 Found 1062 hits with Last Name = 'lee' and Initial = 'n'
Found 1062 hits with Last Name = 'lee' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase 17B
(Homo sapiens (Human)) | BDBM50166121
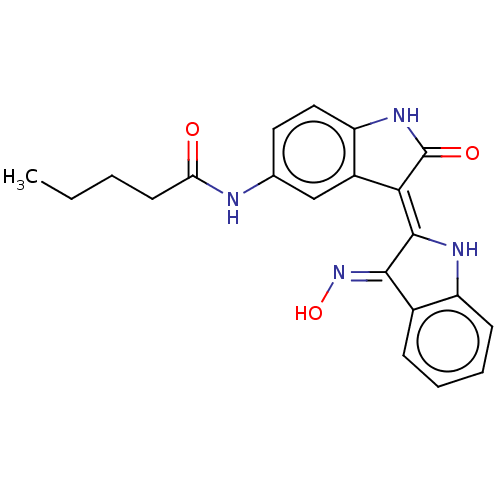
(CHEMBL3797480)Show SMILES CCCCC(=O)Nc1ccc2NC(=O)\C(=C3/Nc4ccccc4/C/3=N\O)c2c1 Show InChI InChI=1S/C21H20N4O3/c1-2-3-8-17(26)22-12-9-10-16-14(11-12)18(21(27)24-16)20-19(25-28)13-6-4-5-7-15(13)23-20/h4-7,9-11,23,28H,2-3,8H2,1H3,(H,22,26)(H,24,27)/b20-18-,25-19+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Competitive inhibition of DRAK2 (unknown origin) using MRLC3 peptide as substrate incubated for 2 hrs by Lineweaver-Burk plot analysis in presence of... |
Bioorg Med Chem Lett 26: 2719-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.111
BindingDB Entry DOI: 10.7270/Q2N29ZTZ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | CHEMBL4782535
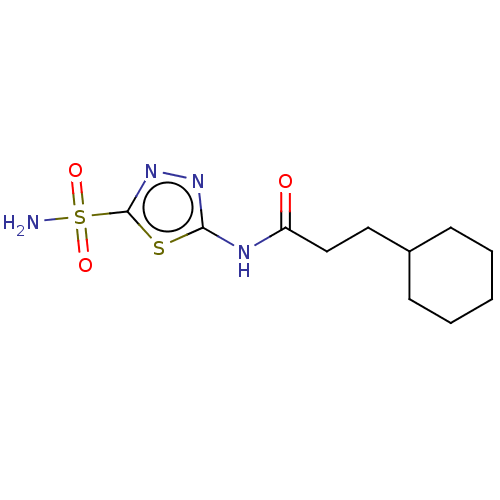
Show InChI InChI=1S/C13H15NO5S2/c1-8(2)7-18-13(15)19-10-4-3-9-5-12(21(14,16)17)20-11(9)6-10/h3-6,8H,7H2,1-2H3,(H2,14,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
| | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antihistaminic activity against Histamine H1 receptor was measured on isolated terminal part of guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50240552
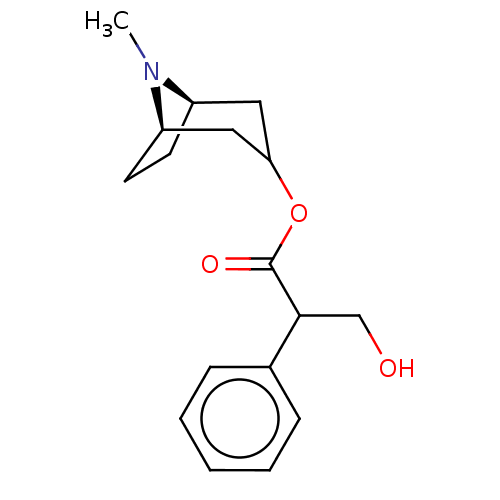
(CHEMBL195)Show SMILES [H][C@@]12CC[C@]([H])(CC(C1)OC(=O)C(CO)c1ccccc1)N2C |r,@@:7,TLB:9:7:21:2.3| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3/t13-,14-,16?/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human M1 AChR expressed in CHO cell membranes after 1 to 2 hrs by liquid scintillation spectrometry method |
Bioorg Med Chem Lett 29: 471-476 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.022
BindingDB Entry DOI: 10.7270/Q2NP27SC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50240552

(CHEMBL195)Show SMILES [H][C@@]12CC[C@]([H])(CC(C1)OC(=O)C(CO)c1ccccc1)N2C |r,@@:7,TLB:9:7:21:2.3| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3/t13-,14-,16?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human M3 AChR expressed in CHO cell membranes after 1 to 2 hrs by liquid scintillation spectrometry method |
Bioorg Med Chem Lett 29: 471-476 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.022
BindingDB Entry DOI: 10.7270/Q2NP27SC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM50240552

(CHEMBL195)Show SMILES [H][C@@]12CC[C@]([H])(CC(C1)OC(=O)C(CO)c1ccccc1)N2C |r,@@:7,TLB:9:7:21:2.3| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3/t13-,14-,16?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human M5 AChR expressed in CHO cell membranes after 1 to 2 hrs by liquid scintillation spectrometry method |
Bioorg Med Chem Lett 29: 471-476 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.022
BindingDB Entry DOI: 10.7270/Q2NP27SC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50130293

(7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...)Show SMILES Clc1cccc(N2CCN(CCCCOc3ccc4CCC(=O)Nc4c3)CC2)c1Cl Show InChI InChI=1S/C23H27Cl2N3O2/c24-19-4-3-5-21(23(19)25)28-13-11-27(12-14-28)10-1-2-15-30-18-8-6-17-7-9-22(29)26-20(17)16-18/h3-6,8,16H,1-2,7,9-15H2,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT2A |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313283

(1-{2-[4-(6-Fluoro-1H-indol-3-yl)-3,6-dihydro-2H-py...)Show SMILES Fc1ccc2c(c[nH]c2c1)C1=CCN(CCN2c3cccc4CCCN(c34)S2(=O)=O)CC1 |t:12| Show InChI InChI=1S/C24H25FN4O2S/c25-19-6-7-20-21(16-26-22(20)15-19)17-8-11-27(12-9-17)13-14-28-23-5-1-3-18-4-2-10-29(24(18)23)32(28,30)31/h1,3,5-8,15-16,26H,2,4,9-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT2A |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50240552

(CHEMBL195)Show SMILES [H][C@@]12CC[C@]([H])(CC(C1)OC(=O)C(CO)c1ccccc1)N2C |r,@@:7,TLB:9:7:21:2.3| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3/t13-,14-,16?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human M2 AChR expressed in CHO cell membranes after 1 to 2 hrs by liquid scintillation spectrometry method |
Bioorg Med Chem Lett 29: 471-476 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.022
BindingDB Entry DOI: 10.7270/Q2NP27SC |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50011322
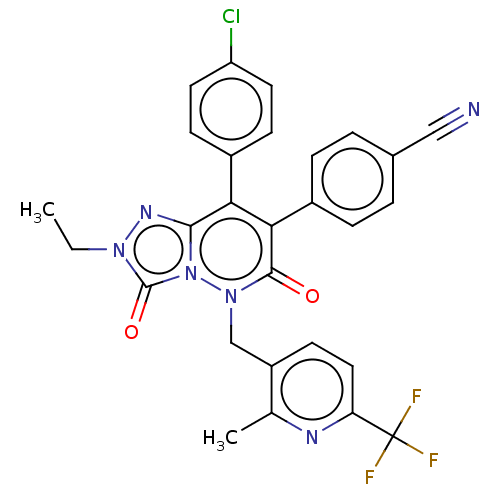
(CHEMBL3260745)Show SMILES CCn1nc2c(-c3ccc(Cl)cc3)c(-c3ccc(cc3)C#N)c(=O)n(Cc3ccc(nc3C)C(F)(F)F)n2c1=O Show InChI InChI=1S/C28H20ClF3N6O2/c1-3-36-27(40)38-25(35-36)23(18-8-11-21(29)12-9-18)24(19-6-4-17(14-33)5-7-19)26(39)37(38)15-20-10-13-22(28(30,31)32)34-16(20)2/h4-13H,3,15H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50011324
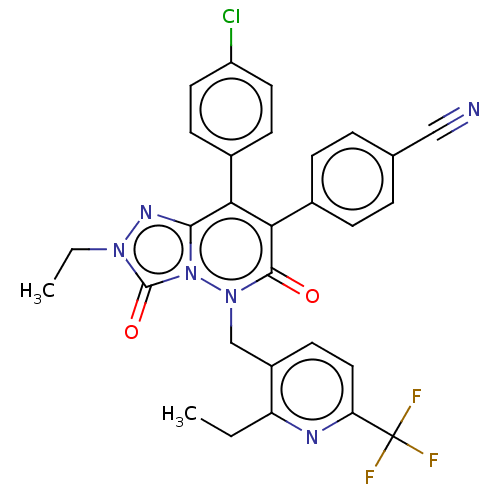
(CHEMBL3260747)Show SMILES CCc1nc(ccc1Cn1n2c(nn(CC)c2=O)c(-c2ccc(Cl)cc2)c(-c2ccc(cc2)C#N)c1=O)C(F)(F)F Show InChI InChI=1S/C29H22ClF3N6O2/c1-3-22-20(11-14-23(35-22)29(31,32)33)16-38-27(40)25(19-7-5-17(15-34)6-8-19)24(18-9-12-21(30)13-10-18)26-36-37(4-2)28(41)39(26)38/h5-14H,3-4,16H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50011328
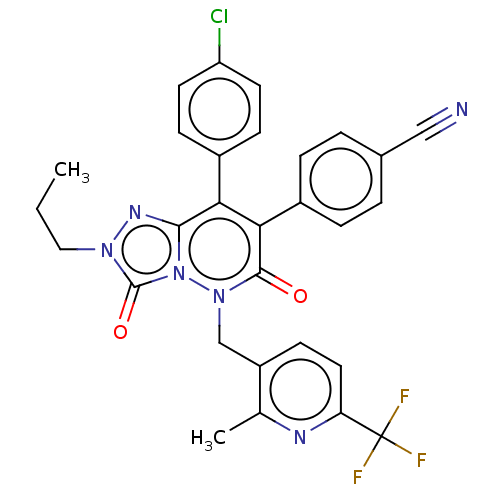
(CHEMBL3259829)Show SMILES CCCn1nc2c(-c3ccc(Cl)cc3)c(-c3ccc(cc3)C#N)c(=O)n(Cc3ccc(nc3C)C(F)(F)F)n2c1=O Show InChI InChI=1S/C29H22ClF3N6O2/c1-3-14-37-28(41)39-26(36-37)24(19-8-11-22(30)12-9-19)25(20-6-4-18(15-34)5-7-20)27(40)38(39)16-21-10-13-23(29(31,32)33)35-17(21)2/h4-13H,3,14,16H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM50518557

(CHEMBL4519301)Show SMILES Fc1ccc(CCN(C(=O)OC2CN3CCC2CC3)c2ccccc2)cc1 |TLB:10:11:14.15:17.18,(18.31,-36.25,;16.97,-35.49,;15.64,-36.26,;14.31,-35.49,;14.31,-33.95,;12.98,-33.18,;11.65,-33.95,;10.31,-33.18,;8.98,-33.95,;8.98,-35.49,;7.65,-33.18,;6.31,-33.95,;5.96,-35.38,;4.48,-34.87,;4.47,-32.84,;5.07,-31.58,;5.1,-33.5,;3.39,-33.95,;2.99,-35.39,;10.31,-31.64,;8.98,-30.87,;8.98,-29.32,;10.31,-28.55,;11.65,-29.32,;11.65,-30.87,;15.64,-33.17,;16.97,-33.94,)| Show InChI InChI=1S/C22H25FN2O2/c23-19-8-6-17(7-9-19)10-15-25(20-4-2-1-3-5-20)22(26)27-21-16-24-13-11-18(21)12-14-24/h1-9,18,21H,10-16H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human M5 AChR expressed in CHO cell membranes after 1 to 2 hrs by liquid scintillation spectrometry method |
Bioorg Med Chem Lett 29: 471-476 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.022
BindingDB Entry DOI: 10.7270/Q2NP27SC |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50011326
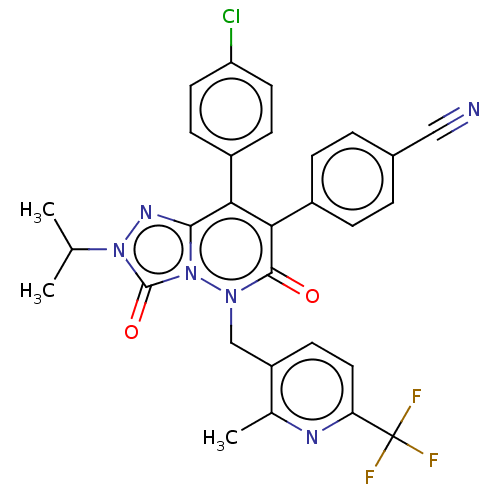
(CHEMBL3260748)Show SMILES CC(C)n1nc2c(-c3ccc(Cl)cc3)c(-c3ccc(cc3)C#N)c(=O)n(Cc3ccc(nc3C)C(F)(F)F)n2c1=O Show InChI InChI=1S/C29H22ClF3N6O2/c1-16(2)38-28(41)39-26(36-38)24(19-8-11-22(30)12-9-19)25(20-6-4-18(14-34)5-7-20)27(40)37(39)15-21-10-13-23(29(31,32)33)35-17(21)3/h4-13,16H,15H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50518557

(CHEMBL4519301)Show SMILES Fc1ccc(CCN(C(=O)OC2CN3CCC2CC3)c2ccccc2)cc1 |TLB:10:11:14.15:17.18,(18.31,-36.25,;16.97,-35.49,;15.64,-36.26,;14.31,-35.49,;14.31,-33.95,;12.98,-33.18,;11.65,-33.95,;10.31,-33.18,;8.98,-33.95,;8.98,-35.49,;7.65,-33.18,;6.31,-33.95,;5.96,-35.38,;4.48,-34.87,;4.47,-32.84,;5.07,-31.58,;5.1,-33.5,;3.39,-33.95,;2.99,-35.39,;10.31,-31.64,;8.98,-30.87,;8.98,-29.32,;10.31,-28.55,;11.65,-29.32,;11.65,-30.87,;15.64,-33.17,;16.97,-33.94,)| Show InChI InChI=1S/C22H25FN2O2/c23-19-8-6-17(7-9-19)10-15-25(20-4-2-1-3-5-20)22(26)27-21-16-24-13-11-18(21)12-14-24/h1-9,18,21H,10-16H2 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human M1 AChR expressed in CHO cell membranes after 1 to 2 hrs by liquid scintillation spectrometry method |
Bioorg Med Chem Lett 29: 471-476 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.022
BindingDB Entry DOI: 10.7270/Q2NP27SC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50518557

(CHEMBL4519301)Show SMILES Fc1ccc(CCN(C(=O)OC2CN3CCC2CC3)c2ccccc2)cc1 |TLB:10:11:14.15:17.18,(18.31,-36.25,;16.97,-35.49,;15.64,-36.26,;14.31,-35.49,;14.31,-33.95,;12.98,-33.18,;11.65,-33.95,;10.31,-33.18,;8.98,-33.95,;8.98,-35.49,;7.65,-33.18,;6.31,-33.95,;5.96,-35.38,;4.48,-34.87,;4.47,-32.84,;5.07,-31.58,;5.1,-33.5,;3.39,-33.95,;2.99,-35.39,;10.31,-31.64,;8.98,-30.87,;8.98,-29.32,;10.31,-28.55,;11.65,-29.32,;11.65,-30.87,;15.64,-33.17,;16.97,-33.94,)| Show InChI InChI=1S/C22H25FN2O2/c23-19-8-6-17(7-9-19)10-15-25(20-4-2-1-3-5-20)22(26)27-21-16-24-13-11-18(21)12-14-24/h1-9,18,21H,10-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human M4 AChR expressed in CHO cell membranes after 1 to 2 hrs by liquid scintillation spectrometry method |
Bioorg Med Chem Lett 29: 471-476 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.022
BindingDB Entry DOI: 10.7270/Q2NP27SC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313283

(1-{2-[4-(6-Fluoro-1H-indol-3-yl)-3,6-dihydro-2H-py...)Show SMILES Fc1ccc2c(c[nH]c2c1)C1=CCN(CCN2c3cccc4CCCN(c34)S2(=O)=O)CC1 |t:12| Show InChI InChI=1S/C24H25FN4O2S/c25-19-6-7-20-21(16-26-22(20)15-19)17-8-11-27(12-9-17)13-14-28-23-5-1-3-18-4-2-10-29(24(18)23)32(28,30)31/h1,3,5-8,15-16,26H,2,4,9-14H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to SERT |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50518557

(CHEMBL4519301)Show SMILES Fc1ccc(CCN(C(=O)OC2CN3CCC2CC3)c2ccccc2)cc1 |TLB:10:11:14.15:17.18,(18.31,-36.25,;16.97,-35.49,;15.64,-36.26,;14.31,-35.49,;14.31,-33.95,;12.98,-33.18,;11.65,-33.95,;10.31,-33.18,;8.98,-33.95,;8.98,-35.49,;7.65,-33.18,;6.31,-33.95,;5.96,-35.38,;4.48,-34.87,;4.47,-32.84,;5.07,-31.58,;5.1,-33.5,;3.39,-33.95,;2.99,-35.39,;10.31,-31.64,;8.98,-30.87,;8.98,-29.32,;10.31,-28.55,;11.65,-29.32,;11.65,-30.87,;15.64,-33.17,;16.97,-33.94,)| Show InChI InChI=1S/C22H25FN2O2/c23-19-8-6-17(7-9-19)10-15-25(20-4-2-1-3-5-20)22(26)27-21-16-24-13-11-18(21)12-14-24/h1-9,18,21H,10-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human M3 AChR expressed in CHO cell membranes after 1 to 2 hrs by liquid scintillation spectrometry method |
Bioorg Med Chem Lett 29: 471-476 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.022
BindingDB Entry DOI: 10.7270/Q2NP27SC |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50011330
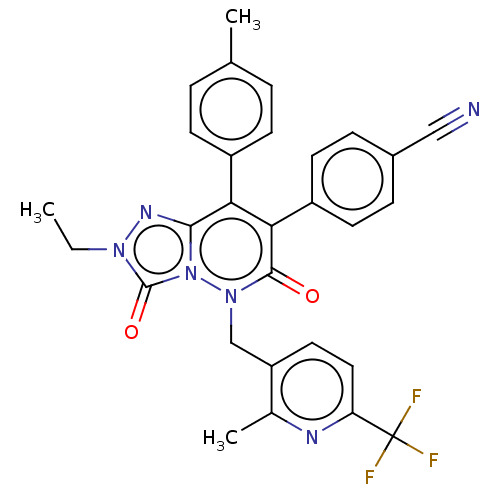
(CHEMBL3260750)Show SMILES CCn1nc2c(-c3ccc(C)cc3)c(-c3ccc(cc3)C#N)c(=O)n(Cc3ccc(nc3C)C(F)(F)F)n2c1=O Show InChI InChI=1S/C29H23F3N6O2/c1-4-36-28(40)38-26(35-36)24(20-9-5-17(2)6-10-20)25(21-11-7-19(15-33)8-12-21)27(39)37(38)16-22-13-14-23(29(30,31)32)34-18(22)3/h5-14H,4,16H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50011323
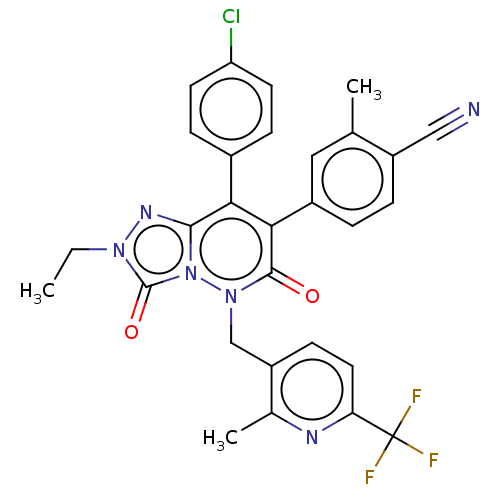
(CHEMBL3260746)Show SMILES CCn1nc2c(-c3ccc(Cl)cc3)c(-c3ccc(C#N)c(C)c3)c(=O)n(Cc3ccc(nc3C)C(F)(F)F)n2c1=O Show InChI InChI=1S/C29H22ClF3N6O2/c1-4-37-28(41)39-26(36-37)24(18-7-10-22(30)11-8-18)25(19-5-6-20(14-34)16(2)13-19)27(40)38(39)15-21-9-12-23(29(31,32)33)35-17(21)3/h5-13H,4,15H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50011334
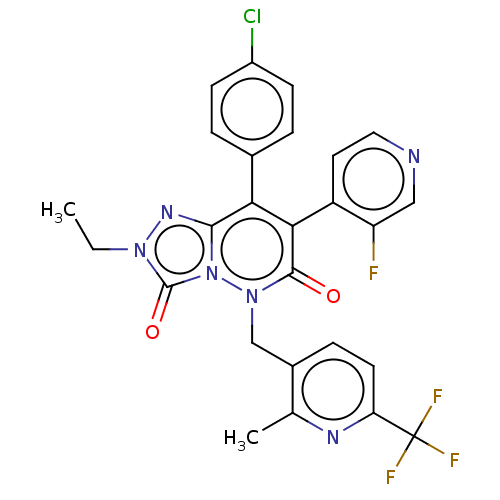
(CHEMBL3260754)Show SMILES CCn1nc2c(-c3ccc(Cl)cc3)c(-c3ccncc3F)c(=O)n(Cc3ccc(nc3C)C(F)(F)F)n2c1=O |(14.54,-16.67,;15.31,-15.33,;14.53,-14,;13,-13.84,;12.68,-12.33,;11.35,-11.56,;10.01,-12.34,;10.02,-13.88,;8.68,-14.65,;7.35,-13.89,;6.01,-14.66,;7.35,-12.34,;8.68,-11.57,;11.34,-10.03,;10.01,-9.26,;8.68,-10.03,;7.35,-9.26,;7.34,-7.72,;8.67,-6.95,;10.01,-7.72,;11.34,-6.95,;12.68,-9.26,;12.67,-7.72,;14.01,-10.02,;15.35,-9.25,;15.34,-7.71,;14,-6.95,;13.98,-5.41,;15.32,-4.62,;16.67,-5.39,;16.67,-6.93,;18.01,-7.69,;15.31,-3.08,;16.64,-2.3,;13.97,-2.32,;15.3,-1.53,;14.01,-11.57,;15.16,-12.6,;16.66,-12.27,)| Show InChI InChI=1S/C26H19ClF4N6O2/c1-3-35-25(39)37-23(34-35)21(15-4-7-17(27)8-5-15)22(18-10-11-32-12-19(18)28)24(38)36(37)13-16-6-9-20(26(29,30)31)33-14(16)2/h4-12H,3,13H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50011310
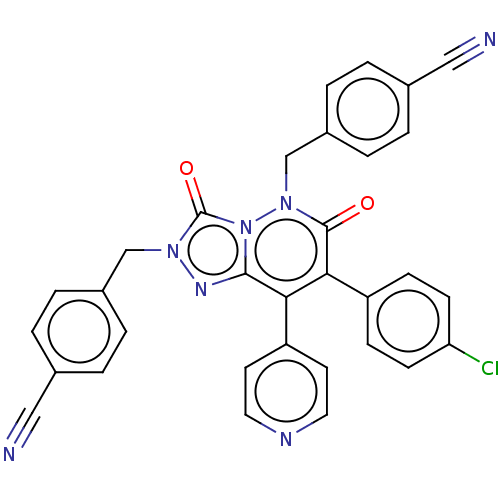
(CHEMBL3260737)Show SMILES Clc1ccc(cc1)-c1c(-c2ccncc2)c2nn(Cc3ccc(cc3)C#N)c(=O)n2n(Cc2ccc(cc2)C#N)c1=O Show InChI InChI=1S/C32H20ClN7O2/c33-27-11-9-25(10-12-27)29-28(26-13-15-36-16-14-26)30-37-38(19-23-5-1-21(17-34)2-6-23)32(42)40(30)39(31(29)41)20-24-7-3-22(18-35)4-8-24/h1-16H,19-20H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50011316
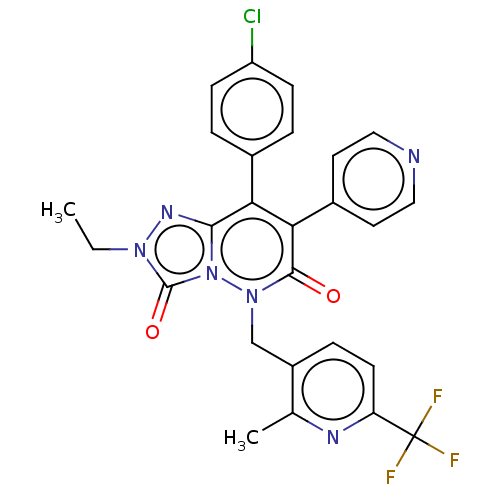
(CHEMBL3260743)Show SMILES CCn1nc2c(-c3ccc(Cl)cc3)c(-c3ccncc3)c(=O)n(Cc3ccc(nc3C)C(F)(F)F)n2c1=O Show InChI InChI=1S/C26H20ClF3N6O2/c1-3-34-25(38)36-23(33-34)21(16-4-7-19(27)8-5-16)22(17-10-12-31-13-11-17)24(37)35(36)14-18-6-9-20(26(28,29)30)32-15(18)2/h4-13H,3,14H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50011333
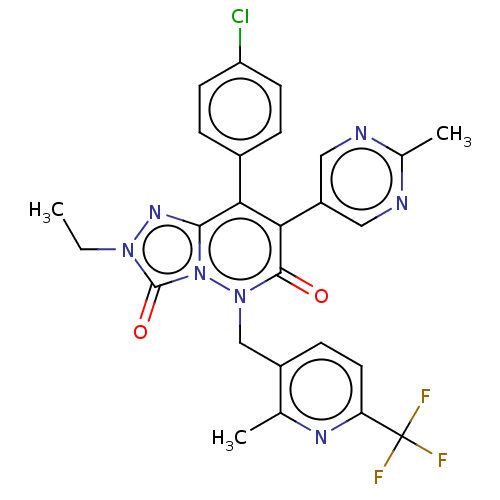
(CHEMBL3260753)Show SMILES CCn1nc2c(-c3ccc(Cl)cc3)c(-c3cnc(C)nc3)c(=O)n(Cc3ccc(nc3C)C(F)(F)F)n2c1=O Show InChI InChI=1S/C26H21ClF3N7O2/c1-4-35-25(39)37-23(34-35)21(16-5-8-19(27)9-6-16)22(18-11-31-15(3)32-12-18)24(38)36(37)13-17-7-10-20(26(28,29)30)33-14(17)2/h5-12H,4,13H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50011332
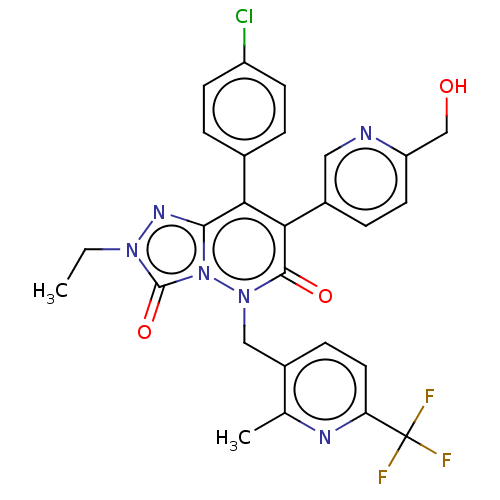
(CHEMBL3260752)Show SMILES CCn1nc2c(-c3ccc(Cl)cc3)c(-c3ccc(CO)nc3)c(=O)n(Cc3ccc(nc3C)C(F)(F)F)n2c1=O Show InChI InChI=1S/C27H22ClF3N6O3/c1-3-35-26(40)37-24(34-35)22(16-4-8-19(28)9-5-16)23(17-6-10-20(14-38)32-12-17)25(39)36(37)13-18-7-11-21(27(29,30)31)33-15(18)2/h4-12,38H,3,13-14H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50011331
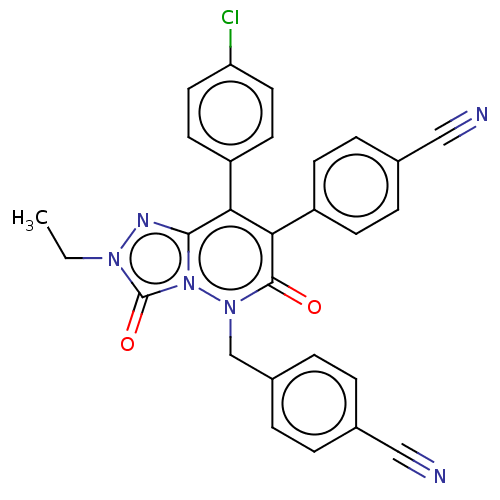
(CHEMBL3260751)Show SMILES CCn1nc2c(-c3ccc(Cl)cc3)c(-c3ccc(cc3)C#N)c(=O)n(Cc3ccc(cc3)C#N)n2c1=O Show InChI InChI=1S/C28H19ClN6O2/c1-2-33-28(37)35-26(32-33)24(21-11-13-23(29)14-12-21)25(22-9-7-19(16-31)8-10-22)27(36)34(35)17-20-5-3-18(15-30)4-6-20/h3-14H,2,17H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50069447
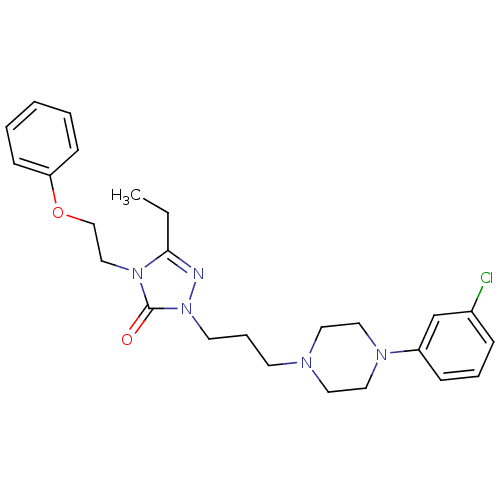
(1-(3-(4-(m-Chlorophenyl)-1-piperazinyl)propyl)-3-e...)Show SMILES CCc1nn(CCCN2CCN(CC2)c2cccc(Cl)c2)c(=O)n1CCOc1ccccc1 Show InChI InChI=1S/C25H32ClN5O2/c1-2-24-27-31(25(32)30(24)18-19-33-23-10-4-3-5-11-23)13-7-12-28-14-16-29(17-15-28)22-9-6-8-21(26)20-22/h3-6,8-11,20H,2,7,12-19H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT2A |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50518559
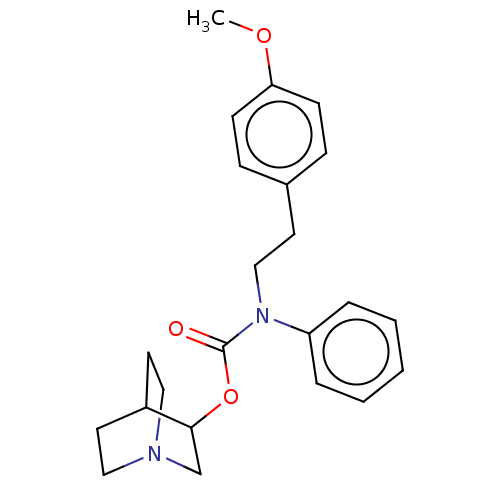
(CHEMBL4585326)Show SMILES COc1ccc(CCN(C(=O)OC2CN3CCC2CC3)c2ccccc2)cc1 |TLB:11:12:15.16:18.19,(19.07,-20.84,;17.73,-21.62,;16.4,-20.85,;15.06,-21.62,;13.73,-20.85,;13.74,-19.31,;12.4,-18.54,;11.07,-19.31,;9.74,-18.54,;8.4,-19.31,;8.4,-20.85,;7.07,-18.54,;5.74,-19.31,;5.39,-20.74,;3.9,-20.23,;3.89,-18.2,;4.49,-16.94,;4.52,-18.86,;2.82,-19.32,;2.41,-20.75,;9.74,-17,;8.4,-16.23,;8.41,-14.68,;9.73,-13.91,;11.07,-14.68,;11.07,-16.23,;15.06,-18.54,;16.39,-19.3,)| Show InChI InChI=1S/C23H28N2O3/c1-27-21-9-7-18(8-10-21)11-16-25(20-5-3-2-4-6-20)23(26)28-22-17-24-14-12-19(22)13-15-24/h2-10,19,22H,11-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human M3 AChR expressed in CHO cell membranes after 1 to 2 hrs by liquid scintillation spectrometry method |
Bioorg Med Chem Lett 29: 471-476 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.022
BindingDB Entry DOI: 10.7270/Q2NP27SC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | CHEMBL4741159

Show InChI InChI=1S/C10H10N2O3S2/c1-6(13)12-8-3-2-7-4-10(17(11,14)15)16-9(7)5-8/h2-5H,1H3,(H,12,13)(H2,11,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
CHEMBL
PC cid
PC sid
UniChem
| | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antihistaminic activity against Histamine H1 receptor was measured on isolated terminal part of guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50011317
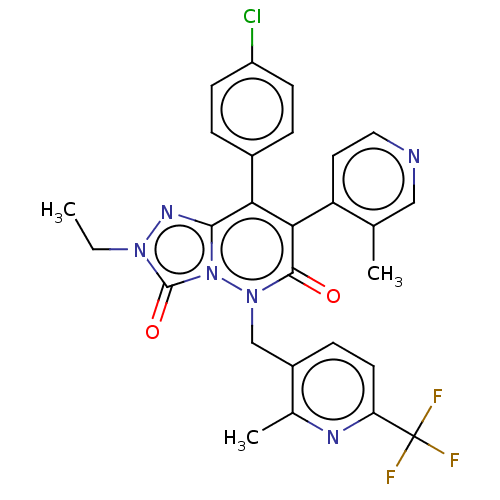
(CHEMBL3260744)Show SMILES CCn1nc2c(-c3ccc(Cl)cc3)c(-c3ccncc3C)c(=O)n(Cc3ccc(nc3C)C(F)(F)F)n2c1=O |(16.96,-15.83,;17.73,-14.5,;16.96,-13.17,;15.43,-13.01,;15.11,-11.5,;13.78,-10.73,;12.44,-11.51,;12.44,-13.05,;11.11,-13.82,;9.77,-13.05,;8.44,-13.82,;9.77,-11.51,;11.11,-10.74,;13.77,-9.2,;12.44,-8.43,;11.11,-9.2,;9.77,-8.43,;9.77,-6.89,;11.1,-6.12,;12.44,-6.89,;13.78,-5.75,;15.1,-8.42,;15.1,-6.89,;16.44,-9.19,;17.77,-8.42,;17.76,-6.87,;16.42,-6.12,;16.41,-4.58,;17.75,-3.79,;19.09,-4.55,;19.1,-6.1,;20.44,-6.86,;17.74,-2.25,;19.06,-1.47,;16.4,-1.49,;17.72,-.7,;16.44,-10.73,;17.58,-11.76,;19.09,-11.44,)| Show InChI InChI=1S/C27H22ClF3N6O2/c1-4-35-26(39)37-24(34-35)22(17-5-8-19(28)9-6-17)23(20-11-12-32-13-15(20)2)25(38)36(37)14-18-7-10-21(27(29,30)31)33-16(18)3/h5-13H,4,14H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
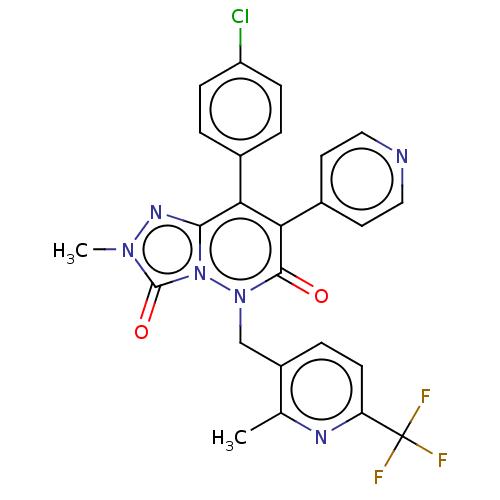
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50011314
(CHEMBL3260741)Show SMILES Cc1nc(ccc1Cn1n2c(nn(C)c2=O)c(-c2ccc(Cl)cc2)c(-c2ccncc2)c1=O)C(F)(F)F Show InChI InChI=1S/C25H18ClF3N6O2/c1-14-17(5-8-19(31-14)25(27,28)29)13-34-23(36)21(16-9-11-30-12-10-16)20(15-3-6-18(26)7-4-15)22-32-33(2)24(37)35(22)34/h3-12H,13H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50518559

(CHEMBL4585326)Show SMILES COc1ccc(CCN(C(=O)OC2CN3CCC2CC3)c2ccccc2)cc1 |TLB:11:12:15.16:18.19,(19.07,-20.84,;17.73,-21.62,;16.4,-20.85,;15.06,-21.62,;13.73,-20.85,;13.74,-19.31,;12.4,-18.54,;11.07,-19.31,;9.74,-18.54,;8.4,-19.31,;8.4,-20.85,;7.07,-18.54,;5.74,-19.31,;5.39,-20.74,;3.9,-20.23,;3.89,-18.2,;4.49,-16.94,;4.52,-18.86,;2.82,-19.32,;2.41,-20.75,;9.74,-17,;8.4,-16.23,;8.41,-14.68,;9.73,-13.91,;11.07,-14.68,;11.07,-16.23,;15.06,-18.54,;16.39,-19.3,)| Show InChI InChI=1S/C23H28N2O3/c1-27-21-9-7-18(8-10-21)11-16-25(20-5-3-2-4-6-20)23(26)28-22-17-24-14-12-19(22)13-15-24/h2-10,19,22H,11-17H2,1H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human M1 AChR expressed in CHO cell membranes after 1 to 2 hrs by liquid scintillation spectrometry method |
Bioorg Med Chem Lett 29: 471-476 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.022
BindingDB Entry DOI: 10.7270/Q2NP27SC |
More data for this
Ligand-Target Pair | |
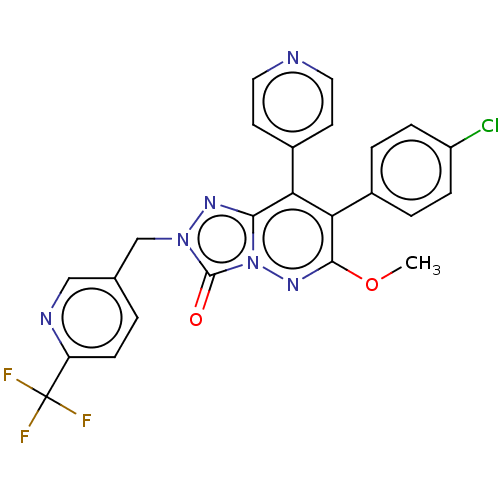
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50011307
(CHEMBL3260734)Show SMILES COc1nn2c(nn(Cc3ccc(nc3)C(F)(F)F)c2=O)c(-c2ccncc2)c1-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H16ClF3N6O2/c1-36-22-20(15-3-5-17(25)6-4-15)19(16-8-10-29-11-9-16)21-31-33(23(35)34(21)32-22)13-14-2-7-18(30-12-14)24(26,27)28/h2-12H,13H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM50518559

(CHEMBL4585326)Show SMILES COc1ccc(CCN(C(=O)OC2CN3CCC2CC3)c2ccccc2)cc1 |TLB:11:12:15.16:18.19,(19.07,-20.84,;17.73,-21.62,;16.4,-20.85,;15.06,-21.62,;13.73,-20.85,;13.74,-19.31,;12.4,-18.54,;11.07,-19.31,;9.74,-18.54,;8.4,-19.31,;8.4,-20.85,;7.07,-18.54,;5.74,-19.31,;5.39,-20.74,;3.9,-20.23,;3.89,-18.2,;4.49,-16.94,;4.52,-18.86,;2.82,-19.32,;2.41,-20.75,;9.74,-17,;8.4,-16.23,;8.41,-14.68,;9.73,-13.91,;11.07,-14.68,;11.07,-16.23,;15.06,-18.54,;16.39,-19.3,)| Show InChI InChI=1S/C23H28N2O3/c1-27-21-9-7-18(8-10-21)11-16-25(20-5-3-2-4-6-20)23(26)28-22-17-24-14-12-19(22)13-15-24/h2-10,19,22H,11-17H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human M5 AChR expressed in CHO cell membranes after 1 to 2 hrs by liquid scintillation spectrometry method |
Bioorg Med Chem Lett 29: 471-476 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.022
BindingDB Entry DOI: 10.7270/Q2NP27SC |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50011329
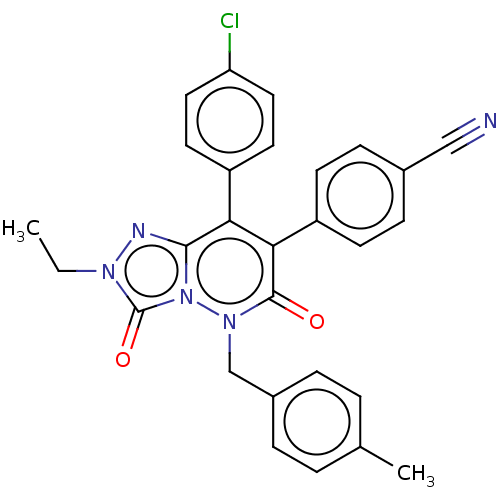
(CHEMBL3260749)Show SMILES CCn1nc2c(-c3ccc(Cl)cc3)c(-c3ccc(cc3)C#N)c(=O)n(Cc3ccc(C)cc3)n2c1=O Show InChI InChI=1S/C28H22ClN5O2/c1-3-32-28(36)34-26(31-32)24(21-12-14-23(29)15-13-21)25(22-10-8-19(16-30)9-11-22)27(35)33(34)17-20-6-4-18(2)5-7-20/h4-15H,3,17H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50356013
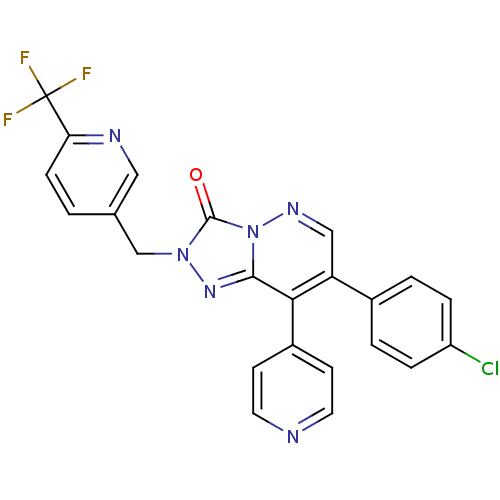
(CHEMBL1911374 | CHEMBL1911375)Show SMILES FC(F)(F)c1ccc(Cn2nc3c(-c4ccncc4)c(cnn3c2=O)-c2ccc(Cl)cc2)cn1 Show InChI InChI=1S/C23H14ClF3N6O/c24-17-4-2-15(3-5-17)18-12-30-33-21(20(18)16-7-9-28-10-8-16)31-32(22(33)34)13-14-1-6-19(29-11-14)23(25,26)27/h1-12H,13H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antihistaminic activity against Histamine H1 receptor was measured on isolated terminal part of guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50518557

(CHEMBL4519301)Show SMILES Fc1ccc(CCN(C(=O)OC2CN3CCC2CC3)c2ccccc2)cc1 |TLB:10:11:14.15:17.18,(18.31,-36.25,;16.97,-35.49,;15.64,-36.26,;14.31,-35.49,;14.31,-33.95,;12.98,-33.18,;11.65,-33.95,;10.31,-33.18,;8.98,-33.95,;8.98,-35.49,;7.65,-33.18,;6.31,-33.95,;5.96,-35.38,;4.48,-34.87,;4.47,-32.84,;5.07,-31.58,;5.1,-33.5,;3.39,-33.95,;2.99,-35.39,;10.31,-31.64,;8.98,-30.87,;8.98,-29.32,;10.31,-28.55,;11.65,-29.32,;11.65,-30.87,;15.64,-33.17,;16.97,-33.94,)| Show InChI InChI=1S/C22H25FN2O2/c23-19-8-6-17(7-9-19)10-15-25(20-4-2-1-3-5-20)22(26)27-21-16-24-13-11-18(21)12-14-24/h1-9,18,21H,10-16H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human M2 AChR expressed in CHO cell membranes after 1 to 2 hrs by liquid scintillation spectrometry method |
Bioorg Med Chem Lett 29: 471-476 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.022
BindingDB Entry DOI: 10.7270/Q2NP27SC |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50011315
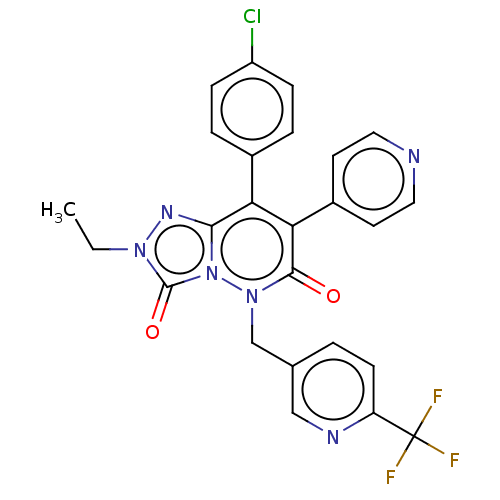
(CHEMBL3260742)Show SMILES CCn1nc2c(-c3ccc(Cl)cc3)c(-c3ccncc3)c(=O)n(Cc3ccc(nc3)C(F)(F)F)n2c1=O Show InChI InChI=1S/C25H18ClF3N6O2/c1-2-33-24(37)35-22(32-33)20(16-4-6-18(26)7-5-16)21(17-9-11-30-12-10-17)23(36)34(35)14-15-3-8-19(31-13-15)25(27,28)29/h3-13H,2,14H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 13
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human carbonic anhydrase 13 expressed in Escherichia coliBL21(DE3) pLysS incubated for 10 mins by phenol red dye based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00734
BindingDB Entry DOI: 10.7270/Q2FR019P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50011309
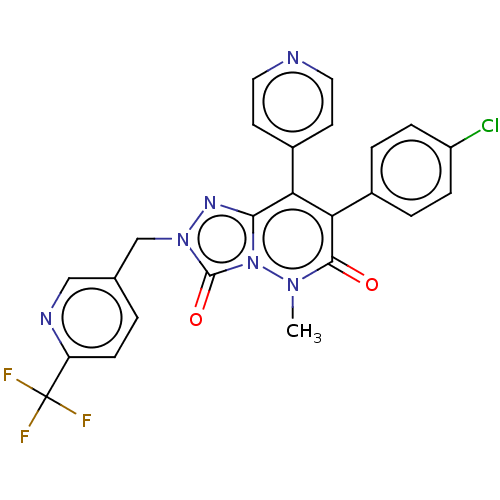
(CHEMBL3260736)Show SMILES Cn1n2c(nn(Cc3ccc(nc3)C(F)(F)F)c2=O)c(-c2ccncc2)c(-c2ccc(Cl)cc2)c1=O Show InChI InChI=1S/C24H16ClF3N6O2/c1-32-22(35)20(15-3-5-17(25)6-4-15)19(16-8-10-29-11-9-16)21-31-33(23(36)34(21)32)13-14-2-7-18(30-12-14)24(26,27)28/h2-12H,13H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50518567

(CHEMBL4576768)Show SMILES O=C(OCc1ccc2OCOc2c1)N(C1CN2CCC1CC2)c1ccccc1 |TLB:13:14:17.18:20.21,(54.21,-33.97,;54.27,-32.43,;55.63,-31.71,;56.93,-32.53,;58.29,-31.81,;58.34,-30.28,;59.7,-29.55,;61.01,-30.37,;62.49,-29.95,;63.35,-31.22,;62.41,-32.43,;60.96,-31.91,;59.6,-32.63,;52.96,-31.61,;51.6,-32.34,;51.25,-33.77,;49.76,-33.26,;49.75,-31.23,;50.35,-29.97,;50.38,-31.89,;48.68,-32.34,;48.28,-33.78,;53.02,-30.07,;54.38,-29.36,;54.44,-27.82,;53.13,-27,;51.76,-27.73,;51.71,-29.27,)| Show InChI InChI=1S/C22H24N2O4/c25-22(26-14-16-6-7-20-21(12-16)28-15-27-20)24(18-4-2-1-3-5-18)19-13-23-10-8-17(19)9-11-23/h1-7,12,17,19H,8-11,13-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human M3 AChR expressed in CHO cell membranes after 1 to 2 hrs by liquid scintillation spectrometry method |
Bioorg Med Chem Lett 29: 471-476 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.022
BindingDB Entry DOI: 10.7270/Q2NP27SC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50547697
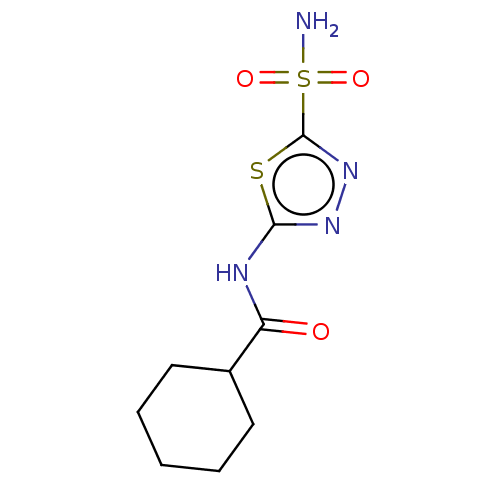
(CHEMBL4739913) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antihistaminic activity against Histamine H1 receptor was measured on isolated terminal part of guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50518570
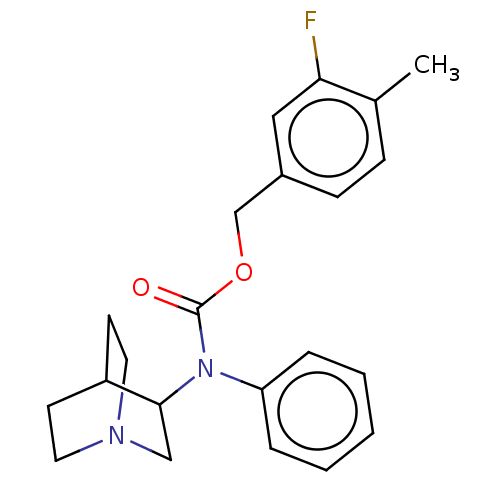
(CHEMBL4462631)Show SMILES Cc1ccc(COC(=O)N(C2CN3CCC2CC3)c2ccccc2)cc1F |TLB:9:10:13.14:16.17,(39.78,-53.75,;38.42,-54.48,;38.36,-56.02,;37,-56.74,;35.7,-55.91,;34.34,-56.63,;33.03,-55.81,;31.67,-56.54,;31.62,-58.07,;30.37,-55.72,;29.01,-56.44,;28.66,-57.87,;27.17,-57.37,;27.16,-55.34,;27.76,-54.07,;27.79,-55.99,;26.09,-56.45,;25.68,-57.88,;30.42,-54.18,;31.78,-53.46,;31.84,-51.93,;30.53,-51.11,;29.17,-51.84,;29.12,-53.37,;35.75,-54.38,;37.1,-53.66,;37.15,-52.12,)| Show InChI InChI=1S/C22H25FN2O2/c1-16-7-8-17(13-20(16)23)15-27-22(26)25(19-5-3-2-4-6-19)21-14-24-11-9-18(21)10-12-24/h2-8,13,18,21H,9-12,14-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human M3 AChR expressed in CHO cell membranes after 1 to 2 hrs by liquid scintillation spectrometry method |
Bioorg Med Chem Lett 29: 471-476 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.022
BindingDB Entry DOI: 10.7270/Q2NP27SC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313284

((S)-2-((7-fluoro-2,3-dihydro-1H-inden-4-yloxy)meth...)Show InChI InChI=1S/C14H18FNO2/c15-13-4-5-14(12-3-1-2-11(12)13)18-9-10-8-16-6-7-17-10/h4-5,10,16H,1-3,6-9H2/t10-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to SERT |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | CHEMBL4750765
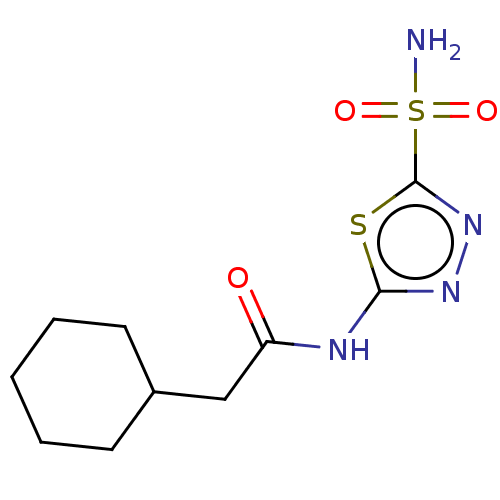
Show InChI InChI=1S/C9H9NO3S2/c1-13-7-2-3-8-6(4-7)5-9(14-8)15(10,11)12/h2-5H,1H3,(H2,10,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antihistaminic activity against Histamine H1 receptor was measured on isolated terminal part of guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50518559

(CHEMBL4585326)Show SMILES COc1ccc(CCN(C(=O)OC2CN3CCC2CC3)c2ccccc2)cc1 |TLB:11:12:15.16:18.19,(19.07,-20.84,;17.73,-21.62,;16.4,-20.85,;15.06,-21.62,;13.73,-20.85,;13.74,-19.31,;12.4,-18.54,;11.07,-19.31,;9.74,-18.54,;8.4,-19.31,;8.4,-20.85,;7.07,-18.54,;5.74,-19.31,;5.39,-20.74,;3.9,-20.23,;3.89,-18.2,;4.49,-16.94,;4.52,-18.86,;2.82,-19.32,;2.41,-20.75,;9.74,-17,;8.4,-16.23,;8.41,-14.68,;9.73,-13.91,;11.07,-14.68,;11.07,-16.23,;15.06,-18.54,;16.39,-19.3,)| Show InChI InChI=1S/C23H28N2O3/c1-27-21-9-7-18(8-10-21)11-16-25(20-5-3-2-4-6-20)23(26)28-22-17-24-14-12-19(22)13-15-24/h2-10,19,22H,11-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human M4 AChR expressed in CHO cell membranes after 1 to 2 hrs by liquid scintillation spectrometry method |
Bioorg Med Chem Lett 29: 471-476 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.022
BindingDB Entry DOI: 10.7270/Q2NP27SC |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50011313
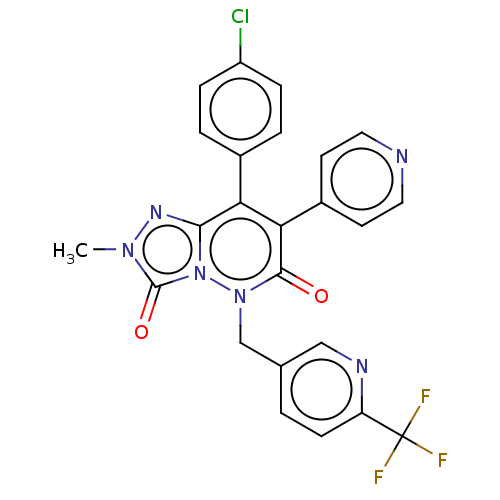
(CHEMBL3260740)Show SMILES Cn1nc2c(-c3ccc(Cl)cc3)c(-c3ccncc3)c(=O)n(Cc3ccc(nc3)C(F)(F)F)n2c1=O Show InChI InChI=1S/C24H16ClF3N6O2/c1-32-23(36)34-21(31-32)19(15-3-5-17(25)6-4-15)20(16-8-10-29-11-9-16)22(35)33(34)13-14-2-7-18(30-12-14)24(26,27)28/h2-12H,13H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM16669
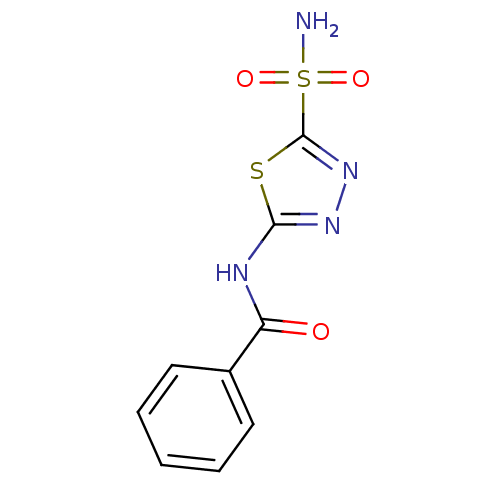
(CHEMBL281376 | N-(5-sulfamoyl-1,3,4-thiadiazol-2-y...)Show InChI InChI=1S/C9H8N4O3S2/c10-18(15,16)9-13-12-8(17-9)11-7(14)6-4-2-1-3-5-6/h1-5H,(H2,10,15,16)(H,11,12,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antihistaminic activity against Histamine H1 receptor was measured on isolated terminal part of guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50011312
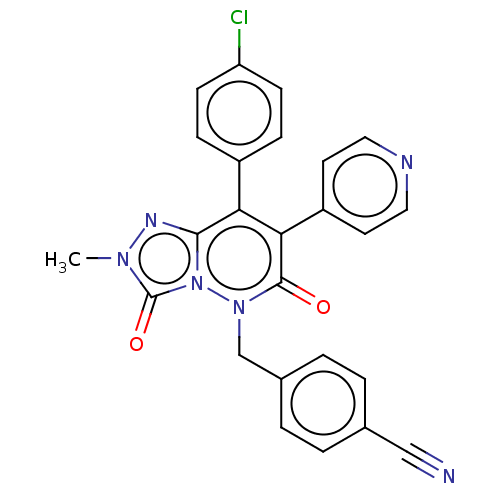
(CHEMBL3260739)Show SMILES Cn1nc2c(-c3ccc(Cl)cc3)c(-c3ccncc3)c(=O)n(Cc3ccc(cc3)C#N)n2c1=O Show InChI InChI=1S/C25H17ClN6O2/c1-30-25(34)32-23(29-30)21(18-6-8-20(26)9-7-18)22(19-10-12-28-13-11-19)24(33)31(32)15-17-4-2-16(14-27)3-5-17/h2-13H,15H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50518567

(CHEMBL4576768)Show SMILES O=C(OCc1ccc2OCOc2c1)N(C1CN2CCC1CC2)c1ccccc1 |TLB:13:14:17.18:20.21,(54.21,-33.97,;54.27,-32.43,;55.63,-31.71,;56.93,-32.53,;58.29,-31.81,;58.34,-30.28,;59.7,-29.55,;61.01,-30.37,;62.49,-29.95,;63.35,-31.22,;62.41,-32.43,;60.96,-31.91,;59.6,-32.63,;52.96,-31.61,;51.6,-32.34,;51.25,-33.77,;49.76,-33.26,;49.75,-31.23,;50.35,-29.97,;50.38,-31.89,;48.68,-32.34,;48.28,-33.78,;53.02,-30.07,;54.38,-29.36,;54.44,-27.82,;53.13,-27,;51.76,-27.73,;51.71,-29.27,)| Show InChI InChI=1S/C22H24N2O4/c25-22(26-14-16-6-7-20-21(12-16)28-15-27-20)24(18-4-2-1-3-5-18)19-13-23-10-8-17(19)9-11-23/h1-7,12,17,19H,8-11,13-15H2 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human M1 AChR expressed in CHO cell membranes after 1 to 2 hrs by liquid scintillation spectrometry method |
Bioorg Med Chem Lett 29: 471-476 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.022
BindingDB Entry DOI: 10.7270/Q2NP27SC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data