 Found 128 hits with Last Name = 'lelais' and Initial = 'g'
Found 128 hits with Last Name = 'lelais' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Lon protease homolog, mitochondrial
(Homo sapiens) | BDBM50571496

(CHEMBL4876525)Show SMILES CCC[C@@H](NC(=O)c1cnccn1)C(=O)N[C@@H](CCCc1ccccc1)B(O)O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of LONP1 (unknown origin) using QXL520-YRGITCSGRQK(5-FAM)-NH2 peptide as substrate incubated for 50 mins in presence of ATP by HTRF assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02152
BindingDB Entry DOI: 10.7270/Q25Q50WZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lon protease homolog, mitochondrial
(Homo sapiens) | BDBM50571500

(CHEMBL4856865)Show SMILES OB(O)[C@H](CCCc1ccccc1)NC(=O)[C@@H](Cc1ccccc1)NC(=O)c1cnccn1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of LONP1 (unknown origin) using QXL520-YRGITCSGRQK(5-FAM)-NH2 peptide as substrate incubated for 50 mins in presence of ATP by HTRF assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02152
BindingDB Entry DOI: 10.7270/Q25Q50WZ |
More data for this
Ligand-Target Pair | |
Lon protease homolog, mitochondrial
(Homo sapiens) | BDBM50571499
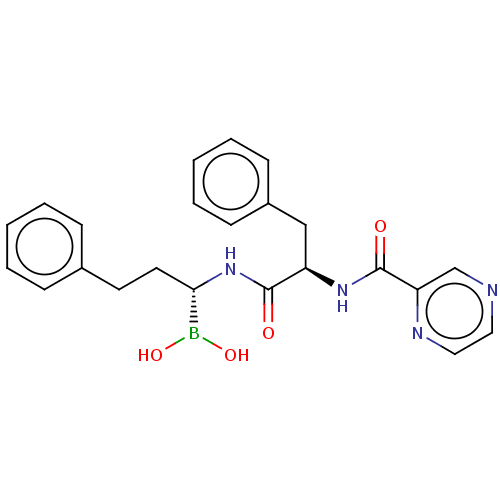
(CHEMBL4864108)Show SMILES OB(O)[C@H](CCc1ccccc1)NC(=O)[C@@H](Cc1ccccc1)NC(=O)c1cnccn1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of LONP1 (unknown origin) using QXL520-YRGITCSGRQK(5-FAM)-NH2 peptide as substrate incubated for 50 mins in presence of ATP by HTRF assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02152
BindingDB Entry DOI: 10.7270/Q25Q50WZ |
More data for this
Ligand-Target Pair | |
Lon protease homolog, mitochondrial
(Homo sapiens) | BDBM50571493

(CHEMBL4877126)Show SMILES CCCC[C@H](NC(=O)[C@@H](CCC)NC(=O)c1cnccn1)B(O)O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of LONP1 (unknown origin) using QXL520-YRGITCSGRQK(5-FAM)-NH2 peptide as substrate incubated for 50 mins in presence of ATP by HTRF assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02152
BindingDB Entry DOI: 10.7270/Q25Q50WZ |
More data for this
Ligand-Target Pair | |
Lon protease homolog, mitochondrial
(Homo sapiens) | BDBM50571492

(CHEMBL4870013)Show SMILES CCC[C@@H](NC(=O)c1oc(C)nc1C)C(=O)N[C@@H](CCCc1ccccc1)B(O)O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of LONP1 (unknown origin) using QXL520-YRGITCSGRQK(5-FAM)-NH2 peptide as substrate incubated for 50 mins in presence of ATP by HTRF assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02152
BindingDB Entry DOI: 10.7270/Q25Q50WZ |
More data for this
Ligand-Target Pair | |
Lon protease homolog, mitochondrial
(Homo sapiens) | BDBM50571502
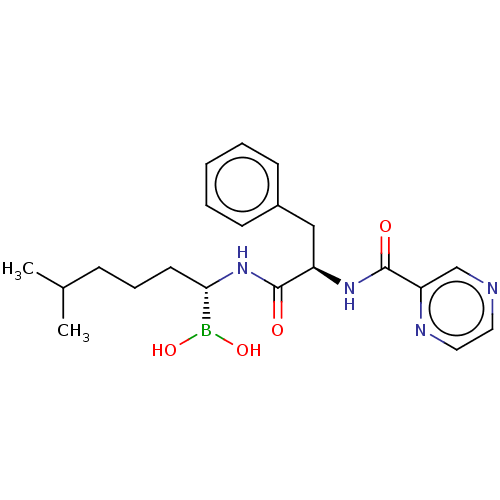
(CHEMBL4862424)Show SMILES CC(C)CCC[C@H](NC(=O)[C@@H](Cc1ccccc1)NC(=O)c1cnccn1)B(O)O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of LONP1 (unknown origin) using QXL520-YRGITCSGRQK(5-FAM)-NH2 peptide as substrate incubated for 50 mins in presence of ATP by HTRF assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02152
BindingDB Entry DOI: 10.7270/Q25Q50WZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50196094
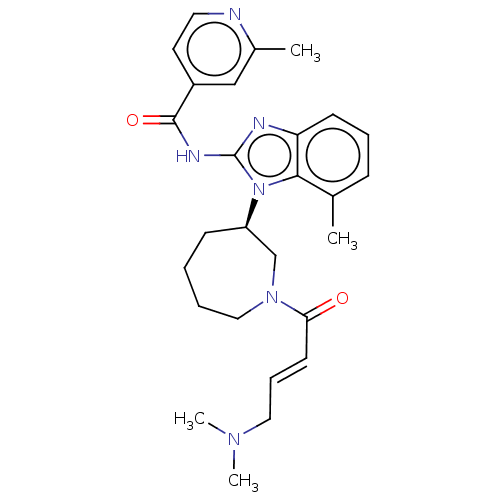
(CHEMBL3960167)Show SMILES CN(C)C\C=C\C(=O)N1CCCC[C@H](C1)n1c(NC(=O)c2ccnc(C)c2)nc2cccc(C)c12 |r| Show InChI InChI=1S/C27H34N6O2/c1-19-9-7-11-23-25(19)33(27(29-23)30-26(35)21-13-14-28-20(2)17-21)22-10-5-6-16-32(18-22)24(34)12-8-15-31(3)4/h7-9,11-14,17,22H,5-6,10,15-16,18H2,1-4H3,(H,29,30,35)/b12-8+/t22-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA method |
J Med Chem 59: 6671-89 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01985
BindingDB Entry DOI: 10.7270/Q2RF5X00 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50196093
(CHEMBL3939913)Show SMILES CN(C)C\C=C\C(=O)N1CCCC[C@H](C1)n1c(NC(=O)c2ccnc(c2)C(F)(F)F)nc2cccc(C)c12 |r| Show InChI InChI=1S/C27H31F3N6O2/c1-18-8-6-10-21-24(18)36(20-9-4-5-15-35(17-20)23(37)11-7-14-34(2)3)26(32-21)33-25(38)19-12-13-31-22(16-19)27(28,29)30/h6-8,10-13,16,20H,4-5,9,14-15,17H2,1-3H3,(H,32,33,38)/b11-7+/t20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA method |
J Med Chem 59: 6671-89 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01985
BindingDB Entry DOI: 10.7270/Q2RF5X00 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA method |
J Med Chem 59: 6671-89 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01985
BindingDB Entry DOI: 10.7270/Q2RF5X00 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lon protease homolog, mitochondrial
(Homo sapiens) | BDBM50571501

(CHEMBL4870902)Show SMILES OB(O)[C@H](CCCCCBr)NC(=O)[C@@H](Cc1ccccc1)NC(=O)c1cnccn1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of LONP1 (unknown origin) using QXL520-YRGITCSGRQK(5-FAM)-NH2 peptide as substrate incubated for 50 mins in presence of ATP by HTRF assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02152
BindingDB Entry DOI: 10.7270/Q25Q50WZ |
More data for this
Ligand-Target Pair | |
Lon protease homolog, mitochondrial
(Homo sapiens) | BDBM50571503
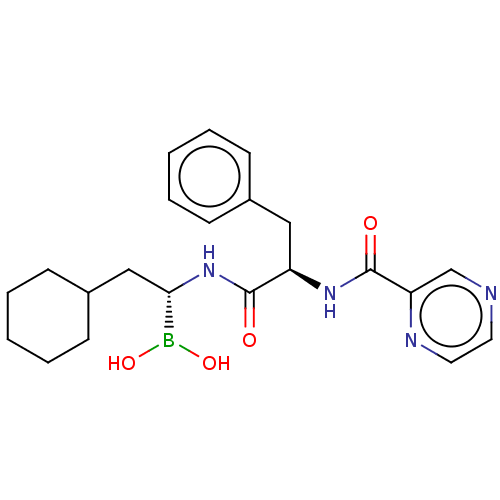
(CHEMBL4850612)Show SMILES OB(O)[C@H](CC1CCCCC1)NC(=O)[C@@H](Cc1ccccc1)NC(=O)c1cnccn1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of LONP1 (unknown origin) using QXL520-YRGITCSGRQK(5-FAM)-NH2 peptide as substrate incubated for 50 mins in presence of ATP by HTRF assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02152
BindingDB Entry DOI: 10.7270/Q25Q50WZ |
More data for this
Ligand-Target Pair | |
Lon protease homolog, mitochondrial
(Homo sapiens) | BDBM50571495

(CHEMBL4876920)Show SMILES CCC[C@@H](NC(=O)c1cnccn1)C(=O)N[C@@H](CCc1ccccc1)B(O)O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of LONP1 (unknown origin) using QXL520-YRGITCSGRQK(5-FAM)-NH2 peptide as substrate incubated for 50 mins in presence of ATP by HTRF assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02152
BindingDB Entry DOI: 10.7270/Q25Q50WZ |
More data for this
Ligand-Target Pair | |
Lon protease homolog, mitochondrial
(Homo sapiens) | BDBM50571508
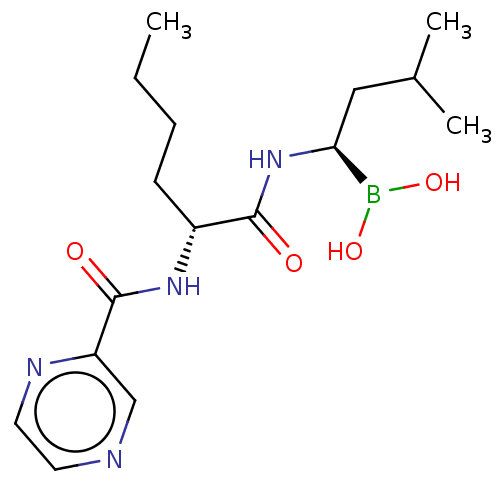
(CHEMBL4856768)Show SMILES CCCC[C@@H](NC(=O)c1cnccn1)C(=O)N[C@@H](CC(C)C)B(O)O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of LONP1 (unknown origin) using QXL520-YRGITCSGRQK(5-FAM)-NH2 peptide as substrate incubated for 50 mins in presence of ATP by HTRF assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02152
BindingDB Entry DOI: 10.7270/Q25Q50WZ |
More data for this
Ligand-Target Pair | |
A5LHX3/O14818/P20618/P25786/P25787/P25788/P25789/P28062/P28065/P28066/P28070/P28072/P28074/P40306/P49720/P49721/P60900/Q8TAA3/Q99436
(Homo sapiens (Human)) | BDBM50069989

((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)c1cnccn1)B(O)O |r| Show InChI InChI=1S/C19H25BN4O4/c1-13(2)10-17(20(27)28)24-18(25)15(11-14-6-4-3-5-7-14)23-19(26)16-12-21-8-9-22-16/h3-9,12-13,15,17,27-28H,10-11H2,1-2H3,(H,23,26)(H,24,25)/t15-,17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocytes 20S proteasome using QXL520-YRGITCSGRQK(5-FAM)-NH2 fluorogenic peptide as substrate preincubated for 10 mins followe... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02152
BindingDB Entry DOI: 10.7270/Q25Q50WZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lon protease homolog, mitochondrial
(Homo sapiens) | BDBM50571498
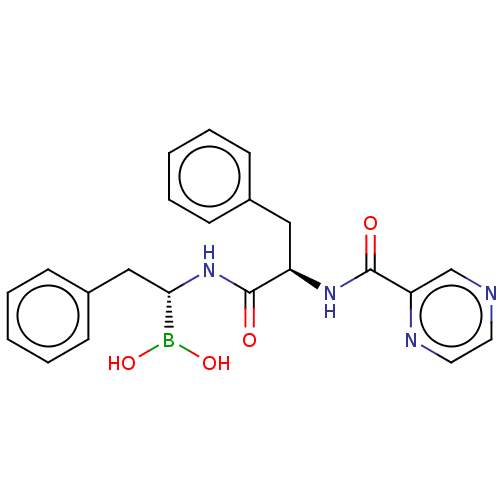
(CHEMBL4847335)Show SMILES OB(O)[C@H](Cc1ccccc1)NC(=O)[C@@H](Cc1ccccc1)NC(=O)c1cnccn1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of LONP1 (unknown origin) using QXL520-YRGITCSGRQK(5-FAM)-NH2 peptide as substrate incubated for 50 mins in presence of ATP by HTRF assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02152
BindingDB Entry DOI: 10.7270/Q25Q50WZ |
More data for this
Ligand-Target Pair | |
Lon protease homolog, mitochondrial
(Homo sapiens) | BDBM50571497
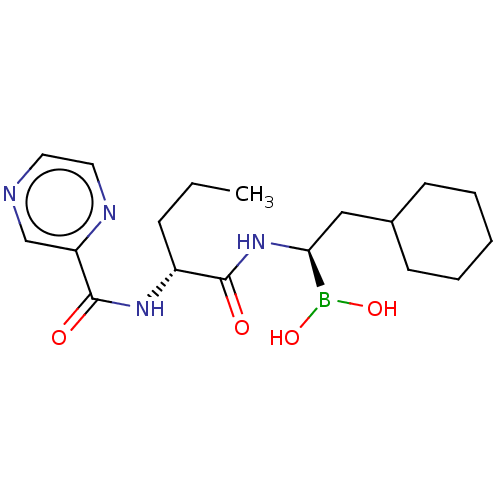
(CHEMBL4863384)Show SMILES CCC[C@@H](NC(=O)c1cnccn1)C(=O)N[C@@H](CC1CCCCC1)B(O)O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 136 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of LONP1 (unknown origin) using QXL520-YRGITCSGRQK(5-FAM)-NH2 peptide as substrate incubated for 50 mins in presence of ATP by HTRF assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02152
BindingDB Entry DOI: 10.7270/Q25Q50WZ |
More data for this
Ligand-Target Pair | |
Lon protease homolog, mitochondrial
(Homo sapiens) | BDBM50571505
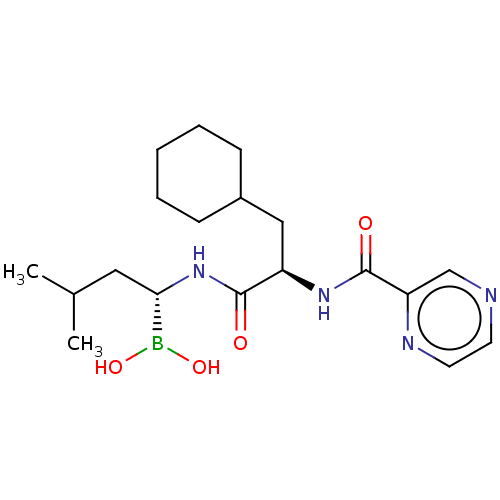
(CHEMBL4847763)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](CC1CCCCC1)NC(=O)c1cnccn1)B(O)O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 137 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of LONP1 (unknown origin) using QXL520-YRGITCSGRQK(5-FAM)-NH2 peptide as substrate incubated for 50 mins in presence of ATP by HTRF assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02152
BindingDB Entry DOI: 10.7270/Q25Q50WZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50160870
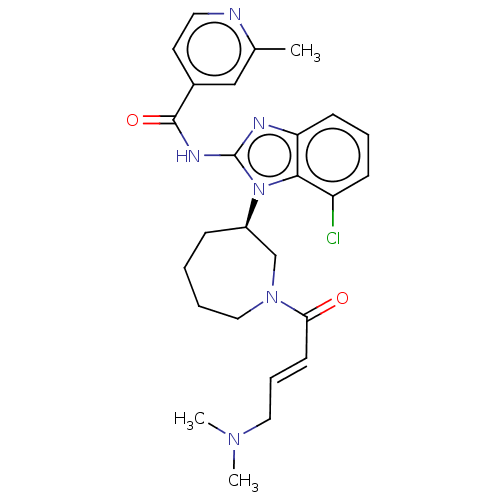
(CHEMBL3787344 | WO2022090481, Example nazartinib)Show SMILES CN(C)C\C=C\C(=O)N1CCCC[C@H](C1)n1c(NC(=O)c2ccnc(C)c2)nc2cccc(Cl)c12 |r| Show InChI InChI=1S/C26H31ClN6O2/c1-18-16-19(12-13-28-18)25(35)30-26-29-22-10-6-9-21(27)24(22)33(26)20-8-4-5-15-32(17-20)23(34)11-7-14-31(2)3/h6-7,9-13,16,20H,4-5,8,14-15,17H2,1-3H3,(H,29,30,35)/b11-7+/t20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 161 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA method |
J Med Chem 59: 6671-89 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01985
BindingDB Entry DOI: 10.7270/Q2RF5X00 |
More data for this
Ligand-Target Pair | |
Lon protease homolog, mitochondrial
(Homo sapiens) | BDBM50069989

((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)c1cnccn1)B(O)O |r| Show InChI InChI=1S/C19H25BN4O4/c1-13(2)10-17(20(27)28)24-18(25)15(11-14-6-4-3-5-7-14)23-19(26)16-12-21-8-9-22-16/h3-9,12-13,15,17,27-28H,10-11H2,1-2H3,(H,23,26)(H,24,25)/t15-,17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 183 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of LONP1 (unknown origin) using QXL520-YRGITCSGRQK(5-FAM)-NH2 peptide as substrate incubated for 50 mins in presence of ATP by HTRF assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02152
BindingDB Entry DOI: 10.7270/Q25Q50WZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lon protease homolog, mitochondrial
(Homo sapiens) | BDBM50571509

(CHEMBL4861871)Show SMILES CCC[C@@H](NC(=O)c1cnccn1)C(=O)N[C@@H](CC(C)C)B(O)O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 187 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of LONP1 (unknown origin) using QXL520-YRGITCSGRQK(5-FAM)-NH2 peptide as substrate incubated for 50 mins in presence of ATP by HTRF assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02152
BindingDB Entry DOI: 10.7270/Q25Q50WZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50196095
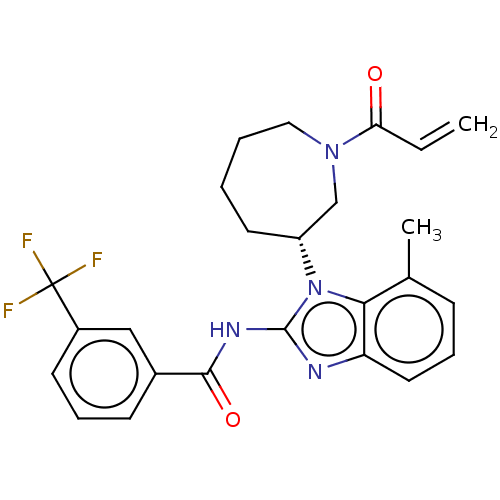
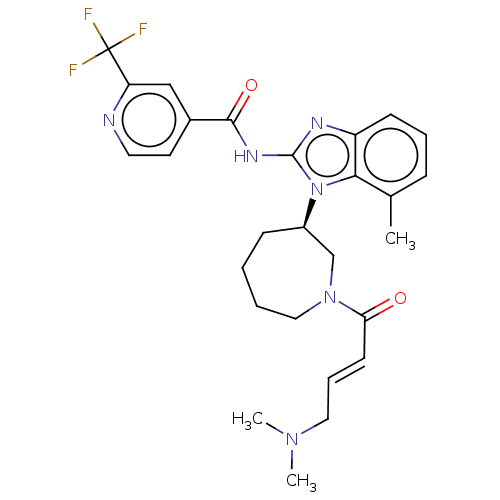
(CHEMBL3972316)Show SMILES Cc1cccc2nc(NC(=O)c3cccc(c3)C(F)(F)F)n([C@@H]3CCCCN(C3)C(=O)C=C)c12 |r| Show InChI InChI=1S/C25H25F3N4O2/c1-3-21(33)31-13-5-4-11-19(15-31)32-22-16(2)8-6-12-20(22)29-24(32)30-23(34)17-9-7-10-18(14-17)25(26,27)28/h3,6-10,12,14,19H,1,4-5,11,13,15H2,2H3,(H,29,30,34)/t19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA method |
J Med Chem 59: 6671-89 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01985
BindingDB Entry DOI: 10.7270/Q2RF5X00 |
More data for this
Ligand-Target Pair | |
Lon protease homolog, mitochondrial
(Homo sapiens) | BDBM50571512

(CHEMBL4858320)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](Cc1ccccc1)NC(=O)c1cnccn1)B(O)O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 253 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of LONP1 (unknown origin) using QXL520-YRGITCSGRQK(5-FAM)-NH2 peptide as substrate incubated for 50 mins in presence of ATP by HTRF assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02152
BindingDB Entry DOI: 10.7270/Q25Q50WZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
A5LHX3/O14818/P20618/P25786/P25787/P25788/P25789/P28062/P28065/P28066/P28070/P28072/P28074/P40306/P49720/P49721/P60900/Q8TAA3/Q99436
(Homo sapiens (Human)) | BDBM50571500

(CHEMBL4856865)Show SMILES OB(O)[C@H](CCCc1ccccc1)NC(=O)[C@@H](Cc1ccccc1)NC(=O)c1cnccn1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 259 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocytes 20S proteasome using QXL520-YRGITCSGRQK(5-FAM)-NH2 fluorogenic peptide as substrate preincubated for 10 mins followe... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02152
BindingDB Entry DOI: 10.7270/Q25Q50WZ |
More data for this
Ligand-Target Pair | |
A5LHX3/O14818/P20618/P25786/P25787/P25788/P25789/P28062/P28065/P28066/P28070/P28072/P28074/P40306/P49720/P49721/P60900/Q8TAA3/Q99436
(Homo sapiens (Human)) | BDBM50571499

(CHEMBL4864108)Show SMILES OB(O)[C@H](CCc1ccccc1)NC(=O)[C@@H](Cc1ccccc1)NC(=O)c1cnccn1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 262 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocytes 20S proteasome using QXL520-YRGITCSGRQK(5-FAM)-NH2 fluorogenic peptide as substrate preincubated for 10 mins followe... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02152
BindingDB Entry DOI: 10.7270/Q25Q50WZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM149404
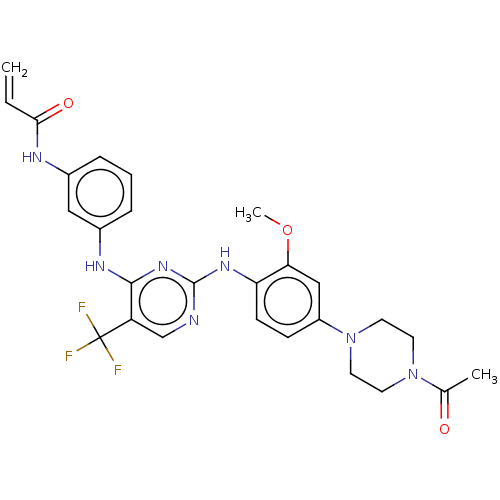
(AVL-301 | CHEMBL3545308 | CNX-419 | CO-1686 | Roci...)Show SMILES COc1cc(ccc1Nc1ncc(c(Nc2cccc(NC(=O)C=C)c2)n1)C(F)(F)F)N1CCN(CC1)C(C)=O Show InChI InChI=1S/C27H28F3N7O3/c1-4-24(39)32-18-6-5-7-19(14-18)33-25-21(27(28,29)30)16-31-26(35-25)34-22-9-8-20(15-23(22)40-3)37-12-10-36(11-13-37)17(2)38/h4-9,14-16H,1,10-13H2,2-3H3,(H,32,39)(H2,31,33,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 262 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA method |
J Med Chem 59: 6671-89 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01985
BindingDB Entry DOI: 10.7270/Q2RF5X00 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50196096
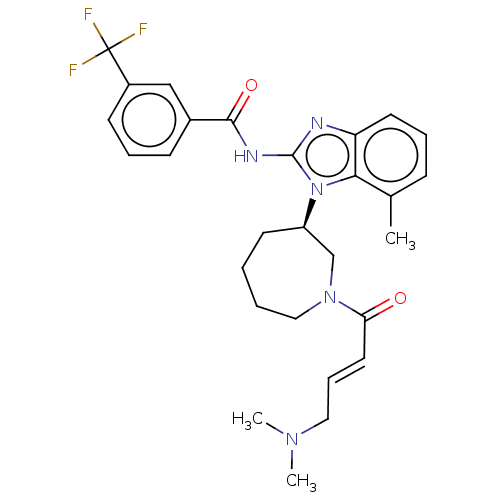
(CHEMBL3951434)Show SMILES CN(C)C\C=C\C(=O)N1CCCC[C@H](C1)n1c(NC(=O)c2cccc(c2)C(F)(F)F)nc2cccc(C)c12 |r| Show InChI InChI=1S/C28H32F3N5O2/c1-19-9-6-13-23-25(19)36(22-12-4-5-16-35(18-22)24(37)14-8-15-34(2)3)27(32-23)33-26(38)20-10-7-11-21(17-20)28(29,30)31/h6-11,13-14,17,22H,4-5,12,15-16,18H2,1-3H3,(H,32,33,38)/b14-8+/t22-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 351 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA method |
J Med Chem 59: 6671-89 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01985
BindingDB Entry DOI: 10.7270/Q2RF5X00 |
More data for this
Ligand-Target Pair | |
Lon protease homolog, mitochondrial
(Homo sapiens) | BDBM50571510
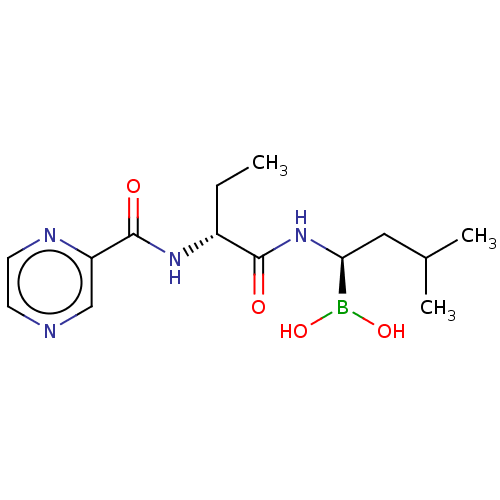
(CHEMBL4853599)Show SMILES CC[C@@H](NC(=O)c1cnccn1)C(=O)N[C@@H](CC(C)C)B(O)O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 408 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of LONP1 (unknown origin) using QXL520-YRGITCSGRQK(5-FAM)-NH2 peptide as substrate incubated for 50 mins in presence of ATP by HTRF assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02152
BindingDB Entry DOI: 10.7270/Q25Q50WZ |
More data for this
Ligand-Target Pair | |
Lon protease homolog, mitochondrial
(Homo sapiens) | BDBM50571511
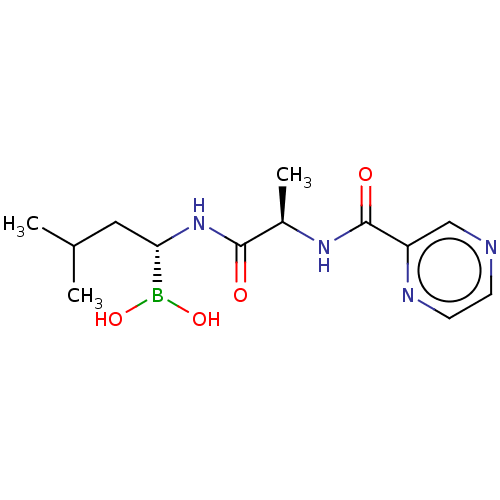
(CHEMBL4864045)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](C)NC(=O)c1cnccn1)B(O)O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 433 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of LONP1 (unknown origin) using QXL520-YRGITCSGRQK(5-FAM)-NH2 peptide as substrate incubated for 50 mins in presence of ATP by HTRF assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02152
BindingDB Entry DOI: 10.7270/Q25Q50WZ |
More data for this
Ligand-Target Pair | |
A5LHX3/O14818/P20618/P25786/P25787/P25788/P25789/P28062/P28065/P28066/P28070/P28072/P28074/P40306/P49720/P49721/P60900/Q8TAA3/Q99436
(Homo sapiens (Human)) | BDBM50571503

(CHEMBL4850612)Show SMILES OB(O)[C@H](CC1CCCCC1)NC(=O)[C@@H](Cc1ccccc1)NC(=O)c1cnccn1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 541 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocytes 20S proteasome using QXL520-YRGITCSGRQK(5-FAM)-NH2 fluorogenic peptide as substrate preincubated for 10 mins followe... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02152
BindingDB Entry DOI: 10.7270/Q25Q50WZ |
More data for this
Ligand-Target Pair | |
Lon protease homolog, mitochondrial
(Homo sapiens) | BDBM50571507
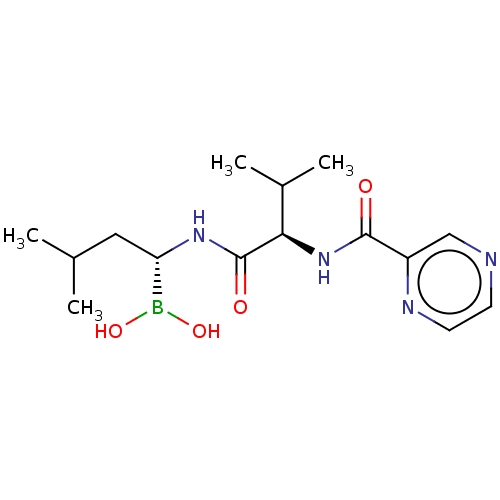
(CHEMBL4859172)Show SMILES CC(C)C[C@H](NC(=O)[C@H](NC(=O)c1cnccn1)C(C)C)B(O)O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 546 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of LONP1 (unknown origin) using QXL520-YRGITCSGRQK(5-FAM)-NH2 peptide as substrate incubated for 50 mins in presence of ATP by HTRF assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02152
BindingDB Entry DOI: 10.7270/Q25Q50WZ |
More data for this
Ligand-Target Pair | |
Lon protease homolog, mitochondrial
(Homo sapiens) | BDBM50571494

(CHEMBL4850826)Show SMILES CCC[C@@H](NC(=O)c1cnccn1)C(=O)N[C@@H](Cc1ccccc1)B(O)O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 556 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of LONP1 (unknown origin) using QXL520-YRGITCSGRQK(5-FAM)-NH2 peptide as substrate incubated for 50 mins in presence of ATP by HTRF assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02152
BindingDB Entry DOI: 10.7270/Q25Q50WZ |
More data for this
Ligand-Target Pair | |
A5LHX3/O14818/P20618/P25786/P25787/P25788/P25789/P28062/P28065/P28066/P28070/P28072/P28074/P40306/P49720/P49721/P60900/Q8TAA3/Q99436
(Homo sapiens (Human)) | BDBM50571498

(CHEMBL4847335)Show SMILES OB(O)[C@H](Cc1ccccc1)NC(=O)[C@@H](Cc1ccccc1)NC(=O)c1cnccn1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 679 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocytes 20S proteasome using QXL520-YRGITCSGRQK(5-FAM)-NH2 fluorogenic peptide as substrate preincubated for 10 mins followe... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02152
BindingDB Entry DOI: 10.7270/Q25Q50WZ |
More data for this
Ligand-Target Pair | |
A5LHX3/O14818/P20618/P25786/P25787/P25788/P25789/P28062/P28065/P28066/P28070/P28072/P28074/P40306/P49720/P49721/P60900/Q8TAA3/Q99436
(Homo sapiens (Human)) | BDBM50571505

(CHEMBL4847763)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](CC1CCCCC1)NC(=O)c1cnccn1)B(O)O |r| | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 843 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocytes 20S proteasome using QXL520-YRGITCSGRQK(5-FAM)-NH2 fluorogenic peptide as substrate preincubated for 10 mins followe... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02152
BindingDB Entry DOI: 10.7270/Q25Q50WZ |
More data for this
Ligand-Target Pair | |
A5LHX3/O14818/P20618/P25786/P25787/P25788/P25789/P28062/P28065/P28066/P28070/P28072/P28074/P40306/P49720/P49721/P60900/Q8TAA3/Q99436
(Homo sapiens (Human)) | BDBM50571502

(CHEMBL4862424)Show SMILES CC(C)CCC[C@H](NC(=O)[C@@H](Cc1ccccc1)NC(=O)c1cnccn1)B(O)O |r| | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocytes 20S proteasome using QXL520-YRGITCSGRQK(5-FAM)-NH2 fluorogenic peptide as substrate preincubated for 10 mins followe... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02152
BindingDB Entry DOI: 10.7270/Q25Q50WZ |
More data for this
Ligand-Target Pair | |
A5LHX3/O14818/P20618/P25786/P25787/P25788/P25789/P28062/P28065/P28066/P28070/P28072/P28074/P40306/P49720/P49721/P60900/Q8TAA3/Q99436
(Homo sapiens (Human)) | BDBM200228

(AB01275474-01 | cid_24871309)Show SMILES CC(C)C[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)c1cnccn1)B(O)O Show InChI InChI=1S/C19H25BN4O4/c1-13(2)10-17(20(27)28)24-18(25)15(11-14-6-4-3-5-7-14)23-19(26)16-12-21-8-9-22-16/h3-9,12-13,15,17,27-28H,10-11H2,1-2H3,(H,23,26)(H,24,25)/t15-,17+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocytes 20S proteasome using QXL520-YRGITCSGRQK(5-FAM)-NH2 fluorogenic peptide as substrate preincubated for 10 mins followe... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02152
BindingDB Entry DOI: 10.7270/Q25Q50WZ |
More data for this
Ligand-Target Pair | |
A5LHX3/O14818/P20618/P25786/P25787/P25788/P25789/P28062/P28065/P28066/P28070/P28072/P28074/P40306/P49720/P49721/P60900/Q8TAA3/Q99436
(Homo sapiens (Human)) | BDBM50571513
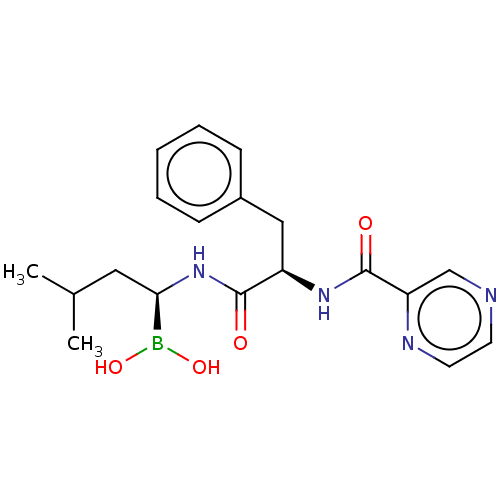
(CHEMBL1530)Show SMILES CC(C)C[C@@H](NC(=O)[C@@H](Cc1ccccc1)NC(=O)c1cnccn1)B(O)O | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocytes 20S proteasome using QXL520-YRGITCSGRQK(5-FAM)-NH2 fluorogenic peptide as substrate preincubated for 10 mins followe... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02152
BindingDB Entry DOI: 10.7270/Q25Q50WZ |
More data for this
Ligand-Target Pair | |
A5LHX3/O14818/P20618/P25786/P25787/P25788/P25789/P28062/P28065/P28066/P28070/P28072/P28074/P40306/P49720/P49721/P60900/Q8TAA3/Q99436
(Homo sapiens (Human)) | BDBM50571501

(CHEMBL4870902)Show SMILES OB(O)[C@H](CCCCCBr)NC(=O)[C@@H](Cc1ccccc1)NC(=O)c1cnccn1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocytes 20S proteasome using QXL520-YRGITCSGRQK(5-FAM)-NH2 fluorogenic peptide as substrate preincubated for 10 mins followe... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02152
BindingDB Entry DOI: 10.7270/Q25Q50WZ |
More data for this
Ligand-Target Pair | |
Lon protease homolog, mitochondrial
(Homo sapiens) | BDBM200228

(AB01275474-01 | cid_24871309)Show SMILES CC(C)C[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)c1cnccn1)B(O)O Show InChI InChI=1S/C19H25BN4O4/c1-13(2)10-17(20(27)28)24-18(25)15(11-14-6-4-3-5-7-14)23-19(26)16-12-21-8-9-22-16/h3-9,12-13,15,17,27-28H,10-11H2,1-2H3,(H,23,26)(H,24,25)/t15-,17+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of LONP1 (unknown origin) using QXL520-YRGITCSGRQK(5-FAM)-NH2 peptide as substrate incubated for 50 mins in presence of ATP by HTRF assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02152
BindingDB Entry DOI: 10.7270/Q25Q50WZ |
More data for this
Ligand-Target Pair | |
A5LHX3/O14818/P20618/P25786/P25787/P25788/P25789/P28062/P28065/P28066/P28070/P28072/P28074/P40306/P49720/P49721/P60900/Q8TAA3/Q99436
(Homo sapiens (Human)) | BDBM50571511

(CHEMBL4864045)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](C)NC(=O)c1cnccn1)B(O)O |r| | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocytes 20S proteasome using QXL520-YRGITCSGRQK(5-FAM)-NH2 fluorogenic peptide as substrate preincubated for 10 mins followe... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02152
BindingDB Entry DOI: 10.7270/Q25Q50WZ |
More data for this
Ligand-Target Pair | |
Lon protease homolog, mitochondrial
(Homo sapiens) | BDBM50571506

(CHEMBL4862987)Show SMILES CC(C)C[C@H](NC(=O)[C@H](NC(=O)c1cnccn1)C(C)(C)C)B(O)O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of LONP1 (unknown origin) using QXL520-YRGITCSGRQK(5-FAM)-NH2 peptide as substrate incubated for 50 mins in presence of ATP by HTRF assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02152
BindingDB Entry DOI: 10.7270/Q25Q50WZ |
More data for this
Ligand-Target Pair | |
Lon protease homolog, mitochondrial
(Homo sapiens) | BDBM50571513

(CHEMBL1530)Show SMILES CC(C)C[C@@H](NC(=O)[C@@H](Cc1ccccc1)NC(=O)c1cnccn1)B(O)O | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of LONP1 (unknown origin) using QXL520-YRGITCSGRQK(5-FAM)-NH2 peptide as substrate incubated for 50 mins in presence of ATP by HTRF assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02152
BindingDB Entry DOI: 10.7270/Q25Q50WZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50196095

(CHEMBL3972316)Show SMILES Cc1cccc2nc(NC(=O)c3cccc(c3)C(F)(F)F)n([C@@H]3CCCCN(C3)C(=O)C=C)c12 |r| Show InChI InChI=1S/C25H25F3N4O2/c1-3-21(33)31-13-5-4-11-19(15-31)32-22-16(2)8-6-12-20(22)29-24(32)30-23(34)17-9-7-10-18(14-17)25(26,27)28/h3,6-10,12,14,19H,1,4-5,11,13,15H2,2H3,(H,29,30,34)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
J Med Chem 59: 6671-89 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01985
BindingDB Entry DOI: 10.7270/Q2RF5X00 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50196095

(CHEMBL3972316)Show SMILES Cc1cccc2nc(NC(=O)c3cccc(c3)C(F)(F)F)n([C@@H]3CCCCN(C3)C(=O)C=C)c12 |r| Show InChI InChI=1S/C25H25F3N4O2/c1-3-21(33)31-13-5-4-11-19(15-31)32-22-16(2)8-6-12-20(22)29-24(32)30-23(34)17-9-7-10-18(14-17)25(26,27)28/h3,6-10,12,14,19H,1,4-5,11,13,15H2,2H3,(H,29,30,34)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 59: 6671-89 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01985
BindingDB Entry DOI: 10.7270/Q2RF5X00 |
More data for this
Ligand-Target Pair | |
A5LHX3/O14818/P20618/P25786/P25787/P25788/P25789/P28062/P28065/P28066/P28070/P28072/P28074/P40306/P49720/P49721/P60900/Q8TAA3/Q99436
(Homo sapiens (Human)) | BDBM50571507

(CHEMBL4859172)Show SMILES CC(C)C[C@H](NC(=O)[C@H](NC(=O)c1cnccn1)C(C)C)B(O)O |r| | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocytes 20S proteasome using QXL520-YRGITCSGRQK(5-FAM)-NH2 fluorogenic peptide as substrate preincubated for 10 mins followe... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02152
BindingDB Entry DOI: 10.7270/Q25Q50WZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50196101
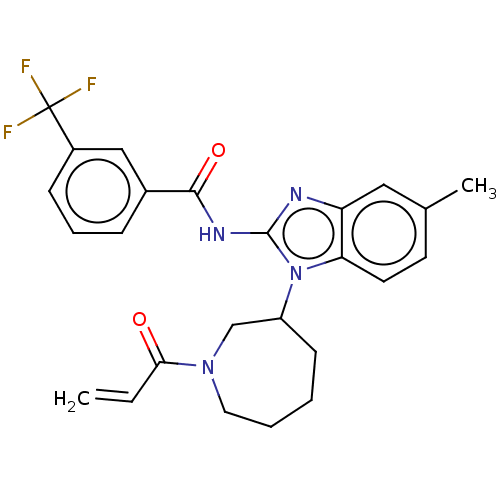
(CHEMBL3901943)Show SMILES Cc1ccc2n(C3CCCCN(C3)C(=O)C=C)c(NC(=O)c3cccc(c3)C(F)(F)F)nc2c1 Show InChI InChI=1S/C25H25F3N4O2/c1-3-22(33)31-12-5-4-9-19(15-31)32-21-11-10-16(2)13-20(21)29-24(32)30-23(34)17-7-6-8-18(14-17)25(26,27)28/h3,6-8,10-11,13-14,19H,1,4-5,9,12,15H2,2H3,(H,29,30,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA method |
J Med Chem 59: 6671-89 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01985
BindingDB Entry DOI: 10.7270/Q2RF5X00 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50196237
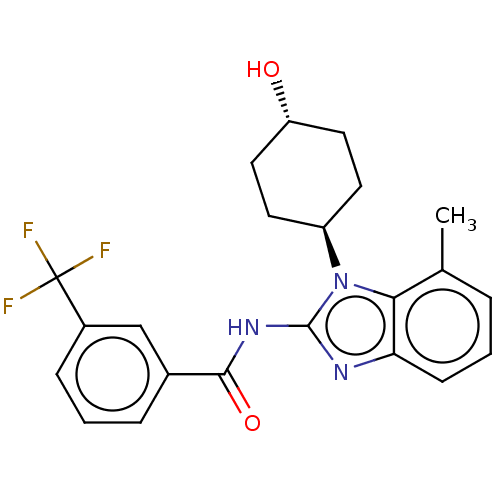
(CHEMBL3937373)Show SMILES Cc1cccc2nc(NC(=O)c3cccc(c3)C(F)(F)F)n([C@H]3CC[C@H](O)CC3)c12 |r,wU:22.22,wD:25.26,(26.54,-21.82,;26.54,-20.28,;25.2,-19.51,;25.2,-17.97,;26.53,-17.2,;27.87,-17.96,;29.34,-17.48,;30.26,-18.73,;31.8,-18.73,;32.57,-17.4,;31.79,-16.07,;34.1,-17.4,;34.87,-16.07,;36.4,-16.06,;37.18,-17.4,;36.41,-18.74,;34.87,-18.74,;37.18,-20.07,;36.41,-21.4,;38.72,-20.07,;37.94,-21.4,;29.34,-19.99,;29.83,-21.46,;31.33,-21.77,;31.8,-23.24,;30.77,-24.39,;31.24,-25.85,;29.26,-24.06,;28.79,-22.6,;27.87,-19.51,)| Show InChI InChI=1S/C22H22F3N3O2/c1-13-4-2-7-18-19(13)28(16-8-10-17(29)11-9-16)21(26-18)27-20(30)14-5-3-6-15(12-14)22(23,24)25/h2-7,12,16-17,29H,8-11H2,1H3,(H,26,27,30)/t16-,17- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6x-HIS-tagged wild type human recombinant EGFR (696 to 1022 residues) expressed in Sf9 cells pre-incubated for 90 mins follo... |
J Med Chem 59: 6671-89 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01985
BindingDB Entry DOI: 10.7270/Q2RF5X00 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50196093

(CHEMBL3939913)Show SMILES CN(C)C\C=C\C(=O)N1CCCC[C@H](C1)n1c(NC(=O)c2ccnc(c2)C(F)(F)F)nc2cccc(C)c12 |r| Show InChI InChI=1S/C27H31F3N6O2/c1-18-8-6-10-21-24(18)36(20-9-4-5-15-35(17-20)23(37)11-7-14-34(2)3)26(32-21)33-25(38)19-12-13-31-22(16-19)27(28,29)30/h6-8,10-13,16,20H,4-5,9,14-15,17H2,1-3H3,(H,32,33,38)/b11-7+/t20-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
J Med Chem 59: 6671-89 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01985
BindingDB Entry DOI: 10.7270/Q2RF5X00 |
More data for this
Ligand-Target Pair | |
Lon protease homolog, mitochondrial
(Homo sapiens) | BDBM50571504
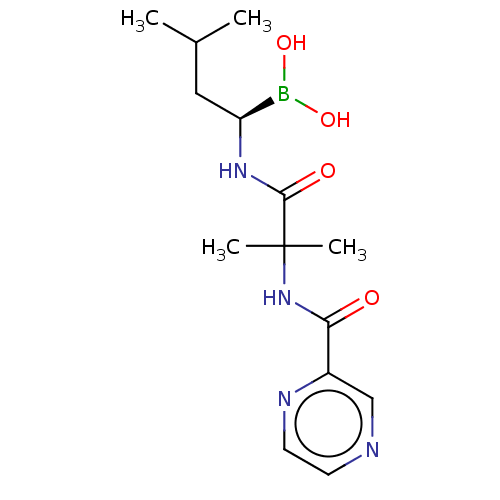
(CHEMBL4854436)Show SMILES CC(C)C[C@H](NC(=O)C(C)(C)NC(=O)c1cnccn1)B(O)O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of LONP1 (unknown origin) using QXL520-YRGITCSGRQK(5-FAM)-NH2 peptide as substrate incubated for 50 mins in presence of ATP by HTRF assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02152
BindingDB Entry DOI: 10.7270/Q25Q50WZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50160870

(CHEMBL3787344 | WO2022090481, Example nazartinib)Show SMILES CN(C)C\C=C\C(=O)N1CCCC[C@H](C1)n1c(NC(=O)c2ccnc(C)c2)nc2cccc(Cl)c12 |r| Show InChI InChI=1S/C26H31ClN6O2/c1-18-16-19(12-13-28-18)25(35)30-26-29-22-10-6-9-21(27)24(22)33(26)20-8-4-5-15-32(17-20)23(34)11-7-14-31(2)3/h6-7,9-13,16,20H,4-5,8,14-15,17H2,1-3H3,(H,29,30,35)/b11-7+/t20-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
J Med Chem 59: 6671-89 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01985
BindingDB Entry DOI: 10.7270/Q2RF5X00 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50196097
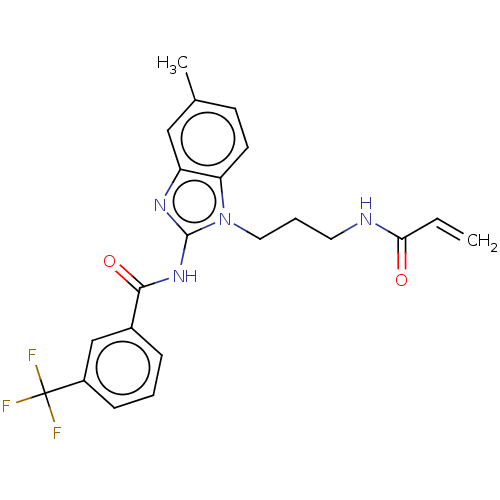
(CHEMBL3966111)Show SMILES Cc1ccc2n(CCCNC(=O)C=C)c(NC(=O)c3cccc(c3)C(F)(F)F)nc2c1 Show InChI InChI=1S/C22H21F3N4O2/c1-3-19(30)26-10-5-11-29-18-9-8-14(2)12-17(18)27-21(29)28-20(31)15-6-4-7-16(13-15)22(23,24)25/h3-4,6-9,12-13H,1,5,10-11H2,2H3,(H,26,30)(H,27,28,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA method |
J Med Chem 59: 6671-89 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01985
BindingDB Entry DOI: 10.7270/Q2RF5X00 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data