

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50090474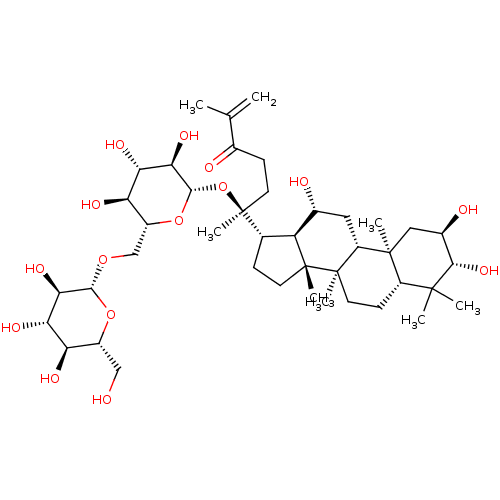 (CHEMBL3581710) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of IL6-induced STAT3 activation in human Hep3B cells after 12 hrs by luciferase assay | J Nat Prod 78: 971-6 (2015) Article DOI: 10.1021/np500803e BindingDB Entry DOI: 10.7270/Q22B90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50090474 (CHEMBL3581710) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of IL6-induced STAT3 activation in human Hep3B cells after 12 hrs by luciferase assay | J Nat Prod 78: 971-6 (2015) Article DOI: 10.1021/np500803e BindingDB Entry DOI: 10.7270/Q22B90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM24567 ((2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]tridecan-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenylbutyrate as substrate assessed as formation of p-nitrophenol preincubated for 15 mins foll... | Bioorg Med Chem Lett 25: 2269-74 (2015) Article DOI: 10.1016/j.bmcl.2015.04.045 BindingDB Entry DOI: 10.7270/Q2GF0W7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM24567 ((2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]tridecan-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenylbutyrate as substrate preincubated for 15 mins prior substrate addition measured after 15 ... | Bioorg Med Chem Lett 22: 2760-3 (2012) Article DOI: 10.1016/j.bmcl.2012.02.088 BindingDB Entry DOI: 10.7270/Q2H41SFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM24567 ((2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]tridecan-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase pre-incubated for 15 mins before p-nitrophenylbutyrate substrate addition by microplate reader based method | Bioorg Med Chem Lett 25: 3455-7 (2015) Article DOI: 10.1016/j.bmcl.2015.07.017 BindingDB Entry DOI: 10.7270/Q2H41T73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50090504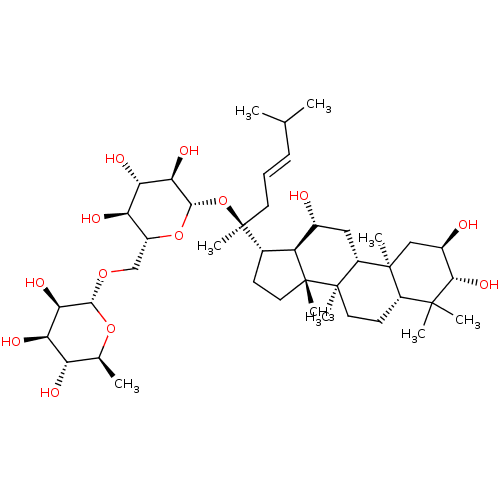 (CHEMBL3581714) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of IL6-induced STAT3 activation in human Hep3B cells after 12 hrs by luciferase assay | J Nat Prod 78: 971-6 (2015) Article DOI: 10.1021/np500803e BindingDB Entry DOI: 10.7270/Q22B90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50090504 (CHEMBL3581714) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of IL6-induced STAT3 activation in human Hep3B cells after 12 hrs by luciferase assay | J Nat Prod 78: 971-6 (2015) Article DOI: 10.1021/np500803e BindingDB Entry DOI: 10.7270/Q22B90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50090483 (CHEMBL3581712) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of IL6-induced STAT3 activation in human Hep3B cells after 12 hrs by luciferase assay | J Nat Prod 78: 971-6 (2015) Article DOI: 10.1021/np500803e BindingDB Entry DOI: 10.7270/Q22B90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50090483 (CHEMBL3581712) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of IL6-induced STAT3 activation in human Hep3B cells after 12 hrs by luciferase assay | J Nat Prod 78: 971-6 (2015) Article DOI: 10.1021/np500803e BindingDB Entry DOI: 10.7270/Q22B90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50090486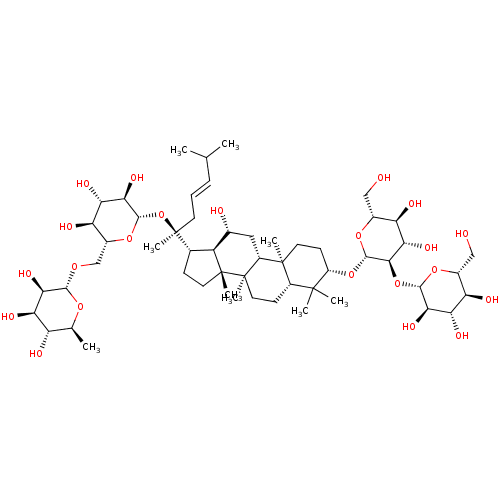 (CHEMBL3581713) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of IL6-induced STAT3 activation in human Hep3B cells after 12 hrs by luciferase assay | J Nat Prod 78: 971-6 (2015) Article DOI: 10.1021/np500803e BindingDB Entry DOI: 10.7270/Q22B90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50090486 (CHEMBL3581713) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of IL6-induced STAT3 activation in human Hep3B cells after 12 hrs by luciferase assay | J Nat Prod 78: 971-6 (2015) Article DOI: 10.1021/np500803e BindingDB Entry DOI: 10.7270/Q22B90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50251013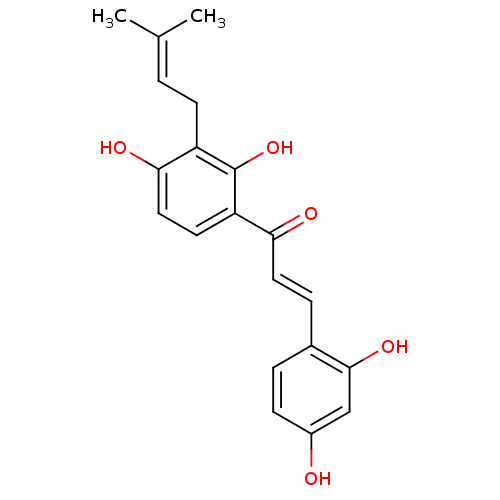 (2,4,2',4'-tetrahydroxy-3'-prenylchalcone | CHEMBL4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenylbutyrate as substrate assessed as formation of p-nitrophenol preincubated for 15 mins foll... | Bioorg Med Chem Lett 25: 2269-74 (2015) Article DOI: 10.1016/j.bmcl.2015.04.045 BindingDB Entry DOI: 10.7270/Q2GF0W7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50193719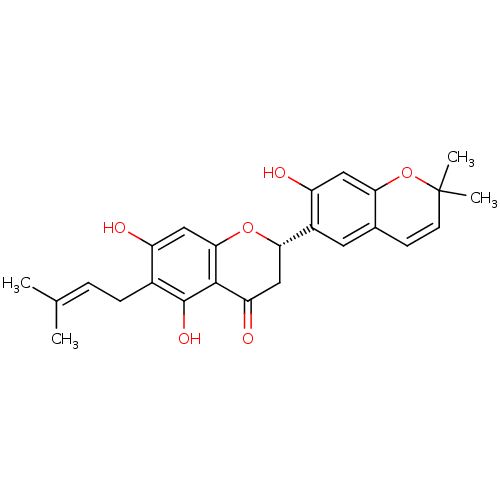 ((S)-5,7,7'-trihydroxy-2',2'-dimethyl-6-(3-methylbu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase pre-incubated for 15 mins before p-nitrophenylbutyrate substrate addition by microplate reader based method | Bioorg Med Chem Lett 25: 3455-7 (2015) Article DOI: 10.1016/j.bmcl.2015.07.017 BindingDB Entry DOI: 10.7270/Q2H41T73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50193723 ((S)-2-(2,4-dihydroxy-5-(3-methylbut-2-enyl)phenyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase pre-incubated for 15 mins before p-nitrophenylbutyrate substrate addition by microplate reader based method | Bioorg Med Chem Lett 25: 3455-7 (2015) Article DOI: 10.1016/j.bmcl.2015.07.017 BindingDB Entry DOI: 10.7270/Q2H41T73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha inhibitor (Homo sapiens (Human)) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sejong University Curated by ChEMBL | Assay Description Inhibition of human recombinant FIH1 | Bioorg Med Chem 17: 7769-74 (2009) Article DOI: 10.1016/j.bmc.2009.09.034 BindingDB Entry DOI: 10.7270/Q2TM7B6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50090480 (CHEMBL3581705) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of IL6-induced STAT3 activation in human Hep3B cells after 12 hrs by luciferase assay | J Nat Prod 78: 971-6 (2015) Article DOI: 10.1021/np500803e BindingDB Entry DOI: 10.7270/Q22B90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50090480 (CHEMBL3581705) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of IL6-induced STAT3 activation in human Hep3B cells after 12 hrs by luciferase assay | J Nat Prod 78: 971-6 (2015) Article DOI: 10.1021/np500803e BindingDB Entry DOI: 10.7270/Q22B90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50090481 (CHEMBL3581711) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of IL6-induced STAT3 activation in human Hep3B cells after 12 hrs by luciferase assay | J Nat Prod 78: 971-6 (2015) Article DOI: 10.1021/np500803e BindingDB Entry DOI: 10.7270/Q22B90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50090481 (CHEMBL3581711) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of IL6-induced STAT3 activation in human Hep3B cells after 12 hrs by luciferase assay | J Nat Prod 78: 971-6 (2015) Article DOI: 10.1021/np500803e BindingDB Entry DOI: 10.7270/Q22B90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50242015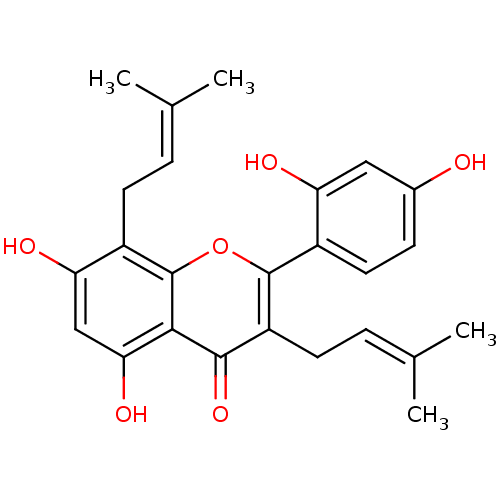 (CHEMBL518543 | Kuwanon C, 4 | kuwanon C) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenylbutyrate as substrate assessed as formation of p-nitrophenol preincubated for 15 mins foll... | Bioorg Med Chem Lett 25: 2269-74 (2015) Article DOI: 10.1016/j.bmcl.2015.04.045 BindingDB Entry DOI: 10.7270/Q2GF0W7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50090475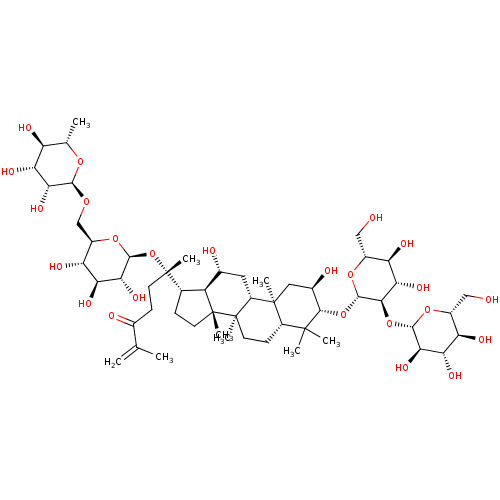 (CHEMBL3581709) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of IL6-induced STAT3 activation in human Hep3B cells after 12 hrs by luciferase assay | J Nat Prod 78: 971-6 (2015) Article DOI: 10.1021/np500803e BindingDB Entry DOI: 10.7270/Q22B90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50090475 (CHEMBL3581709) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of IL6-induced STAT3 activation in human Hep3B cells after 12 hrs by luciferase assay | J Nat Prod 78: 971-6 (2015) Article DOI: 10.1021/np500803e BindingDB Entry DOI: 10.7270/Q22B90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4A [1-973] (Homo sapiens (Human)) | BDBM50193145 (2-(1-chloro-4-hydroxyisoquinoline-3-carboxamido)ac...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Oxford | Assay Description Inhibition assays were carried out in 384-well white ProxiPlates(PerkinElmer) in 10 μL of reaction volume. Standard reaction mixturesconsisted o... | ACS Chem Biol 8: 1488-96 (2013) Article DOI: 10.1021/cb400088q BindingDB Entry DOI: 10.7270/Q29Z93HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4C [1-973] (Homo sapiens (Human)) | BDBM50193145 (2-(1-chloro-4-hydroxyisoquinoline-3-carboxamido)ac...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Oxford | Assay Description Inhibition assays were carried out in 384-well white ProxiPlates(PerkinElmer) in 10 μL of reaction volume. Standard reaction mixturesconsisted o... | ACS Chem Biol 8: 1488-96 (2013) Article DOI: 10.1021/cb400088q BindingDB Entry DOI: 10.7270/Q29Z93HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4E (Homo sapiens (Human)) | BDBM50193145 (2-(1-chloro-4-hydroxyisoquinoline-3-carboxamido)ac...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Oxford | Assay Description Inhibition assays were carried out in 384-well white ProxiPlates(PerkinElmer) in 10 μL of reaction volume. Standard reaction mixturesconsisted o... | ACS Chem Biol 8: 1488-96 (2013) Article DOI: 10.1021/cb400088q BindingDB Entry DOI: 10.7270/Q29Z93HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50193145 (2-(1-chloro-4-hydroxyisoquinoline-3-carboxamido)ac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Oxford | Assay Description Inhibition assays were carried out in 384-well white ProxiPlates(PerkinElmer) in 10 μL of reaction volume. Standard reaction mixturesconsisted o... | ACS Chem Biol 8: 1488-96 (2013) Article DOI: 10.1021/cb400088q BindingDB Entry DOI: 10.7270/Q29Z93HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 2A (Homo sapiens (Human)) | BDBM50193145 (2-(1-chloro-4-hydroxyisoquinoline-3-carboxamido)ac...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Oxford | Assay Description Inhibition assays were carried out in 384-well white ProxiPlates(PerkinElmer) in 10 μL of reaction volume. Standard reaction mixturesconsisted o... | ACS Chem Biol 8: 1488-96 (2013) Article DOI: 10.1021/cb400088q BindingDB Entry DOI: 10.7270/Q29Z93HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 3A (Homo sapiens (Human)) | BDBM50193145 (2-(1-chloro-4-hydroxyisoquinoline-3-carboxamido)ac...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Oxford | Assay Description Inhibition assays were carried out in 384-well white ProxiPlates(PerkinElmer) in 10 μL of reaction volume. Standard reaction mixturesconsisted o... | ACS Chem Biol 8: 1488-96 (2013) Article DOI: 10.1021/cb400088q BindingDB Entry DOI: 10.7270/Q29Z93HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50381001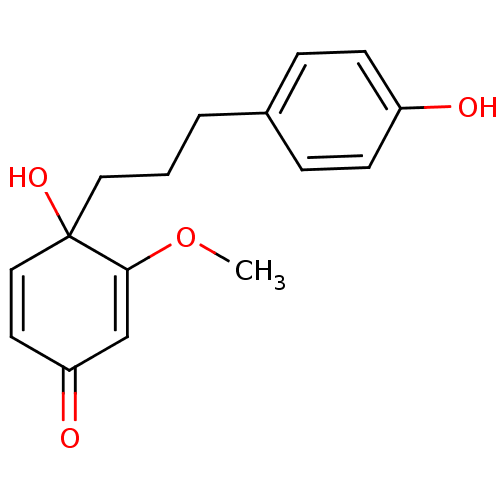 (CHEMBL2017114) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenylbutyrate as substrate preincubated for 15 mins prior substrate addition measured after 15 ... | Bioorg Med Chem Lett 22: 2760-3 (2012) Article DOI: 10.1016/j.bmcl.2012.02.088 BindingDB Entry DOI: 10.7270/Q2H41SFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50083074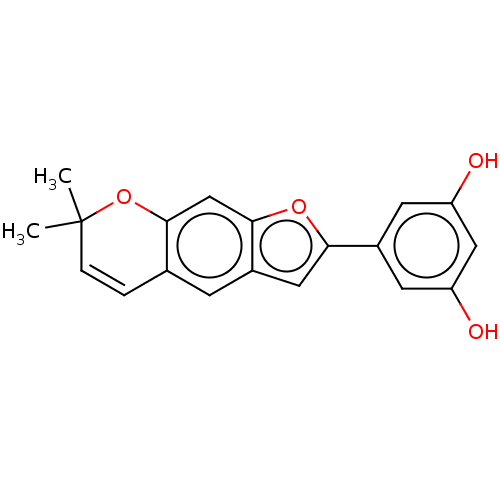 (CHEMBL3422851) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenylbutyrate as substrate assessed as formation of p-nitrophenol preincubated for 15 mins foll... | Bioorg Med Chem Lett 25: 2269-74 (2015) Article DOI: 10.1016/j.bmcl.2015.04.045 BindingDB Entry DOI: 10.7270/Q2GF0W7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50251014 (CHEMBL465881 | moracin N) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenylbutyrate as substrate assessed as formation of p-nitrophenol preincubated for 15 mins foll... | Bioorg Med Chem Lett 25: 2269-74 (2015) Article DOI: 10.1016/j.bmcl.2015.04.045 BindingDB Entry DOI: 10.7270/Q2GF0W7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50090476 (CHEMBL3581708) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of IL6-induced STAT3 activation in human Hep3B cells after 12 hrs by luciferase assay | J Nat Prod 78: 971-6 (2015) Article DOI: 10.1021/np500803e BindingDB Entry DOI: 10.7270/Q22B90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50090476 (CHEMBL3581708) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of IL6-induced STAT3 activation in human Hep3B cells after 12 hrs by luciferase assay | J Nat Prod 78: 971-6 (2015) Article DOI: 10.1021/np500803e BindingDB Entry DOI: 10.7270/Q22B90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50090477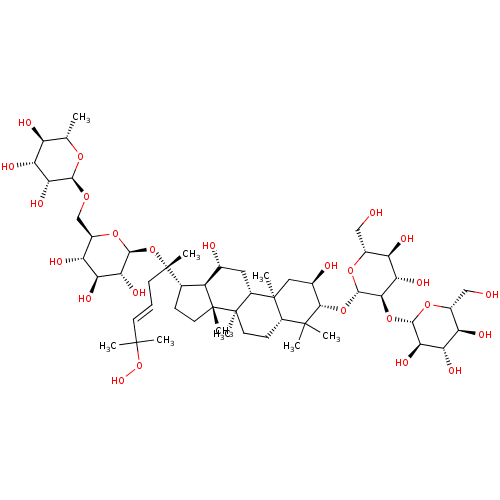 (CHEMBL3581707) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of IL6-induced STAT3 activation in human Hep3B cells after 12 hrs by luciferase assay | J Nat Prod 78: 971-6 (2015) Article DOI: 10.1021/np500803e BindingDB Entry DOI: 10.7270/Q22B90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50090477 (CHEMBL3581707) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of IL6-induced STAT3 activation in human Hep3B cells after 12 hrs by luciferase assay | J Nat Prod 78: 971-6 (2015) Article DOI: 10.1021/np500803e BindingDB Entry DOI: 10.7270/Q22B90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50090478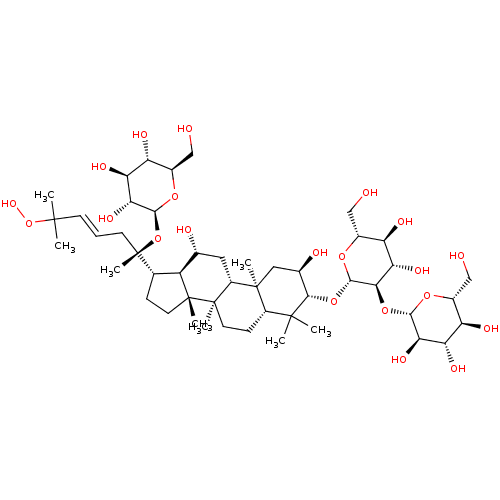 (CHEMBL3581706) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of IL6-induced STAT3 activation in human Hep3B cells after 12 hrs by luciferase assay | J Nat Prod 78: 971-6 (2015) Article DOI: 10.1021/np500803e BindingDB Entry DOI: 10.7270/Q22B90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50090478 (CHEMBL3581706) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of IL6-induced STAT3 activation in human Hep3B cells after 12 hrs by luciferase assay | J Nat Prod 78: 971-6 (2015) Article DOI: 10.1021/np500803e BindingDB Entry DOI: 10.7270/Q22B90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50090479 (CHEMBL3581704) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of IL6-induced STAT3 activation in human Hep3B cells after 12 hrs by luciferase assay | J Nat Prod 78: 971-6 (2015) Article DOI: 10.1021/np500803e BindingDB Entry DOI: 10.7270/Q22B90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50090479 (CHEMBL3581704) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of IL6-induced STAT3 activation in human Hep3B cells after 12 hrs by luciferase assay | J Nat Prod 78: 971-6 (2015) Article DOI: 10.1021/np500803e BindingDB Entry DOI: 10.7270/Q22B90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50381284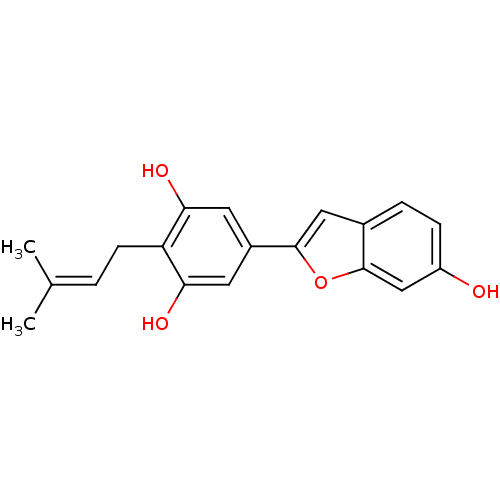 (CHEMBL2018876) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenylbutyrate as substrate assessed as formation of p-nitrophenol preincubated for 15 mins foll... | Bioorg Med Chem Lett 25: 2269-74 (2015) Article DOI: 10.1016/j.bmcl.2015.04.045 BindingDB Entry DOI: 10.7270/Q2GF0W7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha inhibitor (Homo sapiens (Human)) | BDBM50304610 (3-(5-(2-methoxybenzylidene)-4-oxo-2-thioxothiazoli...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sejong University Curated by ChEMBL | Assay Description Inhibition of human recombinant FIH1 | Bioorg Med Chem 17: 7769-74 (2009) Article DOI: 10.1016/j.bmc.2009.09.034 BindingDB Entry DOI: 10.7270/Q2TM7B6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha inhibitor (Homo sapiens (Human)) | BDBM50304612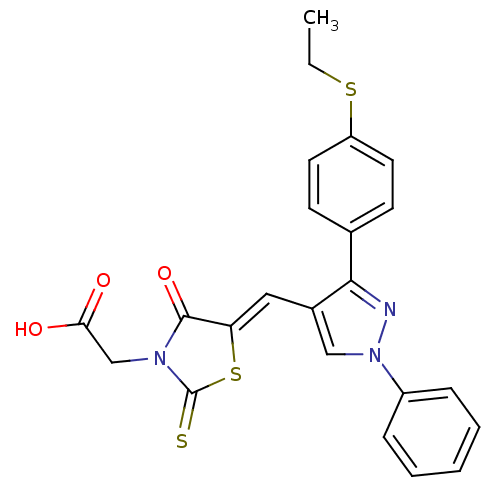 (2-(5-((3-(4-(ethylthio)phenyl)-1-phenyl-1H-pyrazol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sejong University Curated by ChEMBL | Assay Description Inhibition of human recombinant FIH1 | Bioorg Med Chem 17: 7769-74 (2009) Article DOI: 10.1016/j.bmc.2009.09.034 BindingDB Entry DOI: 10.7270/Q2TM7B6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha inhibitor (Homo sapiens (Human)) | BDBM50304617 (6-((2-methoxyphenoxy)methyl)-3-(quinolin-2-yl)thia...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sejong University Curated by ChEMBL | Assay Description Inhibition of human recombinant FIH1 | Bioorg Med Chem 17: 7769-74 (2009) Article DOI: 10.1016/j.bmc.2009.09.034 BindingDB Entry DOI: 10.7270/Q2TM7B6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4A [1-973] (Homo sapiens (Human)) | BDBM103845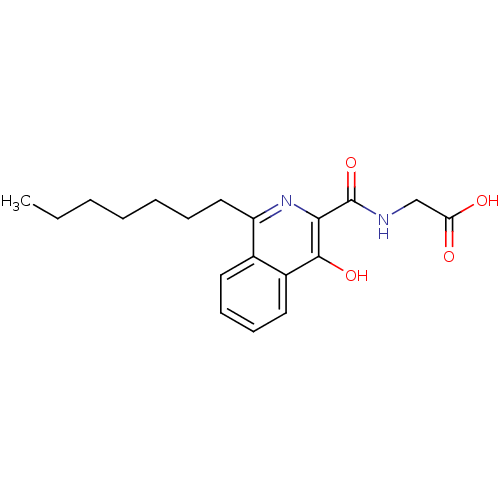 (4HQ derivative 8) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Oxford | Assay Description Inhibition assays were carried out in 384-well white ProxiPlates(PerkinElmer) in 10 μL of reaction volume. Standard reaction mixturesconsisted o... | ACS Chem Biol 8: 1488-96 (2013) Article DOI: 10.1021/cb400088q BindingDB Entry DOI: 10.7270/Q29Z93HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha inhibitor (Homo sapiens (Human)) | BDBM50304614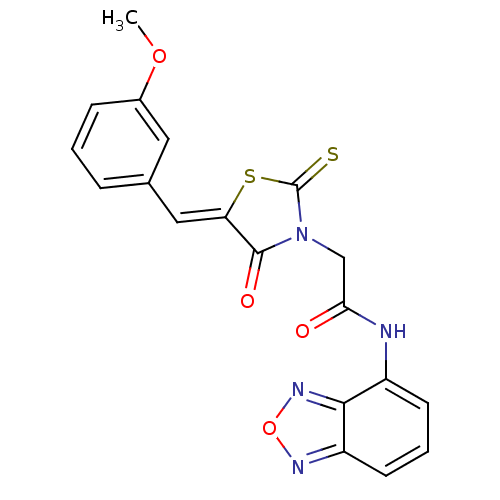 (CHEMBL594893 | N-(benzo[c][1,2,5]oxadiazol-4-yl)-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sejong University Curated by ChEMBL | Assay Description Inhibition of human recombinant FIH1 | Bioorg Med Chem 17: 7769-74 (2009) Article DOI: 10.1016/j.bmc.2009.09.034 BindingDB Entry DOI: 10.7270/Q2TM7B6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha inhibitor (Homo sapiens (Human)) | BDBM50304616 (6-(2-methoxystyryl)-3-(quinolin-2-yl)thiazolo[2,3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sejong University Curated by ChEMBL | Assay Description Inhibition of human recombinant FIH1 | Bioorg Med Chem 17: 7769-74 (2009) Article DOI: 10.1016/j.bmc.2009.09.034 BindingDB Entry DOI: 10.7270/Q2TM7B6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha inhibitor (Homo sapiens (Human)) | BDBM50304613 (CHEMBL593127 | N-(benzo[c][1,2,5]oxadiazol-4-yl)-3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sejong University Curated by ChEMBL | Assay Description Inhibition of human recombinant FIH1 | Bioorg Med Chem 17: 7769-74 (2009) Article DOI: 10.1016/j.bmc.2009.09.034 BindingDB Entry DOI: 10.7270/Q2TM7B6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 2A (Homo sapiens (Human)) | BDBM103843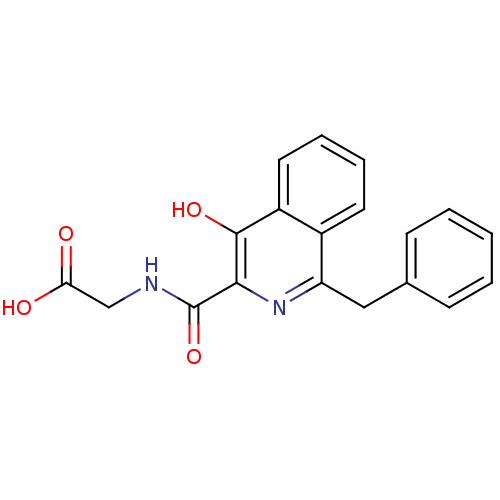 (4HQ derivative 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Oxford | Assay Description Inhibition assays were carried out in 384-well white ProxiPlates(PerkinElmer) in 10 μL of reaction volume. Standard reaction mixturesconsisted o... | ACS Chem Biol 8: 1488-96 (2013) Article DOI: 10.1021/cb400088q BindingDB Entry DOI: 10.7270/Q29Z93HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50114928 (CHEMBL3609152) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase pre-incubated for 15 mins before p-nitrophenylbutyrate substrate addition by microplate reader based method | Bioorg Med Chem Lett 25: 3455-7 (2015) Article DOI: 10.1016/j.bmcl.2015.07.017 BindingDB Entry DOI: 10.7270/Q2H41T73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50083070 (CHEMBL3422848) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenylbutyrate as substrate assessed as formation of p-nitrophenol preincubated for 15 mins foll... | Bioorg Med Chem Lett 25: 2269-74 (2015) Article DOI: 10.1016/j.bmcl.2015.04.045 BindingDB Entry DOI: 10.7270/Q2GF0W7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 119 total ) | Next | Last >> |