 Found 663 hits with Last Name = 'lee' and Initial = 'yj'
Found 663 hits with Last Name = 'lee' and Initial = 'yj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314290
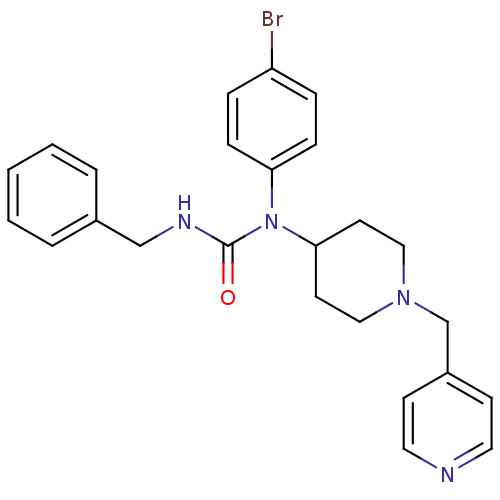
(3-benzyl-1-(4-bromophenyl)-1-(1-(pyridin-4-ylmethy...)Show SMILES Brc1ccc(cc1)N(C1CCN(Cc2ccncc2)CC1)C(=O)NCc1ccccc1 Show InChI InChI=1S/C25H27BrN4O/c26-22-6-8-23(9-7-22)30(25(31)28-18-20-4-2-1-3-5-20)24-12-16-29(17-13-24)19-21-10-14-27-15-11-21/h1-11,14-15,24H,12-13,16-19H2,(H,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314288
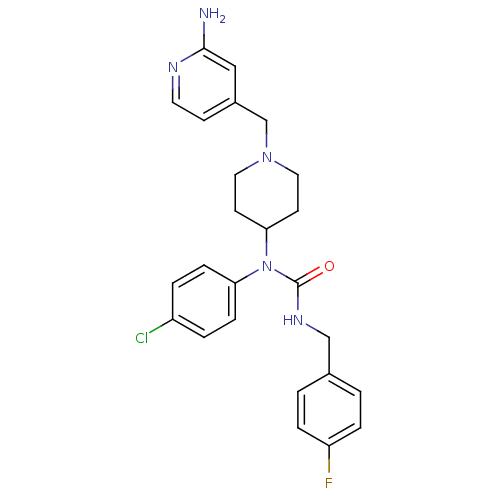
(1-(1-((2-aminopyridin-4-yl)methyl)piperidin-4-yl)-...)Show SMILES Nc1cc(CN2CCC(CC2)N(C(=O)NCc2ccc(F)cc2)c2ccc(Cl)cc2)ccn1 Show InChI InChI=1S/C25H27ClFN5O/c26-20-3-7-22(8-4-20)32(25(33)30-16-18-1-5-21(27)6-2-18)23-10-13-31(14-11-23)17-19-9-12-29-24(28)15-19/h1-9,12,15,23H,10-11,13-14,16-17H2,(H2,28,29)(H,30,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50230294
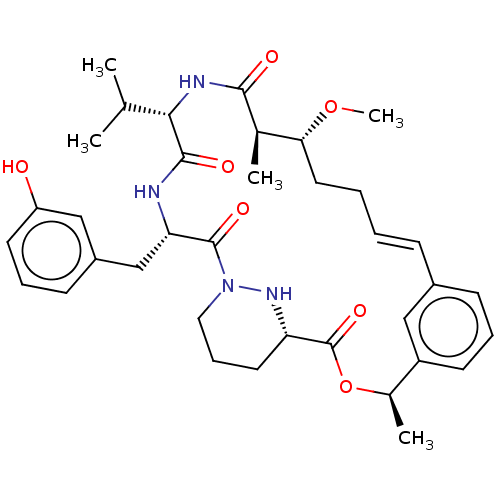
(CHEMBL4071740)Show SMILES CO[C@@H]1CC\C=C\c2cccc(c2)[C@@H](C)OC(=O)[C@@H]2CCCN(N2)C(=O)[C@H](Cc2cccc(O)c2)NC(=O)[C@@H](NC(=O)[C@@H]1C)C(C)C |r,t:5| Show InChI InChI=1S/C36H48N4O7/c1-22(2)32-34(43)37-30(21-26-13-9-15-28(41)20-26)35(44)40-18-10-16-29(39-40)36(45)47-24(4)27-14-8-12-25(19-27)11-6-7-17-31(46-5)23(3)33(42)38-32/h6,8-9,11-15,19-20,22-24,29-32,39,41H,7,10,16-18,21H2,1-5H3,(H,37,43)(H,38,42)/b11-6+/t23-,24-,29+,30+,31-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selcia Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human N-terminal His8-tagged Cyclophilin A (1 to 169 residues) expressed in Escherichia coli BL21(DE3) using Su... |
J Med Chem 60: 1000-1017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01329
BindingDB Entry DOI: 10.7270/Q2JQ137K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50230211
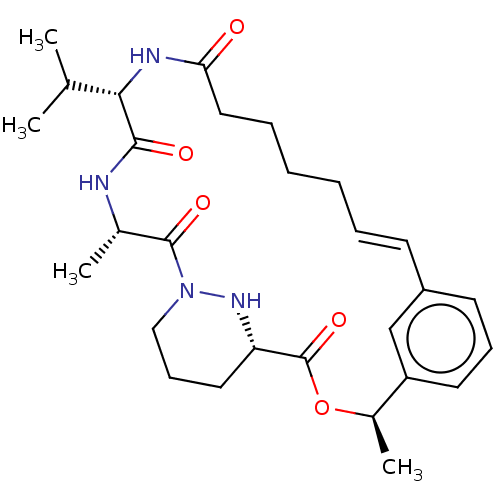
(CHEMBL4063126)Show SMILES CC(C)[C@@H]1NC(=O)CCCC\C=C\c2cccc(c2)[C@@H](C)OC(=O)[C@@H]2CCCN(N2)C(=O)[C@H](C)NC1=O |r,t:11| Show InChI InChI=1S/C28H40N4O5/c1-18(2)25-26(34)29-19(3)27(35)32-16-10-14-23(31-32)28(36)37-20(4)22-13-9-12-21(17-22)11-7-5-6-8-15-24(33)30-25/h7,9,11-13,17-20,23,25,31H,5-6,8,10,14-16H2,1-4H3,(H,29,34)(H,30,33)/b11-7+/t19-,20+,23-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selcia Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human N-terminal His8-tagged Cyclophilin A (1 to 169 residues) expressed in Escherichia coli BL21(DE3) using Su... |
J Med Chem 60: 1000-1017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01329
BindingDB Entry DOI: 10.7270/Q2JQ137K |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50022815

((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C |r| Show InChI InChI=1S/C62H111N11O12/c1-25-27-28-40(15)52(75)51-56(79)65-43(26-2)58(81)67(18)33-48(74)68(19)44(29-34(3)4)55(78)66-49(38(11)12)61(84)69(20)45(30-35(5)6)54(77)63-41(16)53(76)64-42(17)57(80)70(21)46(31-36(7)8)59(82)71(22)47(32-37(9)10)60(83)72(23)50(39(13)14)62(85)73(51)24/h25,27,34-47,49-52,75H,26,28-33H2,1-24H3,(H,63,77)(H,64,76)(H,65,79)(H,66,78)/b27-25+/t40-,41+,42-,43+,44+,45+,46+,47+,49+,50+,51+,52-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selcia Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human N-terminal His8-tagged Cyclophilin A (1 to 169 residues) expressed in Escherichia coli BL21(DE3) using Su... |
J Med Chem 60: 1000-1017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01329
BindingDB Entry DOI: 10.7270/Q2JQ137K |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50230212

(CHEMBL4084776)Show SMILES CO[C@@H]1CC\C=C\c2cccc(c2)[C@@H](C)OC(=O)[C@@H]2CCCN(N2)C(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@@H]1C)C(C)C |r,t:5| Show InChI InChI=1S/C30H44N4O6/c1-18(2)26-28(36)31-20(4)29(37)34-16-10-14-24(33-34)30(38)40-21(5)23-13-9-12-22(17-23)11-7-8-15-25(39-6)19(3)27(35)32-26/h7,9,11-13,17-21,24-26,33H,8,10,14-16H2,1-6H3,(H,31,36)(H,32,35)/b11-7+/t19-,20+,21-,24+,25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selcia Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human N-terminal His8-tagged Cyclophilin A (1 to 169 residues) expressed in Escherichia coli BL21(DE3) using Su... |
J Med Chem 60: 1000-1017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01329
BindingDB Entry DOI: 10.7270/Q2JQ137K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314294
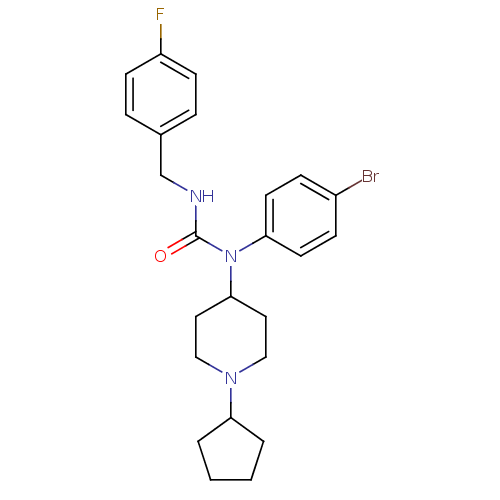
(1-(4-bromophenyl)-1-(1-cyclopentylpiperidin-4-yl)-...)Show SMILES Fc1ccc(CNC(=O)N(C2CCN(CC2)C2CCCC2)c2ccc(Br)cc2)cc1 Show InChI InChI=1S/C24H29BrFN3O/c25-19-7-11-22(12-8-19)29(24(30)27-17-18-5-9-20(26)10-6-18)23-13-15-28(16-14-23)21-3-1-2-4-21/h5-12,21,23H,1-4,13-17H2,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314296

(1-(4-chlorophenyl)-1-(1-ethylpiperidin-4-yl)-3-(4-...)Show SMILES CCN1CCC(CC1)N(C(=O)NCc1ccc(F)cc1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H25ClFN3O/c1-2-25-13-11-20(12-14-25)26(19-9-5-17(22)6-10-19)21(27)24-15-16-3-7-18(23)8-4-16/h3-10,20H,2,11-15H2,1H3,(H,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314291
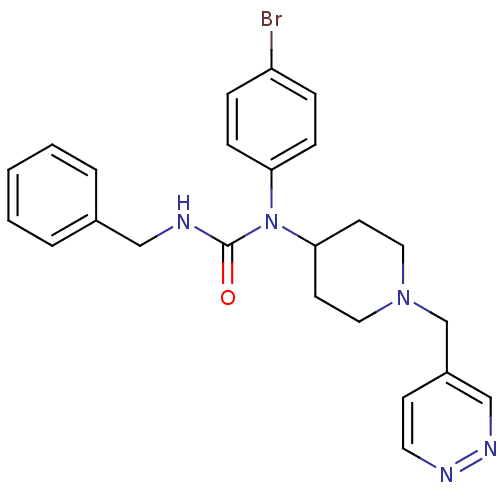
(3-benzyl-1-(4-bromophenyl)-1-(1-(pyridazin-4-ylmet...)Show SMILES Brc1ccc(cc1)N(C1CCN(Cc2ccnnc2)CC1)C(=O)NCc1ccccc1 Show InChI InChI=1S/C24H26BrN5O/c25-21-6-8-22(9-7-21)30(24(31)26-16-19-4-2-1-3-5-19)23-11-14-29(15-12-23)18-20-10-13-27-28-17-20/h1-10,13,17,23H,11-12,14-16,18H2,(H,26,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314292
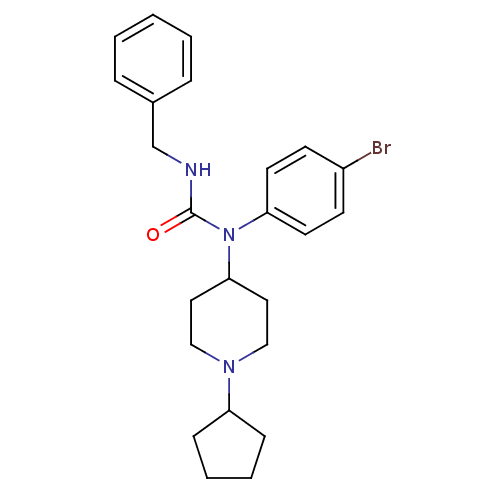
(3-benzyl-1-(4-bromophenyl)-1-(1-cyclopentylpiperid...)Show SMILES Brc1ccc(cc1)N(C1CCN(CC1)C1CCCC1)C(=O)NCc1ccccc1 Show InChI InChI=1S/C24H30BrN3O/c25-20-10-12-22(13-11-20)28(24(29)26-18-19-6-2-1-3-7-19)23-14-16-27(17-15-23)21-8-4-5-9-21/h1-3,6-7,10-13,21,23H,4-5,8-9,14-18H2,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50230213

(CHEMBL4092526)Show SMILES CO[C@@H]1CC\C=C\c2cccc(COC(=O)[C@@H]3CCCN(N3)C(=O)[C@H](Cc3cccc(O)c3)NC(=O)[C@@H](NC(=O)[C@@H]1C)C(C)C)c2 |r,t:5| Show InChI InChI=1S/C35H46N4O7/c1-22(2)31-33(42)36-29(20-25-12-8-14-27(40)19-25)34(43)39-17-9-15-28(38-39)35(44)46-21-26-13-7-11-24(18-26)10-5-6-16-30(45-4)23(3)32(41)37-31/h5,7-8,10-14,18-19,22-23,28-31,38,40H,6,9,15-17,20-21H2,1-4H3,(H,36,42)(H,37,41)/b10-5+/t23-,28+,29+,30-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selcia Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human N-terminal His8-tagged Cyclophilin A (1 to 169 residues) expressed in Escherichia coli BL21(DE3) using Su... |
J Med Chem 60: 1000-1017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01329
BindingDB Entry DOI: 10.7270/Q2JQ137K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314293

(1-(4-bromophenyl)-1-(1-cyclopentylpiperidin-4-yl)-...)Show SMILES Brc1ccc(cc1)N(C1CCN(CC1)C1CCCC1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C23H28BrN3O/c24-18-10-12-21(13-11-18)27(23(28)25-19-6-2-1-3-7-19)22-14-16-26(17-15-22)20-8-4-5-9-20/h1-3,6-7,10-13,20,22H,4-5,8-9,14-17H2,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50230210
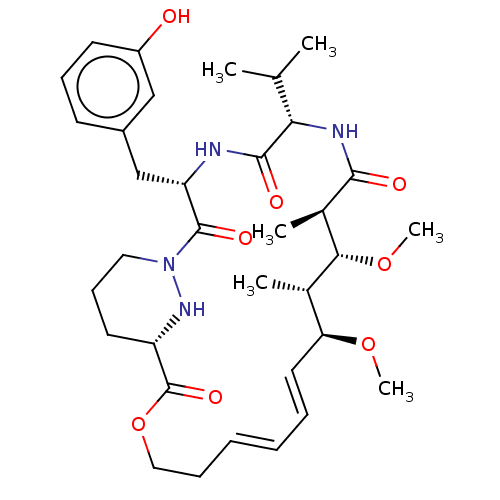
(CHEMBL4090107)Show SMILES CO[C@H]1\C=C\C=C\CCOC(=O)[C@@H]2CCCN(N2)C(=O)[C@H](Cc2cccc(O)c2)NC(=O)[C@@H](NC(=O)[C@H](C)[C@H](OC)[C@H]1C)C(C)C |r,t:3,5| Show InChI InChI=1S/C34H50N4O8/c1-21(2)29-32(41)35-27(20-24-13-11-14-25(39)19-24)33(42)38-17-12-15-26(37-38)34(43)46-18-10-8-7-9-16-28(44-5)22(3)30(45-6)23(4)31(40)36-29/h7-9,11,13-14,16,19,21-23,26-30,37,39H,10,12,15,17-18,20H2,1-6H3,(H,35,41)(H,36,40)/b8-7+,16-9+/t22-,23+,26-,27-,28-,29-,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selcia Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human N-terminal His8-tagged Cyclophilin A (1 to 169 residues) expressed in Escherichia coli BL21(DE3) using Su... |
J Med Chem 60: 1000-1017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01329
BindingDB Entry DOI: 10.7270/Q2JQ137K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314311

(1-(4-chlorophenyl)-3-(4-fluorobenzyl)-1-(1-(pyridi...)Show SMILES Fc1ccc(CNC(=O)N(C2CCN(Cc3ccncc3)CC2)c2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C25H26ClFN4O/c26-21-3-7-23(8-4-21)31(25(32)29-17-19-1-5-22(27)6-2-19)24-11-15-30(16-12-24)18-20-9-13-28-14-10-20/h1-10,13-14,24H,11-12,15-18H2,(H,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314295
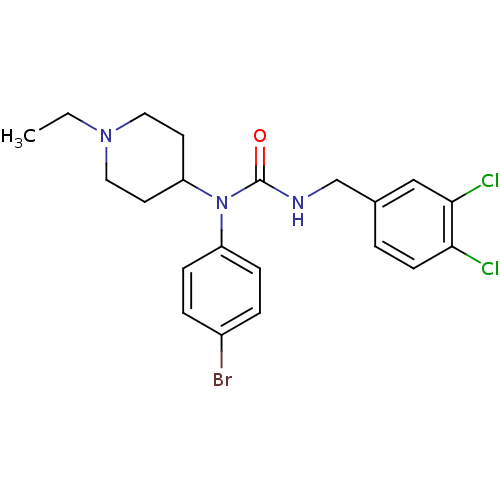
(1-(4-bromophenyl)-3-(3,4-dichlorobenzyl)-1-(1-ethy...)Show SMILES CCN1CCC(CC1)N(C(=O)NCc1ccc(Cl)c(Cl)c1)c1ccc(Br)cc1 Show InChI InChI=1S/C21H24BrCl2N3O/c1-2-26-11-9-18(10-12-26)27(17-6-4-16(22)5-7-17)21(28)25-14-15-3-8-19(23)20(24)13-15/h3-8,13,18H,2,9-12,14H2,1H3,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314313

(1-(4-bromophenyl)-1-(1-cyclopentylpiperidin-4-yl)-...)Show SMILES Clc1cc(Cl)cc(CNC(=O)N(C2CCN(CC2)C2CCCC2)c2ccc(Br)cc2)c1 Show InChI InChI=1S/C24H28BrCl2N3O/c25-18-5-7-22(8-6-18)30(23-9-11-29(12-10-23)21-3-1-2-4-21)24(31)28-16-17-13-19(26)15-20(27)14-17/h5-8,13-15,21,23H,1-4,9-12,16H2,(H,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314297

(1-(5-chloropyridin-2-yl)-1-(1-ethylpiperidin-4-yl)...)Show SMILES CCN1CCC(CC1)N(C(=O)NCc1ccc(F)cc1)c1ccc(Cl)cn1 Show InChI InChI=1S/C20H24ClFN4O/c1-2-25-11-9-18(10-12-25)26(19-8-5-16(21)14-23-19)20(27)24-13-15-3-6-17(22)7-4-15/h3-8,14,18H,2,9-13H2,1H3,(H,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314315

(1-(4-bromophenyl)-1-(1-cyclopentylpiperidin-4-yl)-...)Show SMILES COc1ccc(CNC(=O)N(C2CCN(CC2)C2CCCC2)c2ccc(Br)cc2)cc1 Show InChI InChI=1S/C25H32BrN3O2/c1-31-24-12-6-19(7-13-24)18-27-25(30)29(22-10-8-20(26)9-11-22)23-14-16-28(17-15-23)21-4-2-3-5-21/h6-13,21,23H,2-5,14-18H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314289

(1-(4-chlorophenyl)-3-(4-fluorobenzyl)-1-(1-(pyrida...)Show SMILES Fc1ccc(CNC(=O)N(C2CCN(Cc3ccnnc3)CC2)c2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C24H25ClFN5O/c25-20-3-7-22(8-4-20)31(24(32)27-15-18-1-5-21(26)6-2-18)23-10-13-30(14-11-23)17-19-9-12-28-29-16-19/h1-9,12,16,23H,10-11,13-15,17H2,(H,27,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50230209

(CHEMBL4100272)Show SMILES CO[C@@H]1CC\C=C\C=C\CCOC(=O)[C@@H]2CCCN(N2)C(=O)[C@H](Cc2cccc(O)c2)NC(=O)[C@@H](NC(=O)[C@@H]1C)C(C)C |r,t:5,7| Show InChI InChI=1S/C32H46N4O7/c1-21(2)28-30(39)33-26(20-23-13-11-14-24(37)19-23)31(40)36-17-12-15-25(35-36)32(41)43-18-10-8-6-5-7-9-16-27(42-4)22(3)29(38)34-28/h5-8,11,13-14,19,21-22,25-28,35,37H,9-10,12,15-18,20H2,1-4H3,(H,33,39)(H,34,38)/b7-5+,8-6+/t22-,25+,26+,27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selcia Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human N-terminal His8-tagged Cyclophilin A (1 to 169 residues) expressed in Escherichia coli BL21(DE3) using Su... |
J Med Chem 60: 1000-1017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01329
BindingDB Entry DOI: 10.7270/Q2JQ137K |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314307
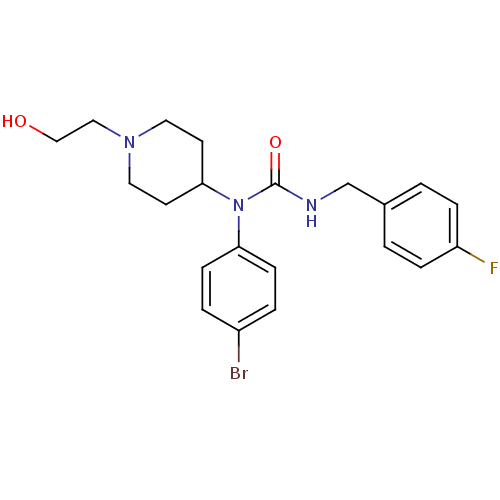
(1-(4-bromophenyl)-3-(4-fluorobenzyl)-1-(1-(2-hydro...)Show SMILES OCCN1CCC(CC1)N(C(=O)NCc1ccc(F)cc1)c1ccc(Br)cc1 Show InChI InChI=1S/C21H25BrFN3O2/c22-17-3-7-19(8-4-17)26(20-9-11-25(12-10-20)13-14-27)21(28)24-15-16-1-5-18(23)6-2-16/h1-8,20,27H,9-15H2,(H,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314306
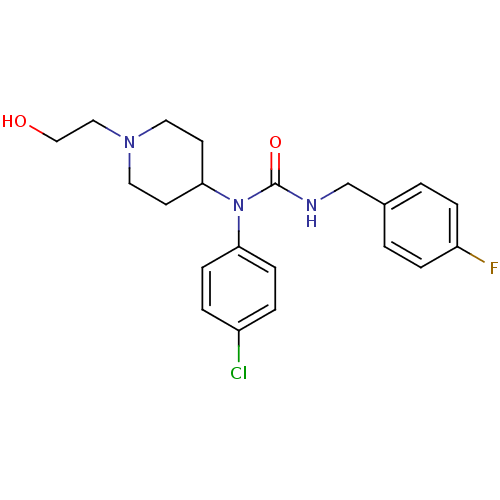
(1-(4-chlorophenyl)-3-(4-fluorobenzyl)-1-(1-(2-hydr...)Show SMILES OCCN1CCC(CC1)N(C(=O)NCc1ccc(F)cc1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H25ClFN3O2/c22-17-3-7-19(8-4-17)26(20-9-11-25(12-10-20)13-14-27)21(28)24-15-16-1-5-18(23)6-2-16/h1-8,20,27H,9-15H2,(H,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314314

(1-(4-bromophenyl)-1-(1-cyclopentylpiperidin-4-yl)-...)Show SMILES Clc1ccc(CNC(=O)N(C2CCN(CC2)C2CCCC2)c2ccc(Br)cc2)cc1Cl Show InChI InChI=1S/C24H28BrCl2N3O/c25-18-6-8-20(9-7-18)30(21-11-13-29(14-12-21)19-3-1-2-4-19)24(31)28-16-17-5-10-22(26)23(27)15-17/h5-10,15,19,21H,1-4,11-14,16H2,(H,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 191 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314298

(1-(1-ethylpiperidin-4-yl)-3-(4-fluorobenzyl)-1-(4-...)Show SMILES CCN1CCC(CC1)N(C(=O)NCc1ccc(F)cc1)c1ccc(F)cc1 Show InChI InChI=1S/C21H25F2N3O/c1-2-25-13-11-20(12-14-25)26(19-9-7-18(23)8-10-19)21(27)24-15-16-3-5-17(22)6-4-16/h3-10,20H,2,11-15H2,1H3,(H,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 447 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314300
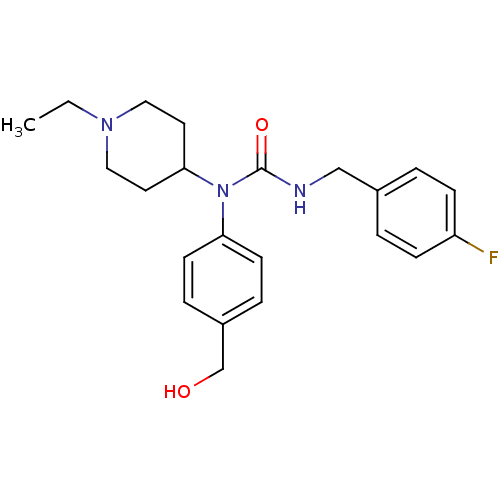
(1-(1-ethylpiperidin-4-yl)-3-(4-fluorobenzyl)-1-(4-...)Show SMILES CCN1CCC(CC1)N(C(=O)NCc1ccc(F)cc1)c1ccc(CO)cc1 Show InChI InChI=1S/C22H28FN3O2/c1-2-25-13-11-21(12-14-25)26(20-9-5-18(16-27)6-10-20)22(28)24-15-17-3-7-19(23)8-4-17/h3-10,21,27H,2,11-16H2,1H3,(H,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314312
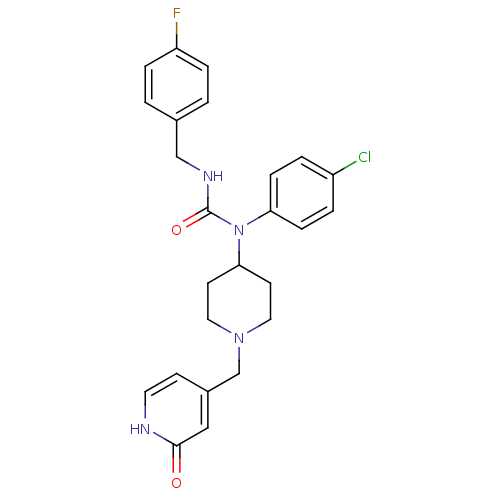
(1-(4-chlorophenyl)-3-(4-fluorobenzyl)-1-(1-((2-hyd...)Show SMILES Fc1ccc(CNC(=O)N(C2CCN(Cc3cc[nH]c(=O)c3)CC2)c2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C25H26ClFN4O2/c26-20-3-7-22(8-4-20)31(25(33)29-16-18-1-5-21(27)6-2-18)23-10-13-30(14-11-23)17-19-9-12-28-24(32)15-19/h1-9,12,15,23H,10-11,13-14,16-17H2,(H,28,32)(H,29,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 624 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50230293

(CHEMBL4082253)Show SMILES CC(C)[C@@H]1NC(=O)CCCC\C=C\C=C\CCOC(=O)[C@@H]2CCCN(N2)C(=O)[C@H](Cc2cccc(O)c2)NC1=O |r,t:11,13| Show InChI InChI=1S/C30H42N4O6/c1-21(2)27-28(37)31-25(20-22-13-11-14-23(35)19-22)29(38)34-17-12-15-24(33-34)30(39)40-18-10-8-6-4-3-5-7-9-16-26(36)32-27/h3-4,6,8,11,13-14,19,21,24-25,27,33,35H,5,7,9-10,12,15-18,20H2,1-2H3,(H,31,37)(H,32,36)/b4-3+,8-6+/t24-,25-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 795 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selcia Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human N-terminal His8-tagged Cyclophilin A (1 to 169 residues) expressed in Escherichia coli BL21(DE3) using Su... |
J Med Chem 60: 1000-1017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01329
BindingDB Entry DOI: 10.7270/Q2JQ137K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314305

(1-(4-chlorophenyl)-3-(4-fluorobenzyl)-1-(1-(methyl...)Show SMILES CS(=O)(=O)N1CCC(CC1)N(C(=O)NCc1ccc(F)cc1)c1ccc(Cl)cc1 Show InChI InChI=1S/C20H23ClFN3O3S/c1-29(27,28)24-12-10-19(11-13-24)25(18-8-4-16(21)5-9-18)20(26)23-14-15-2-6-17(22)7-3-15/h2-9,19H,10-14H2,1H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 858 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314308

(1-(4-chlorophenyl)-1-(1-(2,3-dihydroxypropyl)piper...)Show SMILES OCC(O)CN1CCC(CC1)N(C(=O)NCc1ccc(F)cc1)c1ccc(Cl)cc1 Show InChI InChI=1S/C22H27ClFN3O3/c23-17-3-7-19(8-4-17)27(20-9-11-26(12-10-20)14-21(29)15-28)22(30)25-13-16-1-5-18(24)6-2-16/h1-8,20-21,28-29H,9-15H2,(H,25,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314304
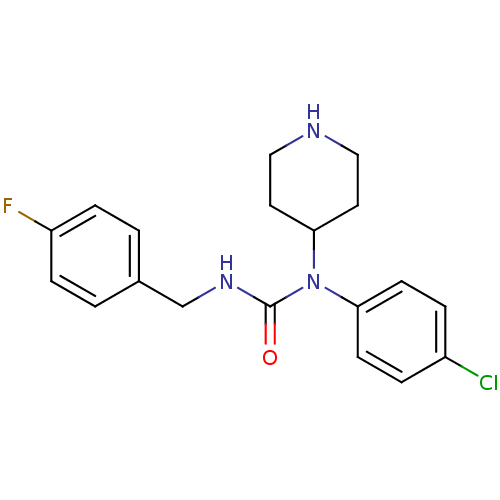
(1-(4-chlorophenyl)-3-(4-fluorobenzyl)-1-(piperidin...)Show InChI InChI=1S/C19H21ClFN3O/c20-15-3-7-17(8-4-15)24(18-9-11-22-12-10-18)19(25)23-13-14-1-5-16(21)6-2-14/h1-8,18,22H,9-13H2,(H,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314310
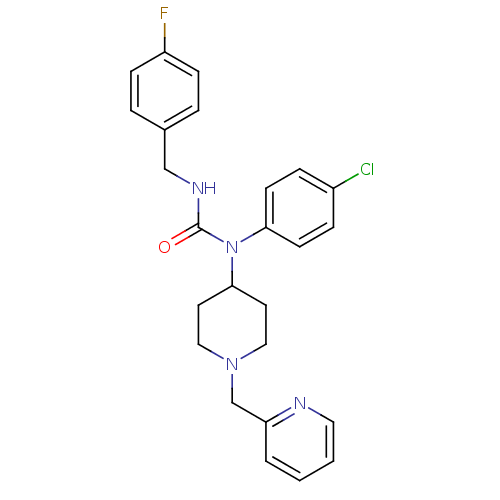
(1-(4-chlorophenyl)-3-(4-fluorobenzyl)-1-(1-(pyridi...)Show SMILES Fc1ccc(CNC(=O)N(C2CCN(Cc3ccccn3)CC2)c2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C25H26ClFN4O/c26-20-6-10-23(11-7-20)31(25(32)29-17-19-4-8-21(27)9-5-19)24-12-15-30(16-13-24)18-22-3-1-2-14-28-22/h1-11,14,24H,12-13,15-18H2,(H,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314302

(1-(1-ethylpiperidin-4-yl)-3-(4-fluorobenzyl)-1-(pi...)Show InChI InChI=1S/C20H31FN4O/c1-2-24-13-9-19(10-14-24)25(18-7-11-22-12-8-18)20(26)23-15-16-3-5-17(21)6-4-16/h3-6,18-19,22H,2,7-15H2,1H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314301
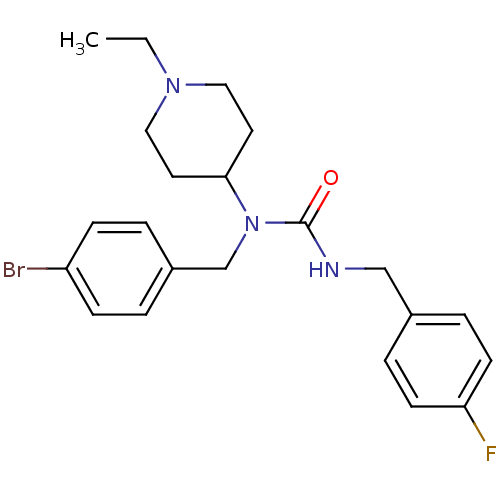
(1-(4-bromobenzyl)-1-(1-ethylpiperidin-4-yl)-3-(4-f...)Show SMILES CCN1CCC(CC1)N(Cc1ccc(Br)cc1)C(=O)NCc1ccc(F)cc1 Show InChI InChI=1S/C22H27BrFN3O/c1-2-26-13-11-21(12-14-26)27(16-18-3-7-19(23)8-4-18)22(28)25-15-17-5-9-20(24)10-6-17/h3-10,21H,2,11-16H2,1H3,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314299
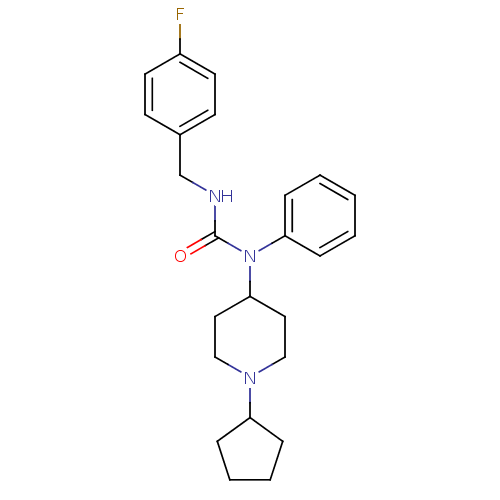
(1-(1-cyclopentylpiperidin-4-yl)-3-(4-fluorobenzyl)...)Show SMILES Fc1ccc(CNC(=O)N(C2CCN(CC2)C2CCCC2)c2ccccc2)cc1 Show InChI InChI=1S/C24H30FN3O/c25-20-12-10-19(11-13-20)18-26-24(29)28(22-8-2-1-3-9-22)23-14-16-27(17-15-23)21-6-4-5-7-21/h1-3,8-13,21,23H,4-7,14-18H2,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314309

(4-(1-(4-chlorophenyl)-3-(4-fluorobenzyl)ureido)-N-...)Show SMILES CCNC(=O)N1CCC(CC1)N(C(=O)NCc1ccc(F)cc1)c1ccc(Cl)cc1 Show InChI InChI=1S/C22H26ClFN4O2/c1-2-25-21(29)27-13-11-20(12-14-27)28(19-9-5-17(23)6-10-19)22(30)26-15-16-3-7-18(24)8-4-16/h3-10,20H,2,11-15H2,1H3,(H,25,29)(H,26,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314303

(1-(1-ethylpiperidin-4-yl)-3-(4-fluorobenzyl)-1-(1-...)Show SMILES CCN1CCC(CC1)N(C1CCN(CCO)CC1)C(=O)NCc1ccc(F)cc1 Show InChI InChI=1S/C22H35FN4O2/c1-2-25-11-7-20(8-12-25)27(21-9-13-26(14-10-21)15-16-28)22(29)24-17-18-3-5-19(23)6-4-18/h3-6,20-21,28H,2,7-17H2,1H3,(H,24,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50381743

(CHEMBL2023619)Show SMILES C[C@]12C[C@H](O)[C@H]3[C@@H](CCC4=Cc5c(C[C@]34C)cnn5-c3ccc(F)cc3)[C@@H]1CC[C@]2(O)C(=O)CSc1nc2ncccc2o1 |r,t:9| Show InChI InChI=1S/C34H35FN4O4S/c1-32-15-19-17-37-39(22-8-6-21(35)7-9-22)25(19)14-20(32)5-10-23-24-11-12-34(42,33(24,2)16-26(40)29(23)32)28(41)18-44-31-38-30-27(43-31)4-3-13-36-30/h3-4,6-9,13-14,17,23-24,26,29,40,42H,5,10-12,15-16,18H2,1-2H3/t23-,24-,26-,29+,32-,33-,34-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Transrepression activity at glucocorticoid receptor in human H292 cells assessed as inhibition of TNF-induced IL8 production |
Bioorg Med Chem Lett 22: 3291-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.015
BindingDB Entry DOI: 10.7270/Q2V69KMZ |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50381724
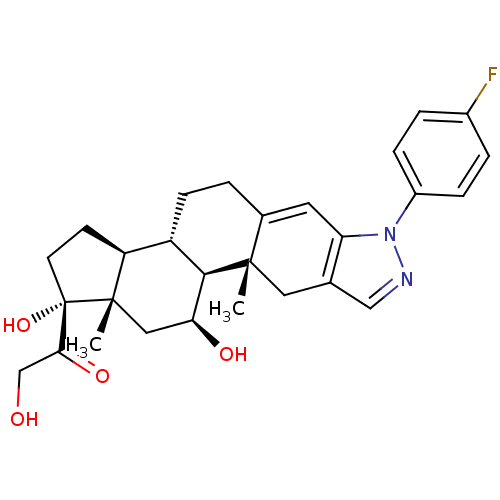
(CHEMBL2022858)Show SMILES C[C@]12C[C@H](O)[C@H]3[C@@H](CCC4=Cc5c(C[C@]34C)cnn5-c3ccc(F)cc3)[C@@H]1CC[C@]2(O)C(=O)CO |r,t:9| Show InChI InChI=1S/C28H33FN2O4/c1-26-12-16-14-30-31(19-6-4-18(29)5-7-19)22(16)11-17(26)3-8-20-21-9-10-28(35,24(34)15-32)27(21,2)13-23(33)25(20)26/h4-7,11,14,20-21,23,25,32-33,35H,3,8-10,12-13,15H2,1-2H3/t20-,21-,23-,25+,26-,27-,28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Transrepression activity at glucocorticoid receptor in human H292 cells assessed as inhibition of TNF-induced IL8 production |
Bioorg Med Chem Lett 22: 3291-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.015
BindingDB Entry DOI: 10.7270/Q2V69KMZ |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50381737

(CHEMBL2021945)Show SMILES C[C@]12C[C@H](O)[C@H]3[C@@H](CCC4=Cc5c(C[C@]34C)cnn5-c3ccc(F)cc3)[C@@H]1CC[C@]2(O)C(=O)CSc1ccccn1 |r,t:9| Show InChI InChI=1S/C33H36FN3O3S/c1-31-16-20-18-36-37(23-9-7-22(34)8-10-23)26(20)15-21(31)6-11-24-25-12-13-33(40,32(25,2)17-27(38)30(24)31)28(39)19-41-29-5-3-4-14-35-29/h3-5,7-10,14-15,18,24-25,27,30,38,40H,6,11-13,16-17,19H2,1-2H3/t24-,25-,27-,30+,31-,32-,33-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Transrepression activity at glucocorticoid receptor in human H292 cells assessed as inhibition of TNF-induced IL8 production |
Bioorg Med Chem Lett 22: 3291-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.015
BindingDB Entry DOI: 10.7270/Q2V69KMZ |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50381744

(CHEMBL2023620)Show SMILES C[C@]12C[C@H](O)[C@H]3[C@@H](CCC4=Cc5c(C[C@]34C)cnn5-c3ccc(F)cc3)[C@@H]1CC[C@]2(O)C(=O)CSc1ncccn1 |r,t:9| Show InChI InChI=1S/C32H35FN4O3S/c1-30-15-19-17-36-37(22-7-5-21(33)6-8-22)25(19)14-20(30)4-9-23-24-10-11-32(40,31(24,2)16-26(38)28(23)30)27(39)18-41-29-34-12-3-13-35-29/h3,5-8,12-14,17,23-24,26,28,38,40H,4,9-11,15-16,18H2,1-2H3/t23-,24-,26-,28+,30-,31-,32-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Transrepression activity at glucocorticoid receptor in human H292 cells assessed as inhibition of TNF-induced IL8 production |
Bioorg Med Chem Lett 22: 3291-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.015
BindingDB Entry DOI: 10.7270/Q2V69KMZ |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50381733
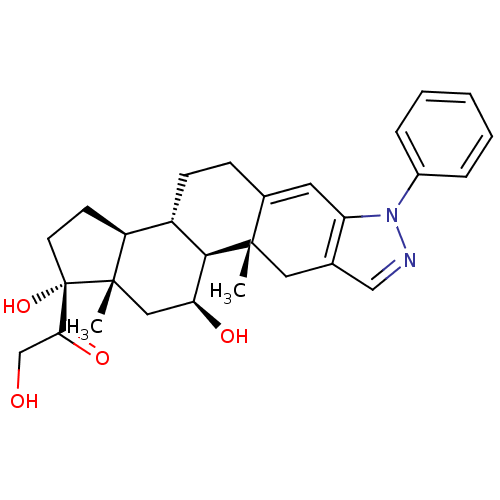
(CHEMBL2022651)Show SMILES C[C@]12C[C@H](O)[C@H]3[C@@H](CCC4=Cc5c(C[C@]34C)cnn5-c3ccccc3)[C@@H]1CC[C@]2(O)C(=O)CO |r,t:9| Show InChI InChI=1S/C28H34N2O4/c1-26-13-17-15-29-30(19-6-4-3-5-7-19)22(17)12-18(26)8-9-20-21-10-11-28(34,24(33)16-31)27(21,2)14-23(32)25(20)26/h3-7,12,15,20-21,23,25,31-32,34H,8-11,13-14,16H2,1-2H3/t20-,21-,23-,25+,26-,27-,28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Transrepression activity at glucocorticoid receptor in human H292 cells assessed as inhibition of TNF-induced IL8 production |
Bioorg Med Chem Lett 22: 3291-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.015
BindingDB Entry DOI: 10.7270/Q2V69KMZ |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50381729

(CHEMBL2023243)Show SMILES C[C@]12C[C@H](O)[C@H]3[C@@H](CCC4=Cc5c(C[C@]34C)cnn5-c3ccc(F)cc3)[C@@H]1CC[C@]2(O)C(=O)CSc1ccc2ccccc2c1 |r,t:9| Show InChI InChI=1S/C38H39FN2O3S/c1-36-19-25-21-40-41(28-11-9-27(39)10-12-28)32(25)18-26(36)8-14-30-31-15-16-38(44,37(31,2)20-33(42)35(30)36)34(43)22-45-29-13-7-23-5-3-4-6-24(23)17-29/h3-7,9-13,17-18,21,30-31,33,35,42,44H,8,14-16,19-20,22H2,1-2H3/t30-,31-,33-,35+,36-,37-,38-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Transrepression activity at glucocorticoid receptor in human H292 cells assessed as inhibition of TNF-induced IL8 production |
Bioorg Med Chem Lett 22: 3291-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.015
BindingDB Entry DOI: 10.7270/Q2V69KMZ |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50381748
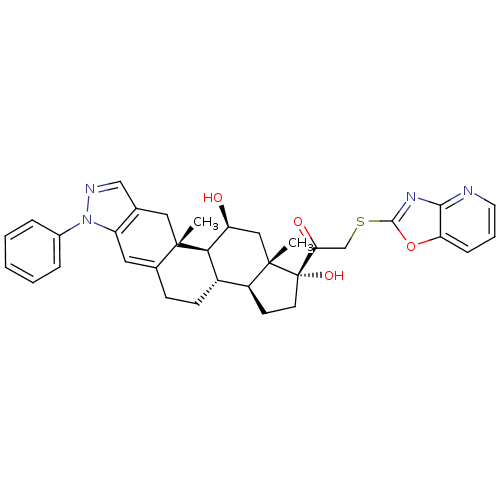
(CHEMBL2022656)Show SMILES C[C@]12C[C@H](O)[C@H]3[C@@H](CCC4=Cc5c(C[C@]34C)cnn5-c3ccccc3)[C@@H]1CC[C@]2(O)C(=O)CSc1nc2ncccc2o1 |r,t:9| Show InChI InChI=1S/C34H36N4O4S/c1-32-16-20-18-36-38(22-7-4-3-5-8-22)25(20)15-21(32)10-11-23-24-12-13-34(41,33(24,2)17-26(39)29(23)32)28(40)19-43-31-37-30-27(42-31)9-6-14-35-30/h3-9,14-15,18,23-24,26,29,39,41H,10-13,16-17,19H2,1-2H3/t23-,24-,26-,29+,32-,33-,34-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Transrepression activity at glucocorticoid receptor in human H292 cells assessed as inhibition of TNF-induced IL8 production |
Bioorg Med Chem Lett 22: 3291-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.015
BindingDB Entry DOI: 10.7270/Q2V69KMZ |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 9
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Life Science and National Research Laboratory of Proteolysis
Curated by ChEMBL
| Assay Description
In vitro inhibition of A disintegrin and metalloproteinase domain 9 (ADAM9) |
Bioorg Med Chem Lett 14: 6071-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.082
BindingDB Entry DOI: 10.7270/Q29Z94DH |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50381730

(CHEMBL2023244)Show SMILES C[C@]12C[C@H](O)[C@H]3[C@@H](CCC4=Cc5c(C[C@]34C)cnn5-c3ccc(F)cc3)[C@@H]1CC[C@]2(O)C(=O)COc1ccc2ccccc2c1 |r,t:9| Show InChI InChI=1S/C38H39FN2O4/c1-36-19-25-21-40-41(28-11-9-27(39)10-12-28)32(25)18-26(36)8-14-30-31-15-16-38(44,37(31,2)20-33(42)35(30)36)34(43)22-45-29-13-7-23-5-3-4-6-24(23)17-29/h3-7,9-13,17-18,21,30-31,33,35,42,44H,8,14-16,19-20,22H2,1-2H3/t30-,31-,33-,35+,36-,37-,38-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.06 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Transrepression activity at glucocorticoid receptor in human H292 cells assessed as inhibition of TNF-induced IL8 production |
Bioorg Med Chem Lett 22: 3291-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.015
BindingDB Entry DOI: 10.7270/Q2V69KMZ |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50381728
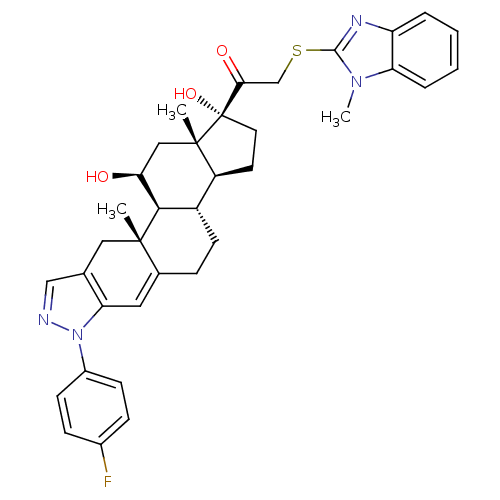
(CHEMBL2023241)Show SMILES Cn1c(SCC(=O)[C@@]2(O)CC[C@H]3[C@@H]4CCC5=Cc6c(C[C@]5(C)[C@H]4[C@@H](O)C[C@]23C)cnn6-c2ccc(F)cc2)nc2ccccc12 |r,t:15| Show InChI InChI=1S/C36H39FN4O3S/c1-34-17-21-19-38-41(24-11-9-23(37)10-12-24)29(21)16-22(34)8-13-25-26-14-15-36(44,35(26,2)18-30(42)32(25)34)31(43)20-45-33-39-27-6-4-5-7-28(27)40(33)3/h4-7,9-12,16,19,25-26,30,32,42,44H,8,13-15,17-18,20H2,1-3H3/t25-,26-,30-,32+,34-,35-,36-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Transrepression activity at glucocorticoid receptor in human H292 cells assessed as inhibition of TNF-induced IL8 production |
Bioorg Med Chem Lett 22: 3291-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.015
BindingDB Entry DOI: 10.7270/Q2V69KMZ |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50381749
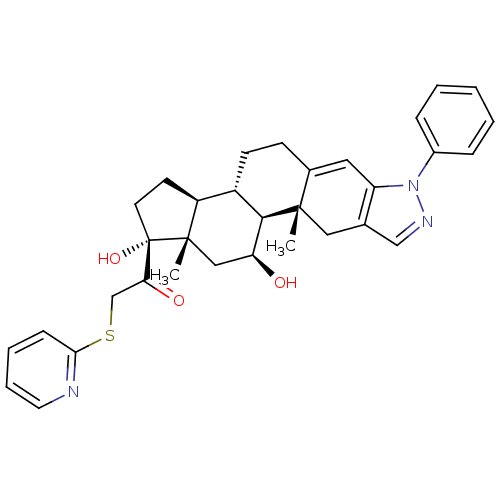
(CHEMBL2022657)Show SMILES C[C@]12C[C@H](O)[C@H]3[C@@H](CCC4=Cc5c(C[C@]34C)cnn5-c3ccccc3)[C@@H]1CC[C@]2(O)C(=O)CSc1ccccn1 |r,t:9| Show InChI InChI=1S/C33H37N3O3S/c1-31-17-21-19-35-36(23-8-4-3-5-9-23)26(21)16-22(31)11-12-24-25-13-14-33(39,32(25,2)18-27(37)30(24)31)28(38)20-40-29-10-6-7-15-34-29/h3-10,15-16,19,24-25,27,30,37,39H,11-14,17-18,20H2,1-2H3/t24-,25-,27-,30+,31-,32-,33-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Transrepression activity at glucocorticoid receptor in human H292 cells assessed as inhibition of TNF-induced IL8 production |
Bioorg Med Chem Lett 22: 3291-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.015
BindingDB Entry DOI: 10.7270/Q2V69KMZ |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50362450

(CHEMBL1940557)Show SMILES C[C@@H]1C[C@H]2[C@@H]3CCC4=CC(=O)C=C[C@]4(C)[C@H]3[C@@H](O)C[C@]2(C)[C@@]1(O)C(=O)CO |r,c:11,t:7| Show InChI InChI=1S/C22H30O5/c1-12-8-16-15-5-4-13-9-14(24)6-7-20(13,2)19(15)17(25)10-21(16,3)22(12,27)18(26)11-23/h6-7,9,12,15-17,19,23,25,27H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19-,20+,21+,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Transrepression activity at glucocorticoid receptor in human H292 cells assessed as inhibition of TNF-stimulated IL-8 production |
Bioorg Med Chem Lett 22: 1086-90 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.120
BindingDB Entry DOI: 10.7270/Q2JH3MNV |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50381742
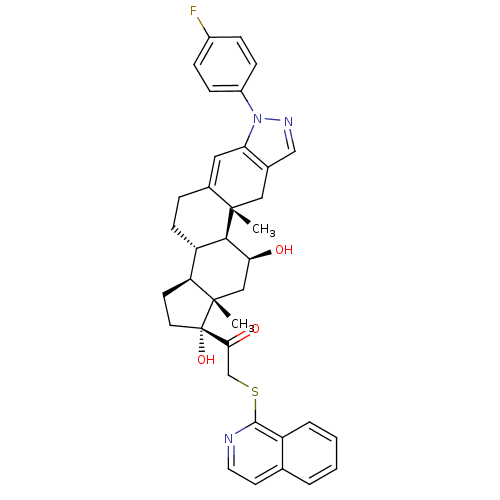
(CHEMBL2023618)Show SMILES C[C@]12C[C@H](O)[C@H]3[C@@H](CCC4=Cc5c(C[C@]34C)cnn5-c3ccc(F)cc3)[C@@H]1CC[C@]2(O)C(=O)CSc1nccc2ccccc12 |r,t:9| Show InChI InChI=1S/C37H38FN3O3S/c1-35-18-23-20-40-41(26-10-8-25(38)9-11-26)30(23)17-24(35)7-12-28-29-13-15-37(44,36(29,2)19-31(42)33(28)35)32(43)21-45-34-27-6-4-3-5-22(27)14-16-39-34/h3-6,8-11,14,16-17,20,28-29,31,33,42,44H,7,12-13,15,18-19,21H2,1-2H3/t28-,29-,31-,33+,35-,36-,37-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Transrepression activity at glucocorticoid receptor in human H292 cells assessed as inhibition of TNF-induced IL8 production |
Bioorg Med Chem Lett 22: 3291-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.015
BindingDB Entry DOI: 10.7270/Q2V69KMZ |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50362458
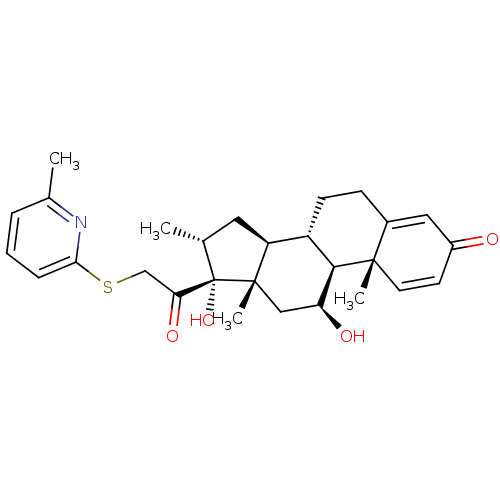
(CHEMBL1940694)Show SMILES C[C@@H]1C[C@H]2[C@@H]3CCC4=CC(=O)C=C[C@]4(C)[C@H]3[C@@H](O)C[C@]2(C)[C@@]1(O)C(=O)CSc1cccc(C)n1 |r,c:11,t:7| Show InChI InChI=1S/C28H35NO4S/c1-16-12-21-20-9-8-18-13-19(30)10-11-26(18,3)25(20)22(31)14-27(21,4)28(16,33)23(32)15-34-24-7-5-6-17(2)29-24/h5-7,10-11,13,16,20-22,25,31,33H,8-9,12,14-15H2,1-4H3/t16-,20+,21+,22+,25-,26+,27+,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.69 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Transrepression activity at glucocorticoid receptor in human H292 cells assessed as inhibition of TNF-stimulated IL-8 production |
Bioorg Med Chem Lett 22: 1086-90 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.120
BindingDB Entry DOI: 10.7270/Q2JH3MNV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data