

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50021345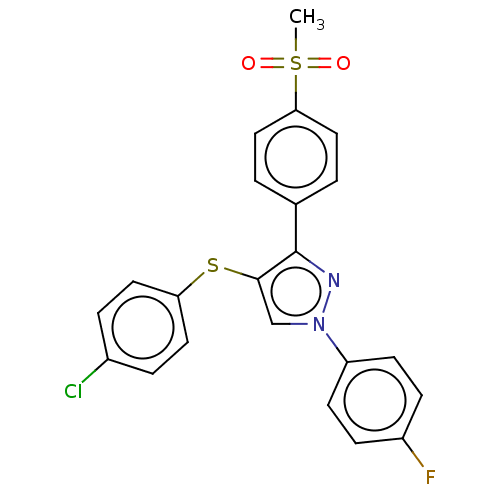 (CHEMBL3287928) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 637 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [35S]MK-499 from human ERG | ACS Med Chem Lett 5: 717-21 (2014) Article DOI: 10.1021/ml5001239 BindingDB Entry DOI: 10.7270/Q2JQ12KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50021331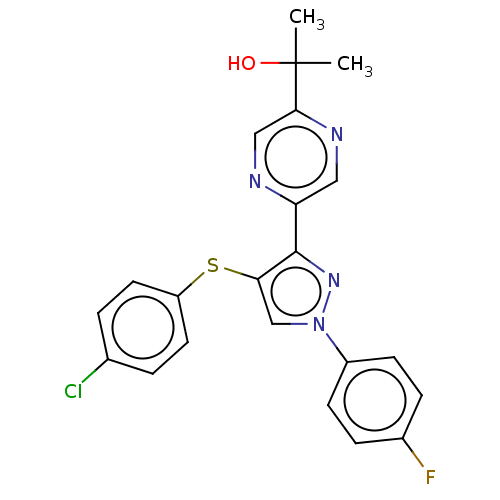 (CHEMBL3287930) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [35S]MK-499 from human ERG | ACS Med Chem Lett 5: 717-21 (2014) Article DOI: 10.1021/ml5001239 BindingDB Entry DOI: 10.7270/Q2JQ12KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50350538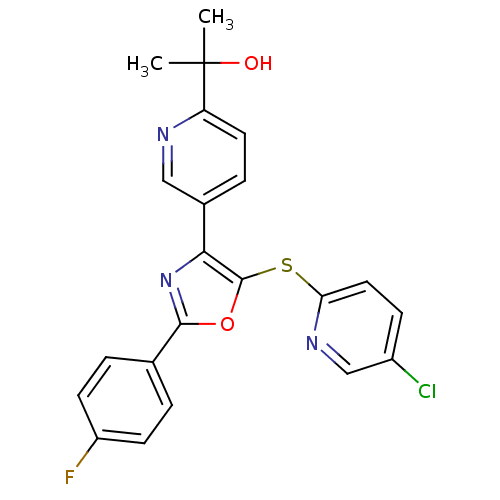 (CHEMBL1812717) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [35S]MK-499 from human ERG | ACS Med Chem Lett 5: 717-21 (2014) Article DOI: 10.1021/ml5001239 BindingDB Entry DOI: 10.7270/Q2JQ12KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50021346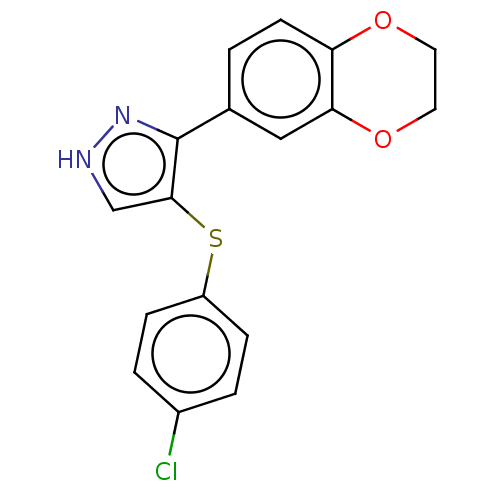 (CHEMBL3287926) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [35S]MK-499 from human ERG | ACS Med Chem Lett 5: 717-21 (2014) Article DOI: 10.1021/ml5001239 BindingDB Entry DOI: 10.7270/Q2JQ12KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50021329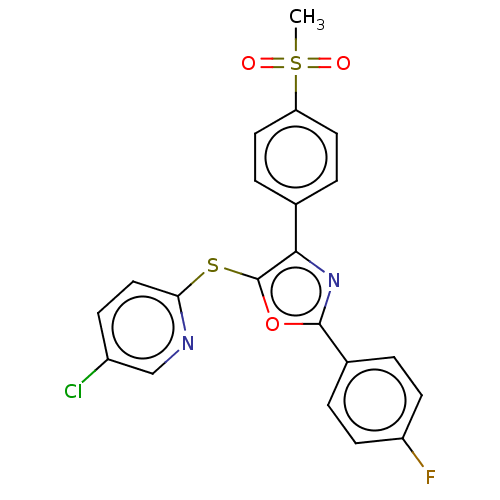 (CHEMBL3287932) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [35S]MK-499 from human ERG | ACS Med Chem Lett 5: 717-21 (2014) Article DOI: 10.1021/ml5001239 BindingDB Entry DOI: 10.7270/Q2JQ12KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50021344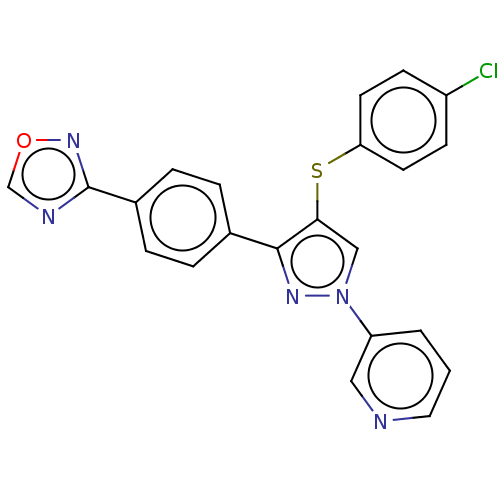 (CHEMBL3287929) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [35S]MK-499 from human ERG | ACS Med Chem Lett 5: 717-21 (2014) Article DOI: 10.1021/ml5001239 BindingDB Entry DOI: 10.7270/Q2JQ12KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50021334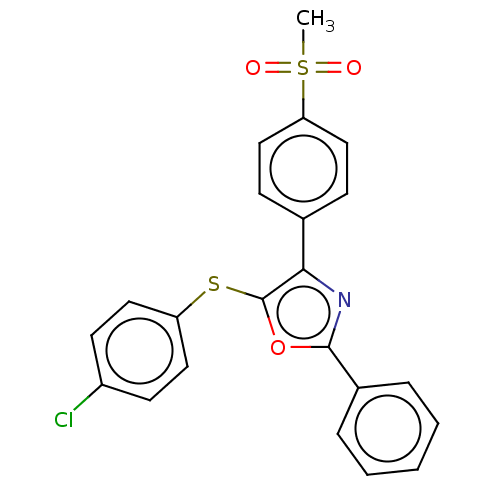 (CHEMBL3287931) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [35S]MK-499 from human ERG | ACS Med Chem Lett 5: 717-21 (2014) Article DOI: 10.1021/ml5001239 BindingDB Entry DOI: 10.7270/Q2JQ12KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50021330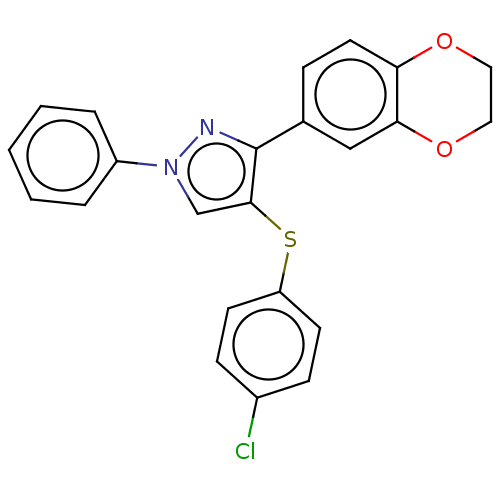 (CHEMBL3287927) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [35S]MK-499 from human ERG | ACS Med Chem Lett 5: 717-21 (2014) Article DOI: 10.1021/ml5001239 BindingDB Entry DOI: 10.7270/Q2JQ12KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50434318 (CHEMBL2386566) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50434329 (CHEMBL2386554) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin p... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50434316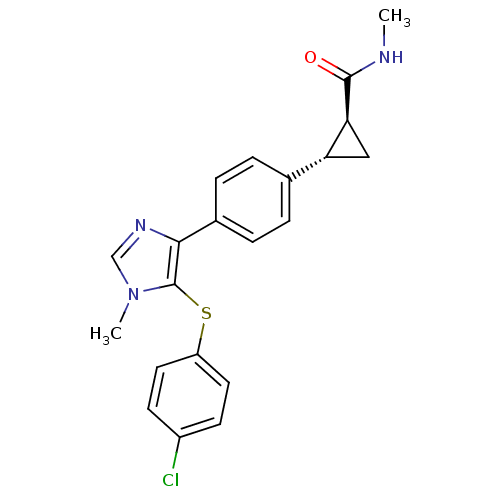 (CHEMBL2386568) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50434329 (CHEMBL2386554) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50434318 (CHEMBL2386566) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin p... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50434317 (CHEMBL2386567) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50434315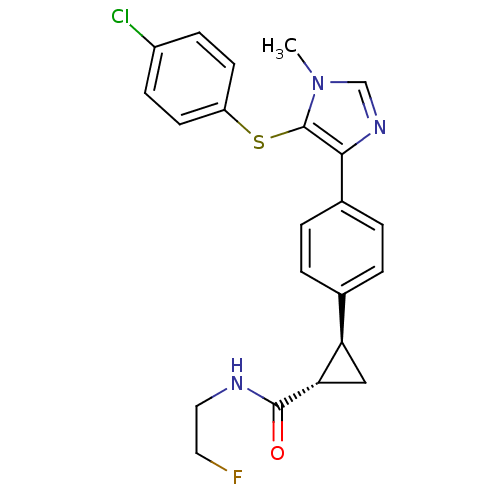 (CHEMBL2386569) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50434313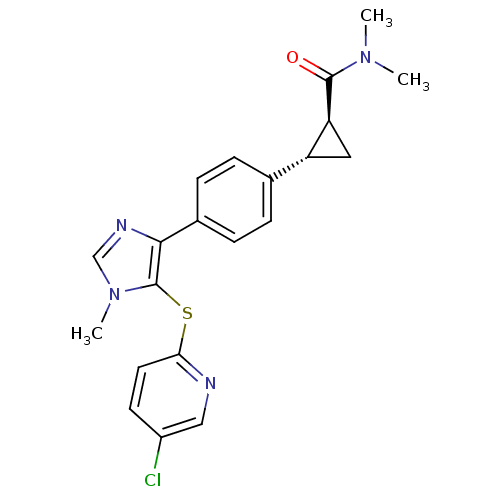 (CHEMBL2386571) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50434314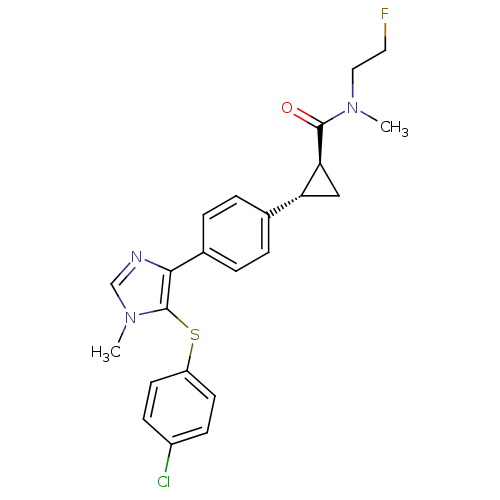 (CHEMBL2386570) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50434322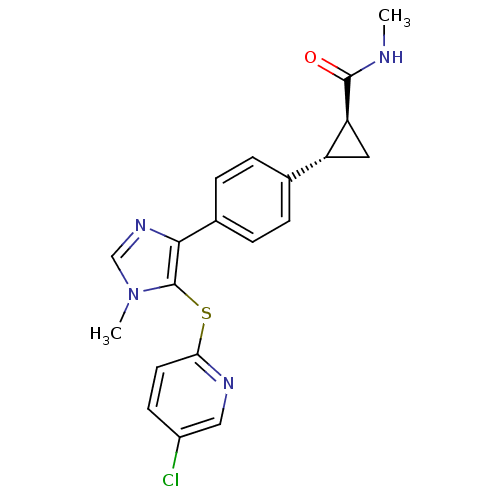 (CHEMBL2386562) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50434314 (CHEMBL2386570) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin p... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50434316 (CHEMBL2386568) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin p... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50434315 (CHEMBL2386569) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin p... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50434320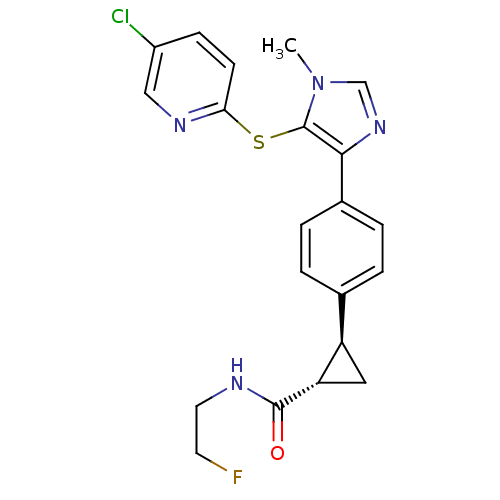 (CHEMBL2386564) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50434313 (CHEMBL2386571) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin p... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50434317 (CHEMBL2386567) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin p... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11225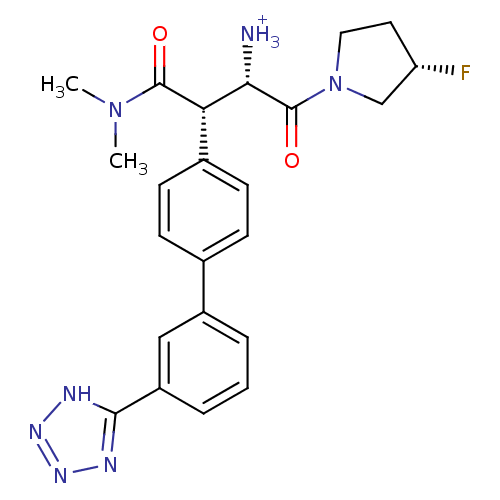 ((1S,2S)-1-(dimethylcarbamoyl)-3-[(3S)-3-fluoropyrr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories | Assay Description The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... | Bioorg Med Chem Lett 15: 3048-52 (2005) Article DOI: 10.1016/j.bmcl.2005.04.028 BindingDB Entry DOI: 10.7270/Q2833Q73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11151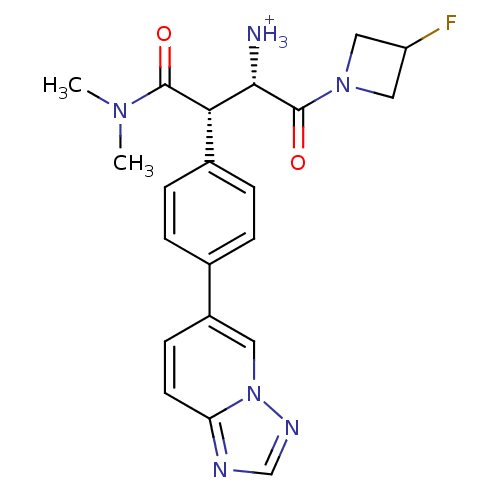 ((1S,2S)-1-(dimethylcarbamoyl)-3-(3-fluoroazetidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Merck Research Laboratories | Assay Description The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... | J Med Chem 49: 3614-27 (2006) Article DOI: 10.1021/jm060015t BindingDB Entry DOI: 10.7270/Q2N87807 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50434319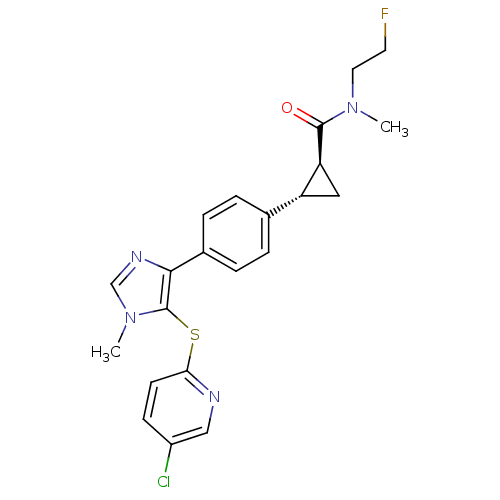 (CHEMBL2386565) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50434319 (CHEMBL2386565) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin p... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50434321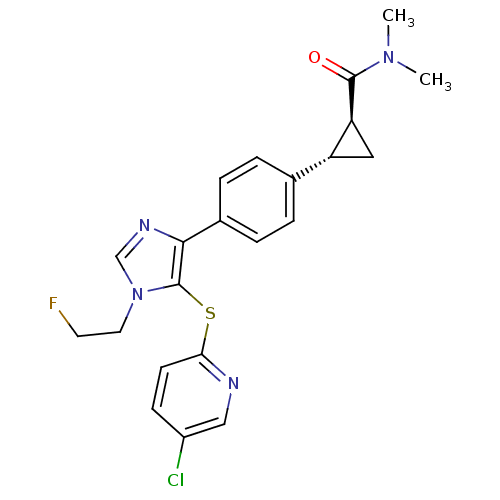 (CHEMBL2386563) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50434323 (CHEMBL2386561) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11218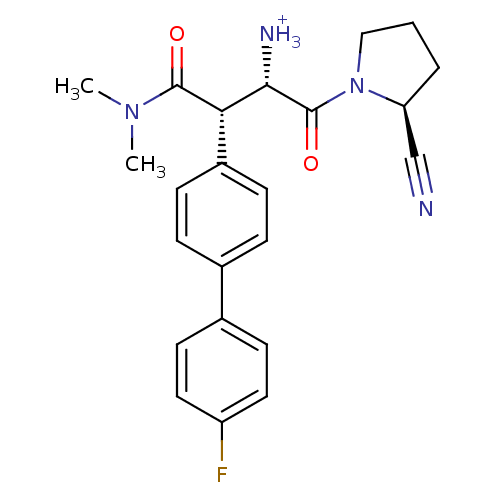 ((2S,3S)-1-[(2S)-2-cyanopyrrolidin-1-yl]-3-(dimethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories | Assay Description The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... | Bioorg Med Chem Lett 15: 3048-52 (2005) Article DOI: 10.1016/j.bmcl.2005.04.028 BindingDB Entry DOI: 10.7270/Q2833Q73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NPC1-like intracellular cholesterol transporter 1 (Homo sapiens (Human)) | BDBM50300992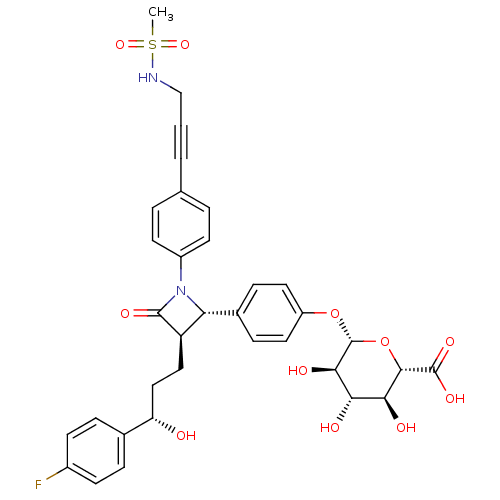 ((2S,3S,4S,5R,6S)-6-(4-{(2S,3R)-3-[(S)-3-(4-Fluoro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [35S](2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-1-(4-(3-(methylsulfonamido)prop-1-ynyl)phenyl)-4-oxoaz... | Bioorg Med Chem Lett 19: 5033-6 (2009) Article DOI: 10.1016/j.bmcl.2009.07.051 BindingDB Entry DOI: 10.7270/Q28P61GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NPC1-like intracellular cholesterol transporter 1 (Rattus norvegicus) | BDBM50300992 ((2S,3S,4S,5R,6S)-6-(4-{(2S,3R)-3-[(S)-3-(4-Fluoro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [35S](2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-1-(4-(3-(methylsulfonamido)prop-1-ynyl)phenyl)-4-oxoaz... | Bioorg Med Chem Lett 19: 5033-6 (2009) Article DOI: 10.1016/j.bmcl.2009.07.051 BindingDB Entry DOI: 10.7270/Q28P61GZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| NPC1-like intracellular cholesterol transporter 1 (Homo sapiens (Human)) | BDBM50300992 ((2S,3S,4S,5R,6S)-6-(4-{(2S,3R)-3-[(S)-3-(4-Fluoro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [35S](2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-1-(4-(3-(methylsulfonamido)prop-1-ynyl)phenyl)-4-oxoaz... | Bioorg Med Chem Lett 19: 5033-6 (2009) Article DOI: 10.1016/j.bmcl.2009.07.051 BindingDB Entry DOI: 10.7270/Q28P61GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NPC1-like intracellular cholesterol transporter 1 (Rattus norvegicus) | BDBM50300992 ((2S,3S,4S,5R,6S)-6-(4-{(2S,3R)-3-[(S)-3-(4-Fluoro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [35S](2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-1-(4-(3-(methylsulfonamido)prop-1-ynyl)phenyl)-4-oxoaz... | Bioorg Med Chem Lett 19: 5033-6 (2009) Article DOI: 10.1016/j.bmcl.2009.07.051 BindingDB Entry DOI: 10.7270/Q28P61GZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11143 ((1S,2S)-1-(dimethylcarbamoyl)-3-[(3S)-3-fluoropyrr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Merck Research Laboratories | Assay Description The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... | J Med Chem 49: 3614-27 (2006) Article DOI: 10.1021/jm060015t BindingDB Entry DOI: 10.7270/Q2N87807 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50434320 (CHEMBL2386564) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin p... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50434328 (CHEMBL2386555) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin p... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11144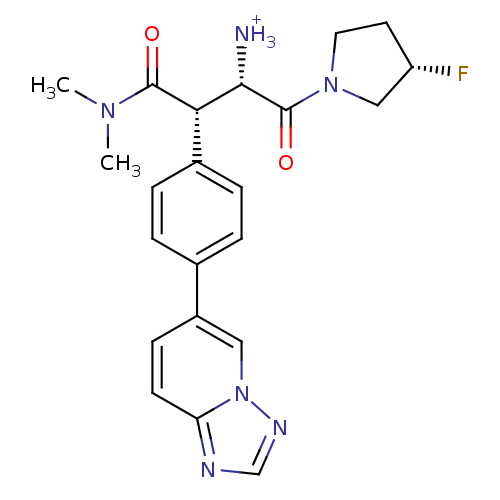 ((1S,2S)-1-(dimethylcarbamoyl)-3-[(3S)-3-fluoropyrr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Merck Research Laboratories | Assay Description The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... | J Med Chem 49: 3614-27 (2006) Article DOI: 10.1021/jm060015t BindingDB Entry DOI: 10.7270/Q2N87807 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50021344 (CHEMBL3287929) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat FAAH lysate using AMCAA as substrate by fluorescence assay | ACS Med Chem Lett 5: 717-21 (2014) Article DOI: 10.1021/ml5001239 BindingDB Entry DOI: 10.7270/Q2JQ12KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50434321 (CHEMBL2386563) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin p... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50434328 (CHEMBL2386555) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11152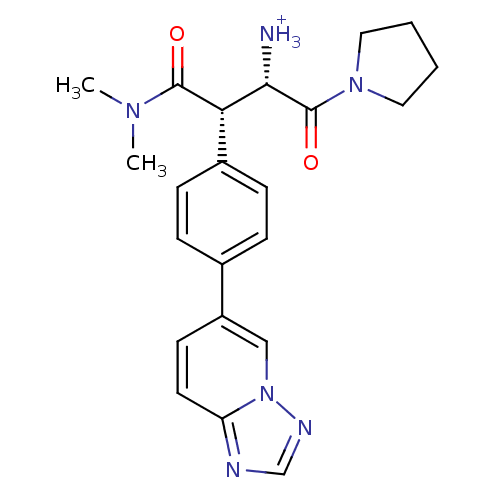 ((1S,2S)-1-(dimethylcarbamoyl)-3-oxo-3-(pyrrolidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Merck Research Laboratories | Assay Description The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... | J Med Chem 49: 3614-27 (2006) Article DOI: 10.1021/jm060015t BindingDB Entry DOI: 10.7270/Q2N87807 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11145 ((1S,2S)-1-(dimethylcarbamoyl)-3-[(3S)-3-fluoropyrr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Merck Research Laboratories | Assay Description The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... | J Med Chem 49: 3614-27 (2006) Article DOI: 10.1021/jm060015t BindingDB Entry DOI: 10.7270/Q2N87807 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50021345 (CHEMBL3287928) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat FAAH lysate using AMCAA as substrate by fluorescence assay | ACS Med Chem Lett 5: 717-21 (2014) Article DOI: 10.1021/ml5001239 BindingDB Entry DOI: 10.7270/Q2JQ12KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50021334 (CHEMBL3287931) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat FAAH lysate using AMCAA as substrate by fluorescence assay | ACS Med Chem Lett 5: 717-21 (2014) Article DOI: 10.1021/ml5001239 BindingDB Entry DOI: 10.7270/Q2JQ12KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50434322 (CHEMBL2386562) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin p... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11154 ((2S,3S)-1-(3,3-difluoropyrrolidin-1-yl)-3-(dimethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Merck Research Laboratories | Assay Description The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... | J Med Chem 49: 3614-27 (2006) Article DOI: 10.1021/jm060015t BindingDB Entry DOI: 10.7270/Q2N87807 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11153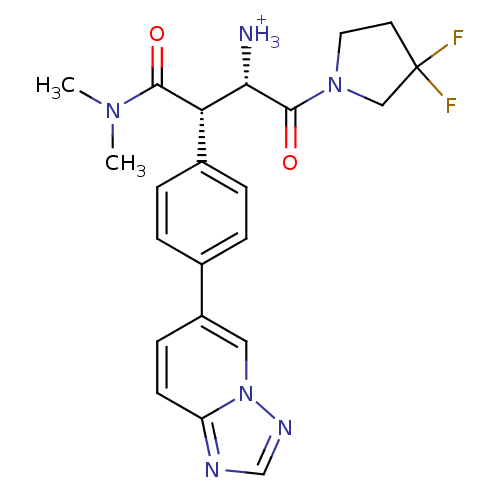 ((2S,3S)-1-(3,3-difluoropyrrolidin-1-yl)-3-(dimethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Merck Research Laboratories | Assay Description The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... | J Med Chem 49: 3614-27 (2006) Article DOI: 10.1021/jm060015t BindingDB Entry DOI: 10.7270/Q2N87807 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11193 ((1S,2S)-1-carboxy-1-[4-(4-fluorophenyl)phenyl]-3-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Merck Research Laboratories | Assay Description The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... | Bioorg Med Chem Lett 15: 3048-52 (2005) Article DOI: 10.1016/j.bmcl.2005.04.028 BindingDB Entry DOI: 10.7270/Q2833Q73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 534 total ) | Next | Last >> |