 Found 224 hits with Last Name = 'lemaire' and Initial = 's'
Found 224 hits with Last Name = 'lemaire' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 4
(RAT) | BDBM85027

(1-(7-Chloro-8-amino-1,4-benzodioxane-5-yl)-3-[1-[3...)Show SMILES COc1ccc(CCCN2CCC(CCC(=O)c3cc(Cl)c(N)c4OCCOc34)CC2)cc1OC Show InChI InChI=1S/C27H35ClN2O5/c1-32-23-8-6-19(16-24(23)33-2)4-3-11-30-12-9-18(10-13-30)5-7-22(31)20-17-21(28)25(29)27-26(20)34-14-15-35-27/h6,8,16-18H,3-5,7,9-15,29H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.000400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM85027

(1-(7-Chloro-8-amino-1,4-benzodioxane-5-yl)-3-[1-[3...)Show SMILES COc1ccc(CCCN2CCC(CCC(=O)c3cc(Cl)c(N)c4OCCOc34)CC2)cc1OC Show InChI InChI=1S/C27H35ClN2O5/c1-32-23-8-6-19(16-24(23)33-2)4-3-11-30-12-9-18(10-13-30)5-7-22(31)20-17-21(28)25(29)27-26(20)34-14-15-35-27/h6,8,16-18H,3-5,7,9-15,29H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.000480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM85027

(1-(7-Chloro-8-amino-1,4-benzodioxane-5-yl)-3-[1-[3...)Show SMILES COc1ccc(CCCN2CCC(CCC(=O)c3cc(Cl)c(N)c4OCCOc34)CC2)cc1OC Show InChI InChI=1S/C27H35ClN2O5/c1-32-23-8-6-19(16-24(23)33-2)4-3-11-30-12-9-18(10-13-30)5-7-22(31)20-17-21(28)25(29)27-26(20)34-14-15-35-27/h6,8,16-18H,3-5,7,9-15,29H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.000580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM85027

(1-(7-Chloro-8-amino-1,4-benzodioxane-5-yl)-3-[1-[3...)Show SMILES COc1ccc(CCCN2CCC(CCC(=O)c3cc(Cl)c(N)c4OCCOc34)CC2)cc1OC Show InChI InChI=1S/C27H35ClN2O5/c1-32-23-8-6-19(16-24(23)33-2)4-3-11-30-12-9-18(10-13-30)5-7-22(31)20-17-21(28)25(29)27-26(20)34-14-15-35-27/h6,8,16-18H,3-5,7,9-15,29H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.000630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM85020
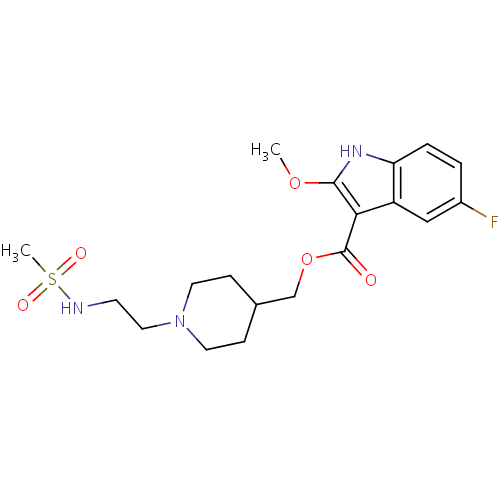
(5-Fluoro-2-methoxy-1H-indole-3-carboxylic acid [1-...)Show SMILES COc1[nH]c2ccc(F)cc2c1C(=O)OCC1CCN(CCN[S](C)(=O)=O)CC1 Show InChI InChI=1S/C19H26FN3O5S/c1-27-18-17(15-11-14(20)3-4-16(15)22-18)19(24)28-12-13-5-8-23(9-6-13)10-7-21-29(2,25)26/h3-4,11,13,21-22H,5-10,12H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.000870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM85020

(5-Fluoro-2-methoxy-1H-indole-3-carboxylic acid [1-...)Show SMILES COc1[nH]c2ccc(F)cc2c1C(=O)OCC1CCN(CCN[S](C)(=O)=O)CC1 Show InChI InChI=1S/C19H26FN3O5S/c1-27-18-17(15-11-14(20)3-4-16(15)22-18)19(24)28-12-13-5-8-23(9-6-13)10-7-21-29(2,25)26/h3-4,11,13,21-22H,5-10,12H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.000910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM85026
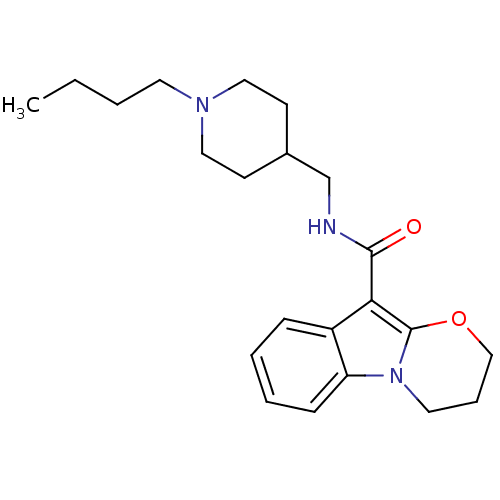
(N-(1-Butylpiperidine-4-ylmethyl)-1,2-(trimethylene...)Show InChI InChI=1S/C22H31N3O2/c1-2-3-11-24-13-9-17(10-14-24)16-23-21(26)20-18-7-4-5-8-19(18)25-12-6-15-27-22(20)25/h4-5,7-8,17H,2-3,6,9-16H2,1H3,(H,23,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.00560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM85020

(5-Fluoro-2-methoxy-1H-indole-3-carboxylic acid [1-...)Show SMILES COc1[nH]c2ccc(F)cc2c1C(=O)OCC1CCN(CCN[S](C)(=O)=O)CC1 Show InChI InChI=1S/C19H26FN3O5S/c1-27-18-17(15-11-14(20)3-4-16(15)22-18)19(24)28-12-13-5-8-23(9-6-13)10-7-21-29(2,25)26/h3-4,11,13,21-22H,5-10,12H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.00890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM85026

(N-(1-Butylpiperidine-4-ylmethyl)-1,2-(trimethylene...)Show InChI InChI=1S/C22H31N3O2/c1-2-3-11-24-13-9-17(10-14-24)16-23-21(26)20-18-7-4-5-8-19(18)25-12-6-15-27-22(20)25/h4-5,7-8,17H,2-3,6,9-16H2,1H3,(H,23,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM85020

(5-Fluoro-2-methoxy-1H-indole-3-carboxylic acid [1-...)Show SMILES COc1[nH]c2ccc(F)cc2c1C(=O)OCC1CCN(CCN[S](C)(=O)=O)CC1 Show InChI InChI=1S/C19H26FN3O5S/c1-27-18-17(15-11-14(20)3-4-16(15)22-18)19(24)28-12-13-5-8-23(9-6-13)10-7-21-29(2,25)26/h3-4,11,13,21-22H,5-10,12H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM29525

(3H-GR113808 | CHEMBL518682 | GR 113808 | [3H] GR 1...)Show SMILES Cn1cc(C(=O)OCC2CCN(CCNS(C)(=O)=O)CC2)c2ccccc12 Show InChI InChI=1S/C19H27N3O4S/c1-21-13-17(16-5-3-4-6-18(16)21)19(23)26-14-15-7-10-22(11-8-15)12-9-20-27(2,24)25/h3-6,13,15,20H,7-12,14H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM29525

(3H-GR113808 | CHEMBL518682 | GR 113808 | [3H] GR 1...)Show SMILES Cn1cc(C(=O)OCC2CCN(CCNS(C)(=O)=O)CC2)c2ccccc12 Show InChI InChI=1S/C19H27N3O4S/c1-21-13-17(16-5-3-4-6-18(16)21)19(23)26-14-15-7-10-22(11-8-15)12-9-20-27(2,24)25/h3-6,13,15,20H,7-12,14H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50155838
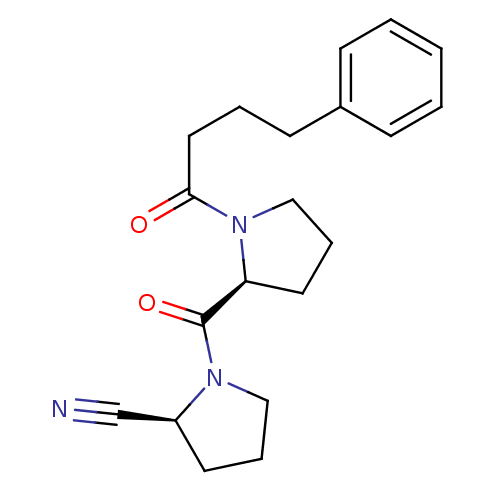
((S)-1-((S)-1-(4-phenylbutanoyl)pyrrolidine-2-carbo...)Show SMILES O=C(CCCc1ccccc1)N1CCC[C@H]1C(=O)N1CCC[C@H]1C#N Show InChI InChI=1S/C20H25N3O2/c21-15-17-10-5-13-22(17)20(25)18-11-6-14-23(18)19(24)12-4-9-16-7-2-1-3-8-16/h1-3,7-8,17-18H,4-6,9-14H2/t17-,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of pig POP |
J Med Chem 53: 3423-38 (2010)
Article DOI: 10.1021/jm901104g
BindingDB Entry DOI: 10.7270/Q261119N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 4
(RAT) | BDBM29525

(3H-GR113808 | CHEMBL518682 | GR 113808 | [3H] GR 1...)Show SMILES Cn1cc(C(=O)OCC2CCN(CCNS(C)(=O)=O)CC2)c2ccccc12 Show InChI InChI=1S/C19H27N3O4S/c1-21-13-17(16-5-3-4-6-18(16)21)19(23)26-14-15-7-10-22(11-8-15)12-9-20-27(2,24)25/h3-6,13,15,20H,7-12,14H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM29525

(3H-GR113808 | CHEMBL518682 | GR 113808 | [3H] GR 1...)Show SMILES Cn1cc(C(=O)OCC2CCN(CCNS(C)(=O)=O)CC2)c2ccccc12 Show InChI InChI=1S/C19H27N3O4S/c1-21-13-17(16-5-3-4-6-18(16)21)19(23)26-14-15-7-10-22(11-8-15)12-9-20-27(2,24)25/h3-6,13,15,20H,7-12,14H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Bos taurus) | BDBM50038879
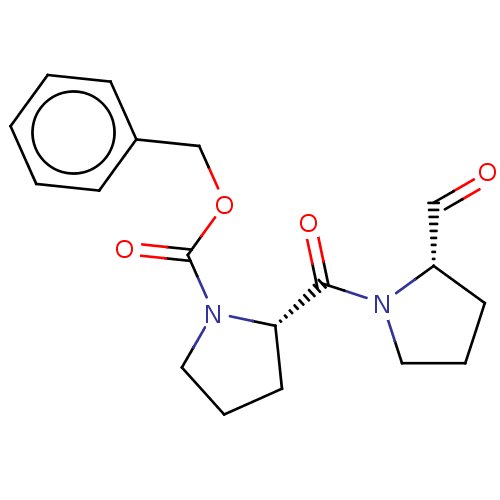
((S)-2-(2-Formyl-pyrrolidine-1-carbonyl)-pyrrolidin...)Show SMILES O=C[C@@H]1CCCN1C(=O)[C@@H]1CCCN1C(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C18H22N2O4/c21-12-15-8-4-10-19(15)17(22)16-9-5-11-20(16)18(23)24-13-14-6-2-1-3-7-14/h1-3,6-7,12,15-16H,4-5,8-11,13H2/t15-,16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain POP |
J Med Chem 53: 3423-38 (2010)
Article DOI: 10.1021/jm901104g
BindingDB Entry DOI: 10.7270/Q261119N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM85026

(N-(1-Butylpiperidine-4-ylmethyl)-1,2-(trimethylene...)Show InChI InChI=1S/C22H31N3O2/c1-2-3-11-24-13-9-17(10-14-24)16-23-21(26)20-18-7-4-5-8-19(18)25-12-6-15-27-22(20)25/h4-5,7-8,17H,2-3,6,9-16H2,1H3,(H,23,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Mus musculus) | BDBM50038879

((S)-2-(2-Formyl-pyrrolidine-1-carbonyl)-pyrrolidin...)Show SMILES O=C[C@@H]1CCCN1C(=O)[C@@H]1CCCN1C(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C18H22N2O4/c21-12-15-8-4-10-19(15)17(22)16-9-5-11-20(16)18(23)24-13-14-6-2-1-3-7-14/h1-3,6-7,12,15-16H,4-5,8-11,13H2/t15-,16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of mouse brain POP |
J Med Chem 53: 3423-38 (2010)
Article DOI: 10.1021/jm901104g
BindingDB Entry DOI: 10.7270/Q261119N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prolyl endopeptidase
(Sus scrofa) | BDBM50170682
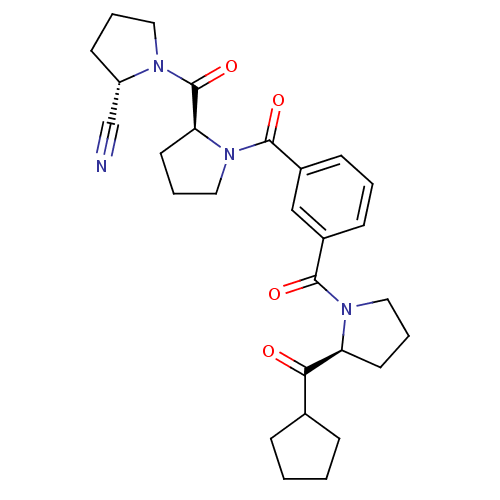
((S)-1-((S)-1-(3-((S)-2-(cyclopentanecarbonyl)pyrro...)Show SMILES O=C(C1CCCC1)[C@@H]1CCCN1C(=O)c1cccc(c1)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C#N Show InChI InChI=1S/C28H34N4O4/c29-18-22-11-4-14-30(22)28(36)24-13-6-16-32(24)27(35)21-10-3-9-20(17-21)26(34)31-15-5-12-23(31)25(33)19-7-1-2-8-19/h3,9-10,17,19,22-24H,1-2,4-8,11-16H2/t22-,23-,24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of pig brain POP |
J Med Chem 53: 3423-38 (2010)
Article DOI: 10.1021/jm901104g
BindingDB Entry DOI: 10.7270/Q261119N |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM85026

(N-(1-Butylpiperidine-4-ylmethyl)-1,2-(trimethylene...)Show InChI InChI=1S/C22H31N3O2/c1-2-3-11-24-13-9-17(10-14-24)16-23-21(26)20-18-7-4-5-8-19(18)25-12-6-15-27-22(20)25/h4-5,7-8,17H,2-3,6,9-16H2,1H3,(H,23,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Flavobacterium meningosepticum) | BDBM50038879

((S)-2-(2-Formyl-pyrrolidine-1-carbonyl)-pyrrolidin...)Show SMILES O=C[C@@H]1CCCN1C(=O)[C@@H]1CCCN1C(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C18H22N2O4/c21-12-15-8-4-10-19(15)17(22)16-9-5-11-20(16)18(23)24-13-14-6-2-1-3-7-14/h1-3,6-7,12,15-16H,4-5,8-11,13H2/t15-,16-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of Flavobacterium meningosepticum POP |
J Med Chem 53: 3423-38 (2010)
Article DOI: 10.1021/jm901104g
BindingDB Entry DOI: 10.7270/Q261119N |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Rattus norvegicus) | BDBM50038881

(CHEMBL294803 | Y-29794 | [2-(8-Dimethylamino-octyl...)Show SMILES CC(C)c1ccc(C(=O)c2cccs2)c(SCCCCCCCCN(C)C)n1 Show InChI InChI=1S/C23H34N2OS2/c1-18(2)20-14-13-19(22(26)21-12-11-17-27-21)23(24-20)28-16-10-8-6-5-7-9-15-25(3)4/h11-14,17-18H,5-10,15-16H2,1-4H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of POP in rat brain homogenate |
J Med Chem 53: 3423-38 (2010)
Article DOI: 10.1021/jm901104g
BindingDB Entry DOI: 10.7270/Q261119N |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Rattus norvegicus) | BDBM50316818
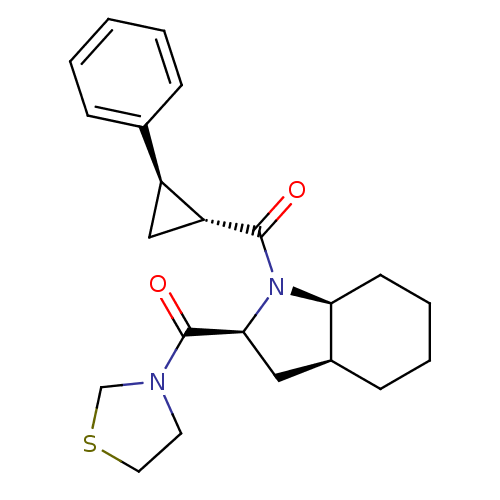
(((2S,3aS,7aS)-1-((1R,2R)-2-phenylcyclopropanecarbo...)Show SMILES O=C([C@@H]1C[C@H]1c1ccccc1)N1[C@H]2CCCC[C@H]2C[C@H]1C(=O)N1CCSC1 |r| Show InChI InChI=1S/C22H28N2O2S/c25-21(18-13-17(18)15-6-2-1-3-7-15)24-19-9-5-4-8-16(19)12-20(24)22(26)23-10-11-27-14-23/h1-3,6-7,16-20H,4-5,8-14H2/t16-,17-,18+,19-,20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
inhibition of rat cortex POP |
J Med Chem 53: 3423-38 (2010)
Article DOI: 10.1021/jm901104g
BindingDB Entry DOI: 10.7270/Q261119N |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Homo sapiens (Human)) | BDBM50316818

(((2S,3aS,7aS)-1-((1R,2R)-2-phenylcyclopropanecarbo...)Show SMILES O=C([C@@H]1C[C@H]1c1ccccc1)N1[C@H]2CCCC[C@H]2C[C@H]1C(=O)N1CCSC1 |r| Show InChI InChI=1S/C22H28N2O2S/c25-21(18-13-17(18)15-6-2-1-3-7-15)24-19-9-5-4-8-16(19)12-20(24)22(26)23-10-11-27-14-23/h1-3,6-7,16-20H,4-5,8-14H2/t16-,17-,18+,19-,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human POP |
J Med Chem 53: 3423-38 (2010)
Article DOI: 10.1021/jm901104g
BindingDB Entry DOI: 10.7270/Q261119N |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Homo sapiens (Human)) | BDBM50200729
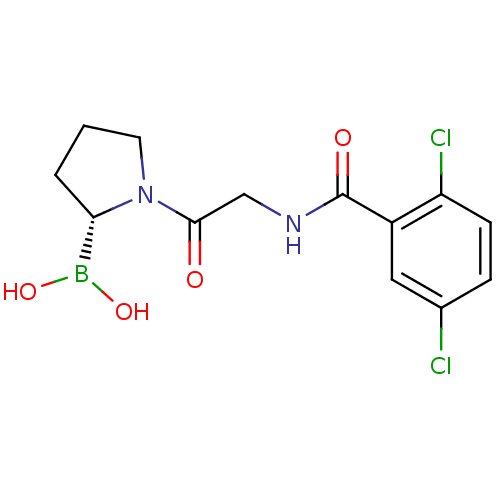
((R)-1-(2-(2,5-dichlorobenzamido)acetyl)pyrrolidin-...)Show SMILES OB(O)[C@@H]1CCCN1C(=O)CNC(=O)c1cc(Cl)ccc1Cl Show InChI InChI=1S/C13H15BCl2N2O4/c15-8-3-4-10(16)9(6-8)13(20)17-7-12(19)18-5-1-2-11(18)14(21)22/h3-4,6,11,21-22H,1-2,5,7H2,(H,17,20)/t11-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant POP |
J Med Chem 53: 3423-38 (2010)
Article DOI: 10.1021/jm901104g
BindingDB Entry DOI: 10.7270/Q261119N |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Mus musculus) | BDBM50316840
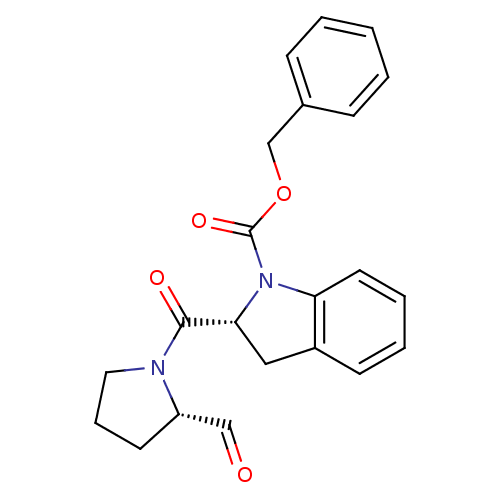
((R)-benzyl 2-((S)-2-formylpyrrolidine-1-carbonyl)i...)Show SMILES O=C[C@@H]1CCCN1C(=O)[C@H]1Cc2ccccc2N1C(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C22H22N2O4/c25-14-18-10-6-12-23(18)21(26)20-13-17-9-4-5-11-19(17)24(20)22(27)28-15-16-7-2-1-3-8-16/h1-5,7-9,11,14,18,20H,6,10,12-13,15H2/t18-,20+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of mouse brain POP |
J Med Chem 53: 3423-38 (2010)
Article DOI: 10.1021/jm901104g
BindingDB Entry DOI: 10.7270/Q261119N |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Mus musculus) | BDBM50279826
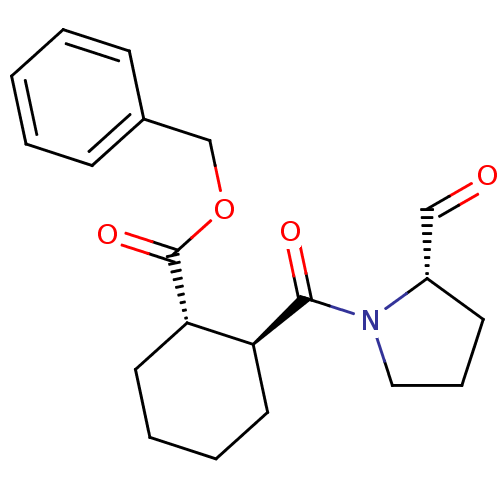
((1S,2S)-2-((S)-2-Formyl-pyrrolidine-1-carbonyl)-cy...)Show SMILES O=C[C@@H]1CCCN1C(=O)[C@H]1CCCC[C@@H]1C(=O)OCc1ccccc1 Show InChI InChI=1S/C20H25NO4/c22-13-16-9-6-12-21(16)19(23)17-10-4-5-11-18(17)20(24)25-14-15-7-2-1-3-8-15/h1-3,7-8,13,16-18H,4-6,9-12,14H2/t16-,17-,18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of mouse brain POP |
J Med Chem 53: 3423-38 (2010)
Article DOI: 10.1021/jm901104g
BindingDB Entry DOI: 10.7270/Q261119N |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Bos taurus) | BDBM50316839

((S)-benzyl 2-((S)-2-formylpyrrolidine-1-carbonyl)-...)Show SMILES O=C[C@@H]1CCCN1C(=O)[C@@H]1CCC(=O)N1C(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C18H20N2O5/c21-11-14-7-4-10-19(14)17(23)15-8-9-16(22)20(15)18(24)25-12-13-5-2-1-3-6-13/h1-3,5-6,11,14-15H,4,7-10,12H2/t14-,15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain POP |
J Med Chem 53: 3423-38 (2010)
Article DOI: 10.1021/jm901104g
BindingDB Entry DOI: 10.7270/Q261119N |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM50007863
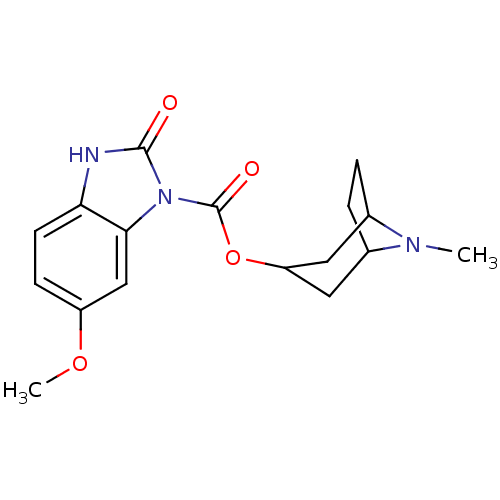
(6-Methoxy-2-oxo-2,3-dihydro-benzoimidazole-1-carbo...)Show SMILES COc1ccc2[nH]c(=O)n(C(=O)OC3CC4CCC(C3)N4C)c2c1 |TLB:12:13:20:16.17| Show InChI InChI=1S/C17H21N3O4/c1-19-10-3-4-11(19)8-13(7-10)24-17(22)20-15-9-12(23-2)5-6-14(15)18-16(20)21/h5-6,9-11,13H,3-4,7-8H2,1-2H3,(H,18,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50007863

(6-Methoxy-2-oxo-2,3-dihydro-benzoimidazole-1-carbo...)Show SMILES COc1ccc2[nH]c(=O)n(C(=O)OC3CC4CCC(C3)N4C)c2c1 |TLB:12:13:20:16.17| Show InChI InChI=1S/C17H21N3O4/c1-19-10-3-4-11(19)8-13(7-10)24-17(22)20-15-9-12(23-2)5-6-14(15)18-16(20)21/h5-6,9-11,13H,3-4,7-8H2,1-2H3,(H,18,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Rattus norvegicus) | BDBM50316819

((S)-1-(4-(4-chlorobenzylamino)-4-oxobutanoyl)pyrro...)Show SMILES OC(=O)[C@@H]1CCCN1C(=O)CCC(=O)NCc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C16H19ClN2O4/c17-12-5-3-11(4-6-12)10-18-14(20)7-8-15(21)19-9-1-2-13(19)16(22)23/h3-6,13H,1-2,7-10H2,(H,18,20)(H,22,23)/t13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
inhibition of rat cortex POP |
J Med Chem 53: 3423-38 (2010)
Article DOI: 10.1021/jm901104g
BindingDB Entry DOI: 10.7270/Q261119N |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50007863

(6-Methoxy-2-oxo-2,3-dihydro-benzoimidazole-1-carbo...)Show SMILES COc1ccc2[nH]c(=O)n(C(=O)OC3CC4CCC(C3)N4C)c2c1 |TLB:12:13:20:16.17| Show InChI InChI=1S/C17H21N3O4/c1-19-10-3-4-11(19)8-13(7-10)24-17(22)20-15-9-12(23-2)5-6-14(15)18-16(20)21/h5-6,9-11,13H,3-4,7-8H2,1-2H3,(H,18,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
alpha-1,2-Mannosidase
(Glycine max) | BDBM50168997
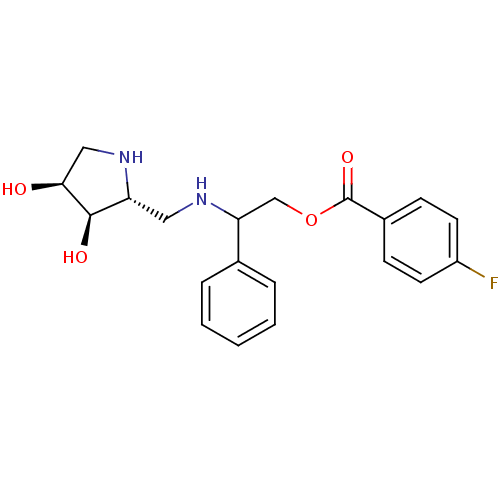
(4-Fluoro-benzoic acid 2-[((2R,3R,4S)-3,4-dihydroxy...)Show SMILES O[C@H]1CN[C@H](CNC(COC(=O)c2ccc(F)cc2)c2ccccc2)[C@H]1O Show InChI InChI=1S/C20H23FN2O4/c21-15-8-6-14(7-9-15)20(26)27-12-17(13-4-2-1-3-5-13)22-10-16-19(25)18(24)11-23-16/h1-9,16-19,22-25H,10-12H2/t16-,17?,18+,19-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Sciences and Engineering
Curated by ChEMBL
| Assay Description
Binding affinity against alpha-Mannosidase isolated from Jack bean |
J Med Chem 48: 4237-46 (2005)
Article DOI: 10.1021/jm0409019
BindingDB Entry DOI: 10.7270/Q24J0DNV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM50056404
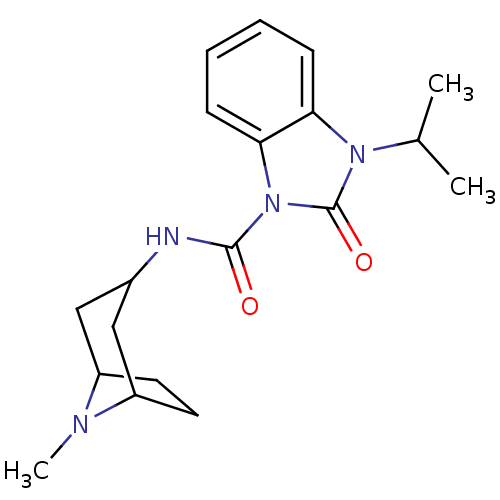
(3-Isopropyl-2-oxo-2,3-dihydro-benzoimidazole-1-car...)Show SMILES CC(C)n1c2ccccc2n(C(=O)NC2CC3CCC(C2)N3C)c1=O |TLB:13:14:21:17.18| Show InChI InChI=1S/C19H26N4O2/c1-12(2)22-16-6-4-5-7-17(16)23(19(22)25)18(24)20-13-10-14-8-9-15(11-13)21(14)3/h4-7,12-15H,8-11H2,1-3H3,(H,20,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 19.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM50007863

(6-Methoxy-2-oxo-2,3-dihydro-benzoimidazole-1-carbo...)Show SMILES COc1ccc2[nH]c(=O)n(C(=O)OC3CC4CCC(C3)N4C)c2c1 |TLB:12:13:20:16.17| Show InChI InChI=1S/C17H21N3O4/c1-19-10-3-4-11(19)8-13(7-10)24-17(22)20-15-9-12(23-2)5-6-14(15)18-16(20)21/h5-6,9-11,13H,3-4,7-8H2,1-2H3,(H,18,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase FAP
(Homo sapiens (Human)) | BDBM50200730
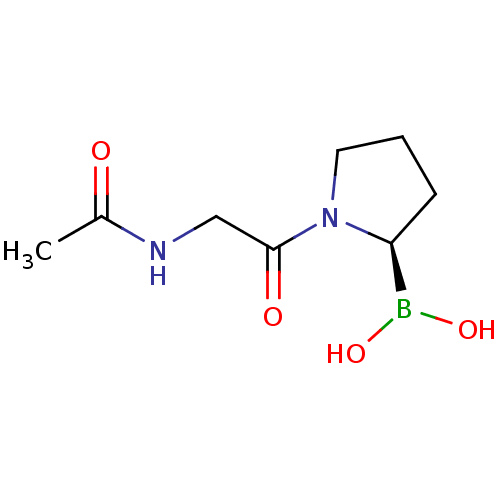
((R)-1-(2-acetamidoacetyl)pyrrolidin-2-ylboronic ac...)Show InChI InChI=1S/C8H15BN2O4/c1-6(12)10-5-8(13)11-4-2-3-7(11)9(14)15/h7,14-15H,2-5H2,1H3,(H,10,12)/t7-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FAPalpha |
J Med Chem 53: 3423-38 (2010)
Article DOI: 10.1021/jm901104g
BindingDB Entry DOI: 10.7270/Q261119N |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase FAP
(Homo sapiens (Human)) | BDBM50200729

((R)-1-(2-(2,5-dichlorobenzamido)acetyl)pyrrolidin-...)Show SMILES OB(O)[C@@H]1CCCN1C(=O)CNC(=O)c1cc(Cl)ccc1Cl Show InChI InChI=1S/C13H15BCl2N2O4/c15-8-3-4-10(16)9(6-8)13(20)17-7-12(19)18-5-1-2-11(18)14(21)22/h3-4,6,11,21-22H,1-2,5,7H2,(H,17,20)/t11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FAPalpha |
J Med Chem 53: 3423-38 (2010)
Article DOI: 10.1021/jm901104g
BindingDB Entry DOI: 10.7270/Q261119N |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Mus musculus) | BDBM50316836
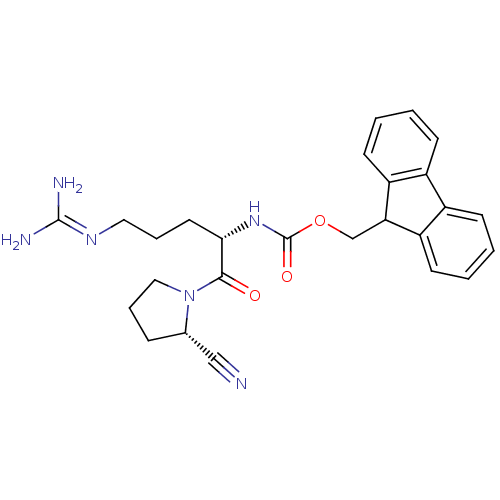
((9H-fluoren-9-yl)methyl(S)-1-((S)-2-cyanopyrrolidi...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#8]-[#6]-[#6]-1-c2ccccc2-c2ccccc-12)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1C#N |r| Show InChI InChI=1S/C26H30N6O3/c27-15-17-7-6-14-32(17)24(33)23(12-5-13-30-25(28)29)31-26(34)35-16-22-20-10-3-1-8-18(20)19-9-2-4-11-21(19)22/h1-4,8-11,17,22-23H,5-7,12-14,16H2,(H,31,34)(H4,28,29,30)/t17-,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of mouse brain POP |
J Med Chem 53: 3423-38 (2010)
Article DOI: 10.1021/jm901104g
BindingDB Entry DOI: 10.7270/Q261119N |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM10755

(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 38.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prolyl endopeptidase
(Bos taurus) | BDBM50316835

((S)-benzyl 2-(thiazolidine-3-carbonyl)pyrrolidine-...)Show InChI InChI=1S/C16H20N2O3S/c19-15(17-9-10-22-12-17)14-7-4-8-18(14)16(20)21-11-13-5-2-1-3-6-13/h1-3,5-6,14H,4,7-12H2/t14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain POP |
J Med Chem 53: 3423-38 (2010)
Article DOI: 10.1021/jm901104g
BindingDB Entry DOI: 10.7270/Q261119N |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50056404

(3-Isopropyl-2-oxo-2,3-dihydro-benzoimidazole-1-car...)Show SMILES CC(C)n1c2ccccc2n(C(=O)NC2CC3CCC(C2)N3C)c1=O |TLB:13:14:21:17.18| Show InChI InChI=1S/C19H26N4O2/c1-12(2)22-16-6-4-5-7-17(16)23(19(22)25)18(24)20-13-10-14-8-9-15(11-13)21(14)3/h4-7,12-15H,8-11H2,1-3H3,(H,20,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 45.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM10755

(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 56.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 4
(RAT) | BDBM82422
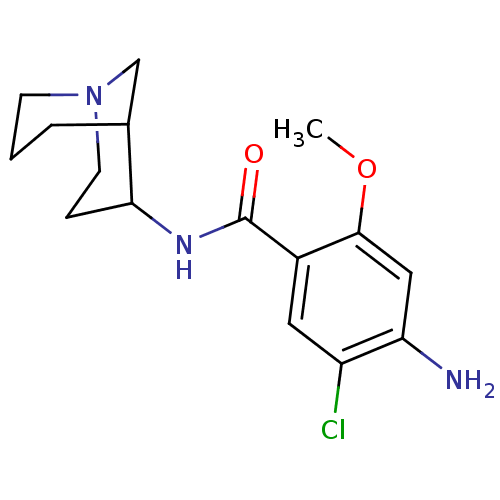
(CAS_109872-41-5 | NSC_3035240 | RENZAPRIDE)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NC1CCN2CCCC1C2 |TLB:12:13:17.18.19:21| Show InChI InChI=1S/C16H22ClN3O2/c1-22-15-8-13(18)12(17)7-11(15)16(21)19-14-4-6-20-5-2-3-10(14)9-20/h7-8,10,14H,2-6,9,18H2,1H3,(H,19,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM82087
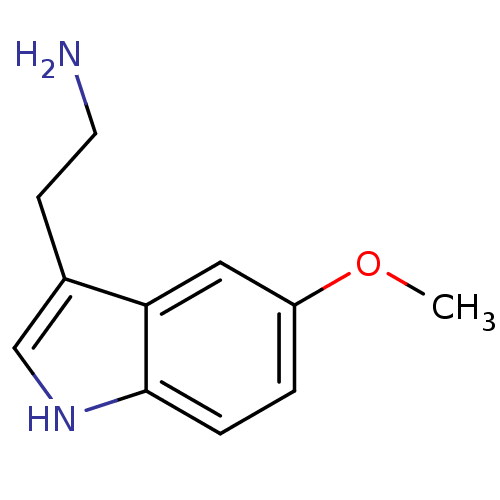
(2-(5-methoxy-1H-indol-3-yl)ethanamine | 5-MT | 5-M...)Show InChI InChI=1S/C11H14N2O/c1-14-9-2-3-11-10(6-9)8(4-5-12)7-13-11/h2-3,6-7,13H,4-5,12H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
alpha-1,2-Mannosidase
(Glycine max) | BDBM50168988
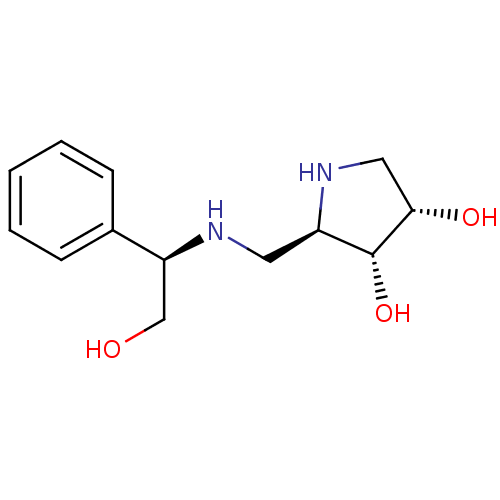
((2R,3R,4S)-2-({[(1R)-2-HYDROXY-1-PHENYLETHYL]AMINO...)Show SMILES OC[C@H](NC[C@H]1NC[C@H](O)[C@@H]1O)c1ccccc1 |r| Show InChI InChI=1S/C13H20N2O3/c16-8-11(9-4-2-1-3-5-9)14-6-10-13(18)12(17)7-15-10/h1-5,10-18H,6-8H2/t10-,11+,12+,13-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 135 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Sciences and Engineering
Curated by ChEMBL
| Assay Description
Binding affinity towards alpha-Mannosidase isolated from Jack bean |
J Med Chem 48: 4237-46 (2005)
Article DOI: 10.1021/jm0409019
BindingDB Entry DOI: 10.7270/Q24J0DNV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM82087

(2-(5-methoxy-1H-indol-3-yl)ethanamine | 5-MT | 5-M...)Show InChI InChI=1S/C11H14N2O/c1-14-9-2-3-11-10(6-9)8(4-5-12)7-13-11/h2-3,6-7,13H,4-5,12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM82422

(CAS_109872-41-5 | NSC_3035240 | RENZAPRIDE)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NC1CCN2CCCC1C2 |TLB:12:13:17.18.19:21| Show InChI InChI=1S/C16H22ClN3O2/c1-22-15-8-13(18)12(17)7-11(15)16(21)19-14-4-6-20-5-2-3-10(14)9-20/h7-8,10,14H,2-6,9,18H2,1H3,(H,19,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 174 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM50000492

((zacopride)4-Amino-N-(1-aza-bicyclo[2.2.2]oct-3-yl...)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NC1CN2CCC1CC2 |(27.19,-33.96,;28.52,-34.73,;28.53,-36.27,;27.2,-37.04,;27.2,-38.58,;25.86,-39.35,;28.53,-39.36,;28.53,-40.89,;29.87,-38.58,;29.86,-37.03,;31.19,-36.26,;31.19,-34.72,;32.53,-37.02,;33.86,-36.25,;35.2,-37.02,;36.52,-36.25,;36.52,-34.71,;35.19,-33.94,;33.85,-34.71,;34.61,-36.04,;35.74,-34.91,)| Show InChI InChI=1S/C15H20ClN3O2/c1-21-14-7-12(17)11(16)6-10(14)15(20)18-13-8-19-4-2-9(13)3-5-19/h6-7,9,13H,2-5,8,17H2,1H3,(H,18,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 186 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Homo sapiens (Human)) | BDBM50200730

((R)-1-(2-acetamidoacetyl)pyrrolidin-2-ylboronic ac...)Show InChI InChI=1S/C8H15BN2O4/c1-6(12)10-5-8(13)11-4-2-3-7(11)9(14)15/h7,14-15H,2-5H2,1H3,(H,10,12)/t7-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 211 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant POP |
J Med Chem 53: 3423-38 (2010)
Article DOI: 10.1021/jm901104g
BindingDB Entry DOI: 10.7270/Q261119N |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50000492

((zacopride)4-Amino-N-(1-aza-bicyclo[2.2.2]oct-3-yl...)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NC1CN2CCC1CC2 |(27.19,-33.96,;28.52,-34.73,;28.53,-36.27,;27.2,-37.04,;27.2,-38.58,;25.86,-39.35,;28.53,-39.36,;28.53,-40.89,;29.87,-38.58,;29.86,-37.03,;31.19,-36.26,;31.19,-34.72,;32.53,-37.02,;33.86,-36.25,;35.2,-37.02,;36.52,-36.25,;36.52,-34.71,;35.19,-33.94,;33.85,-34.71,;34.61,-36.04,;35.74,-34.91,)| Show InChI InChI=1S/C15H20ClN3O2/c1-21-14-7-12(17)11(16)6-10(14)15(20)18-13-8-19-4-2-9(13)3-5-19/h6-7,9,13H,2-5,8,17H2,1H3,(H,18,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 282 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data