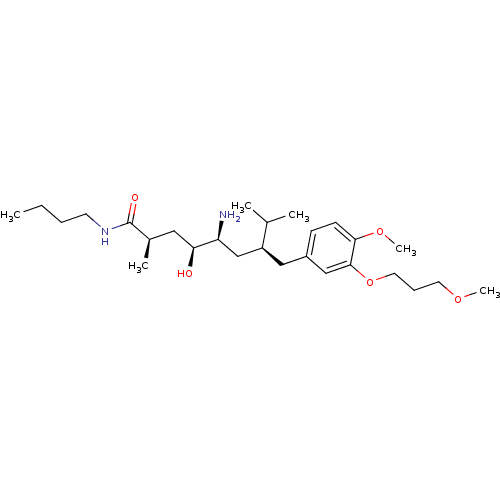 ((2R,4S,5S,7S)-5-amino-N-butyl-4-hydroxy-7-{[4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU) Curated by ChEMBL | Assay Description Inhibition of human renin | Eur J Med Chem 46: 2469-76 (2011) Article DOI: 10.1016/j.ejmech.2011.03.035 BindingDB Entry DOI: 10.7270/Q2GM87NM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM29949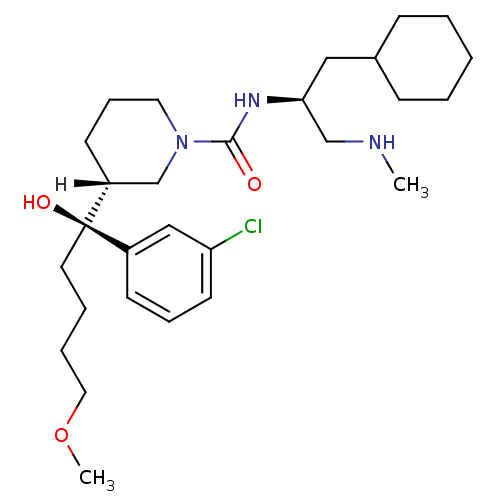 (piperidine-1-carboxamide, 21l) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU) Curated by ChEMBL | Assay Description Inhibition of human renin | Eur J Med Chem 46: 2469-76 (2011) Article DOI: 10.1016/j.ejmech.2011.03.035 BindingDB Entry DOI: 10.7270/Q2GM87NM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50116537 (3-({3-[4-(Biphenyl-4-yloxy)-phenoxy]-propyl}-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU) Curated by ChEMBL | Assay Description Inhibition of human leukotriene A4 hydrolase | Eur J Med Chem 46: 1593-603 (2011) Article DOI: 10.1016/j.ejmech.2011.02.007 BindingDB Entry DOI: 10.7270/Q2K64JDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17950 ((2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2,2-dimethyle...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU) Curated by ChEMBL | Assay Description Inhibition of human renin | Eur J Med Chem 46: 2469-76 (2011) Article DOI: 10.1016/j.ejmech.2011.03.035 BindingDB Entry DOI: 10.7270/Q2GM87NM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50345192 (5-(2-(2-benzyl-3-(tert-butylsulfonyl)propanamido)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU) Curated by ChEMBL | Assay Description Inhibition of human renin | Eur J Med Chem 46: 2469-76 (2011) Article DOI: 10.1016/j.ejmech.2011.03.035 BindingDB Entry DOI: 10.7270/Q2GM87NM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17943 (Renin nonpeptide inhibitor, 3 | methyl (3S)-1-[(5S...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU) Curated by ChEMBL | Assay Description Inhibition of human renin | Eur J Med Chem 46: 2469-76 (2011) Article DOI: 10.1016/j.ejmech.2011.03.035 BindingDB Entry DOI: 10.7270/Q2GM87NM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50345193 (CHEMBL1783186 | N-(4-amino-8-(butylamino)-5-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU) Curated by ChEMBL | Assay Description Inhibition of human renin | Eur J Med Chem 46: 2469-76 (2011) Article DOI: 10.1016/j.ejmech.2011.03.035 BindingDB Entry DOI: 10.7270/Q2GM87NM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077669 ((S)-2-Benzyl-N-[(S)-1-((1S,2R,3S)-1-cyclohexylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU) Curated by ChEMBL | Assay Description Inhibition of human renin | Eur J Med Chem 46: 2469-76 (2011) Article DOI: 10.1016/j.ejmech.2011.03.035 BindingDB Entry DOI: 10.7270/Q2GM87NM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4552 (4-[(2,4-Dichloro-5-methoxyphenyl)amino]-6-methoxy-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Konkuk University Curated by ChEMBL | Assay Description Inhibition of Src kinase (unknown origin) | Bioorg Med Chem 17: 3152-61 (2009) Article DOI: 10.1016/j.bmc.2009.02.054 BindingDB Entry DOI: 10.7270/Q2V9880T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 403 total ) | Next | Last >> |