 Found 289 hits with Last Name = 'leonidas' and Initial = 'dd'
Found 289 hits with Last Name = 'leonidas' and Initial = 'dd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50056221
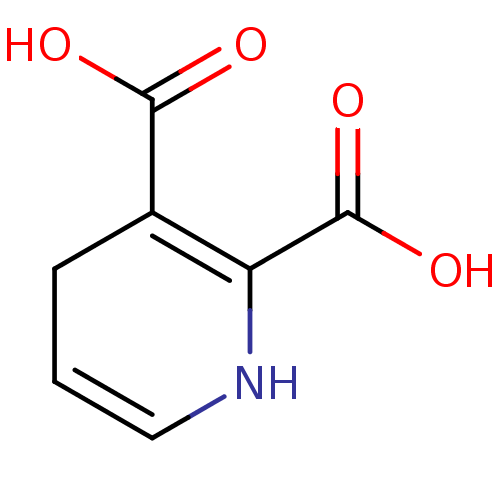
(CHEMBL3322297)Show InChI InChI=1S/C7H7NO4/c9-6(10)4-2-1-3-8-5(4)7(11)12/h1,3,8H,2H2,(H,9,10)(H,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Inhibition of glycogen phosphorylase b (unknown origin) |
Bioorg Med Chem 22: 4810-25 (2014)
Article DOI: 10.1016/j.bmc.2014.06.058
BindingDB Entry DOI: 10.7270/Q2KS6T58 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50148913
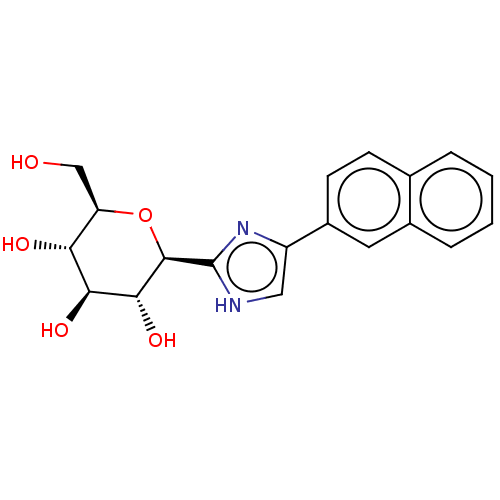
(CHEMBL3770455)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nc(c[nH]1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C19H20N2O5/c22-9-14-15(23)16(24)17(25)18(26-14)19-20-8-13(21-19)12-6-5-10-3-1-2-4-11(10)7-12/h1-8,14-18,22-25H,9H2,(H,20,21)/t14-,15-,16+,17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Competitive inhibition of His6-tagged human liver glycogen phosphorylase-a expressed in Escherichia coli BL21 Gold (DE3) assessed as release of inorg... |
Eur J Med Chem 123: 737-745 (2016)
Article DOI: 10.1016/j.ejmech.2016.06.049
BindingDB Entry DOI: 10.7270/Q2TT4SXG |
More data for this
Ligand-Target Pair | |
Ribonuclease pancreatic
(Homo sapiens (Human)) | BDBM50292713

(5'-phospho-2'-deoxyuridine 3-pyrophosphate (P'->5'...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](OP(O)(=O)OP(O)(=O)OC[C@H]2O[C@H](C[C@@H]2O)n2ccc(=O)[nH]c2=O)[C@H]1O |r| Show InChI InChI=1S/C19H25N7O14P2/c20-16-13-17(22-6-21-16)26(7-23-13)18-14(30)15(9(4-27)38-18)39-42(34,35)40-41(32,33)36-5-10-8(28)3-12(37-10)25-2-1-11(29)24-19(25)31/h1-2,6-10,12,14-15,18,27-28,30H,3-5H2,(H,32,33)(H,34,35)(H2,20,21,22)(H,24,29,31)/t8-,9+,10+,12+,14+,15+,18+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of RNase A |
J Med Chem 52: 932-42 (2009)
Article DOI: 10.1021/jm800724t
BindingDB Entry DOI: 10.7270/Q2H99570 |
More data for this
Ligand-Target Pair | |
Ribonuclease pancreatic
(Homo sapiens (Human)) | BDBM50402338
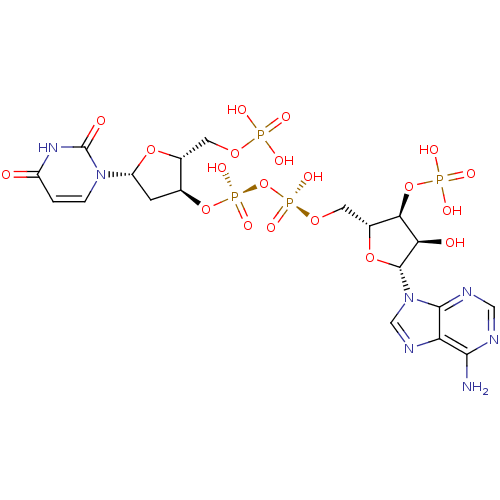
(CHEMBL401150)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@@](O)(=O)O[P@@](O)(=O)O[C@H]2C[C@@H](O[C@@H]2COP(O)(O)=O)n2ccc(=O)[nH]c2=O)[C@@H](OP(O)(O)=O)[C@H]1O |r| Show InChI InChI=1S/C19H27N7O20P4/c20-16-13-17(22-6-21-16)26(7-23-13)18-14(28)15(45-48(33,34)35)10(43-18)5-41-49(36,37)46-50(38,39)44-8-3-12(25-2-1-11(27)24-19(25)29)42-9(8)4-40-47(30,31)32/h1-2,6-10,12,14-15,18,28H,3-5H2,(H,36,37)(H,38,39)(H2,20,21,22)(H,24,27,29)(H2,30,31,32)(H2,33,34,35)/t8-,9+,10+,12+,14+,15+,18+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Inhibition of RNase A |
Bioorg Med Chem 20: 7184-93 (2012)
Article DOI: 10.1016/j.bmc.2012.09.067
BindingDB Entry DOI: 10.7270/Q2M61MDG |
More data for this
Ligand-Target Pair | |
Ribonuclease pancreatic
(Bison bison (American bison)) | BDBM50292721
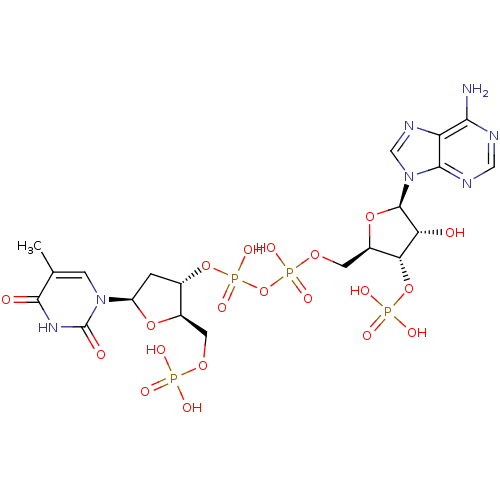
(CHEMBL504858 | [({[(2R,3S,4R,5R)-5-(6-amino-9H-pur...)Show SMILES Cc1cn([C@H]2C[C@H](OP(O)(=O)OP(O)(=O)OC[C@H]3O[C@H]([C@H](O)[C@@H]3OP(O)(O)=O)n3cnc4c(N)ncnc34)[C@@H](COP(O)(O)=O)O2)c(=O)[nH]c1=O Show InChI InChI=1S/C20H29N7O20P4/c1-8-3-26(20(30)25-18(8)29)12-2-9(10(43-12)4-41-48(31,32)33)45-51(39,40)47-50(37,38)42-5-11-15(46-49(34,35)36)14(28)19(44-11)27-7-24-13-16(21)22-6-23-17(13)27/h3,6-7,9-12,14-15,19,28H,2,4-5H2,1H3,(H,37,38)(H,39,40)(H2,21,22,23)(H,25,29,30)(H2,31,32,33)(H2,34,35,36)/t9-,10+,11+,12+,14+,15+,19+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| MMDB
Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of bovine pancreatic RNase A by spectrophotometric method |
J Med Chem 52: 932-42 (2009)
Article DOI: 10.1021/jm800724t
BindingDB Entry DOI: 10.7270/Q2H99570 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50148913

(CHEMBL3770455)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nc(c[nH]1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C19H20N2O5/c22-9-14-15(23)16(24)17(25)18(26-14)19-20-8-13(21-19)12-6-5-10-3-1-2-4-11(10)7-12/h1-8,14-18,22-25H,9H2,(H,20,21)/t14-,15-,16+,17-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Competitive inhibition of rabbit muscle glycogen phosphorylase-a assessed as release of inorganic phosphate using varying levels of glucose-1-phospha... |
Eur J Med Chem 123: 737-745 (2016)
Article DOI: 10.1016/j.ejmech.2016.06.049
BindingDB Entry DOI: 10.7270/Q2TT4SXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50241632
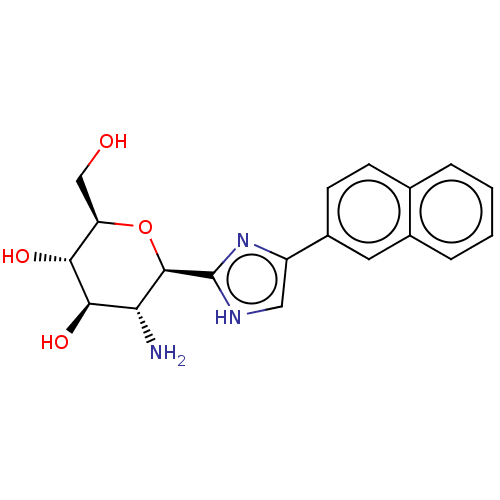
(CHEMBL4101331)Show SMILES N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1c1nc(c[nH]1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C19H21N3O4/c20-15-17(25)16(24)14(9-23)26-18(15)19-21-8-13(22-19)12-6-5-10-3-1-2-4-11(10)7-12/h1-8,14-18,23-25H,9,20H2,(H,21,22)/t14-,15-,16-,17-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Competitive inhibition of rabbit muscle glycogen phosphorylase-a in presence of varying levels of glucose-1-phosphate and constant concentration of g... |
J Med Chem 60: 9251-9262 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01056
BindingDB Entry DOI: 10.7270/Q2ZC851N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50241632

(CHEMBL4101331)Show SMILES N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1c1nc(c[nH]1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C19H21N3O4/c20-15-17(25)16(24)14(9-23)26-18(15)19-21-8-13(22-19)12-6-5-10-3-1-2-4-11(10)7-12/h1-8,14-18,23-25H,9,20H2,(H,21,22)/t14-,15-,16-,17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Competitive inhibition of 6xHis-tagged human liver glycogen phosphorylase-a expressed in Escherichia coli BL21 Gold (DE3) assessed as release of inor... |
J Med Chem 60: 9251-9262 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01056
BindingDB Entry DOI: 10.7270/Q2ZC851N |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50148915
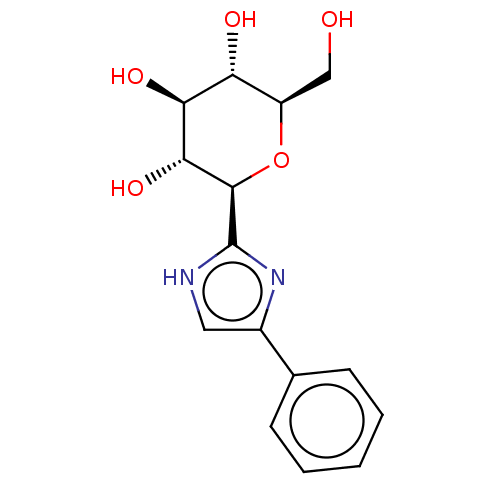
(CHEMBL3770514)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nc(c[nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C15H18N2O5/c18-7-10-11(19)12(20)13(21)14(22-10)15-16-6-9(17-15)8-4-2-1-3-5-8/h1-6,10-14,18-21H,7H2,(H,16,17)/t10-,11-,12+,13-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 156 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Competitive inhibition of His6-tagged human liver glycogen phosphorylase-a expressed in Escherichia coli BL21 Gold (DE3) assessed as release of inorg... |
Eur J Med Chem 123: 737-745 (2016)
Article DOI: 10.1016/j.ejmech.2016.06.049
BindingDB Entry DOI: 10.7270/Q2TT4SXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50437354

(CHEMBL2408225)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nnc([nH]1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C18H19N3O5/c22-8-12-13(23)14(24)15(25)16(26-12)18-19-17(20-21-18)11-6-5-9-3-1-2-4-10(9)7-11/h1-7,12-16,22-25H,8H2,(H,19,20,21)/t12-,13-,14+,15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 172 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Inhibition of human liver GPa using Glc-1-P as substrate incubated for 15 mins |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115196
BindingDB Entry DOI: 10.7270/Q25X2DGG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50437354

(CHEMBL2408225)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nnc([nH]1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C18H19N3O5/c22-8-12-13(23)14(24)15(25)16(26-12)18-19-17(20-21-18)11-6-5-9-3-1-2-4-10(9)7-11/h1-7,12-16,22-25H,8H2,(H,19,20,21)/t12-,13-,14+,15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 172 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Competitive inhibition of human liver glycogen phosphorylase-a assessed as release of inorganic phosphate using varying levels of glucose-1-phosphate... |
J Med Chem 60: 9251-9262 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01056
BindingDB Entry DOI: 10.7270/Q2ZC851N |
More data for this
Ligand-Target Pair | |
Non-secretory ribonuclease
(Homo sapiens (Human)) | BDBM50292713

(5'-phospho-2'-deoxyuridine 3-pyrophosphate (P'->5'...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](OP(O)(=O)OP(O)(=O)OC[C@H]2O[C@H](C[C@@H]2O)n2ccc(=O)[nH]c2=O)[C@H]1O |r| Show InChI InChI=1S/C19H25N7O14P2/c20-16-13-17(22-6-21-16)26(7-23-13)18-14(30)15(9(4-27)38-18)39-42(34,35)40-41(32,33)36-5-10-8(28)3-12(37-10)25-2-1-11(29)24-19(25)31/h1-2,6-10,12,14-15,18,27-28,30H,3-5H2,(H,32,33)(H,34,35)(H2,20,21,22)(H,24,29,31)/t8-,9+,10+,12+,14+,15+,18+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human EDN |
J Med Chem 52: 932-42 (2009)
Article DOI: 10.1021/jm800724t
BindingDB Entry DOI: 10.7270/Q2H99570 |
More data for this
Ligand-Target Pair | |
Non-secretory ribonuclease
(Homo sapiens (Human)) | BDBM50402338

(CHEMBL401150)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@@](O)(=O)O[P@@](O)(=O)O[C@H]2C[C@@H](O[C@@H]2COP(O)(O)=O)n2ccc(=O)[nH]c2=O)[C@@H](OP(O)(O)=O)[C@H]1O |r| Show InChI InChI=1S/C19H27N7O20P4/c20-16-13-17(22-6-21-16)26(7-23-13)18-14(28)15(45-48(33,34)35)10(43-18)5-41-49(36,37)46-50(38,39)44-8-3-12(25-2-1-11(27)24-19(25)29)42-9(8)4-40-47(30,31)32/h1-2,6-10,12,14-15,18,28H,3-5H2,(H,36,37)(H,38,39)(H2,20,21,22)(H,24,27,29)(H2,30,31,32)(H2,33,34,35)/t8-,9+,10+,12+,14+,15+,18+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Inhibition of EDN |
Bioorg Med Chem 20: 7184-93 (2012)
Article DOI: 10.1016/j.bmc.2012.09.067
BindingDB Entry DOI: 10.7270/Q2M61MDG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50241632

(CHEMBL4101331)Show SMILES N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1c1nc(c[nH]1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C19H21N3O4/c20-15-17(25)16(24)14(9-23)26-18(15)19-21-8-13(22-19)12-6-5-10-3-1-2-4-11(10)7-12/h1-8,14-18,23-25H,9,20H2,(H,21,22)/t14-,15-,16-,17-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 191 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Competitive inhibition of rabbit muscle glycogen phosphorylase-b in presence of varying levels of glucose-1-phosphate and constant concentration of g... |
J Med Chem 60: 9251-9262 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01056
BindingDB Entry DOI: 10.7270/Q2ZC851N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50148915

(CHEMBL3770514)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nc(c[nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C15H18N2O5/c18-7-10-11(19)12(20)13(21)14(22-10)15-16-6-9(17-15)8-4-2-1-3-5-8/h1-6,10-14,18-21H,7H2,(H,16,17)/t10-,11-,12+,13-,14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 226 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Competitive inhibition of rabbit muscle glycogen phosphorylase-a assessed as release of inorganic phosphate using varying levels of glucose-1-phospha... |
Eur J Med Chem 123: 737-745 (2016)
Article DOI: 10.1016/j.ejmech.2016.06.049
BindingDB Entry DOI: 10.7270/Q2TT4SXG |
More data for this
Ligand-Target Pair | |
Ribonuclease pancreatic
(Bison bison (American bison)) | BDBM50402337
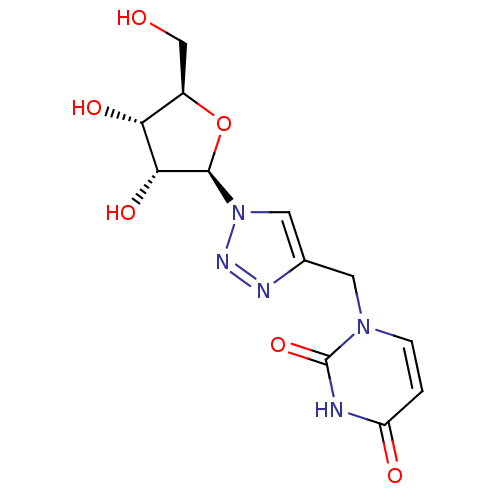
(CHEMBL2206660)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cc(Cn2ccc(=O)[nH]c2=O)nn1 |r| Show InChI InChI=1S/C12H15N5O6/c18-5-7-9(20)10(21)11(23-7)17-4-6(14-15-17)3-16-2-1-8(19)13-12(16)22/h1-2,4,7,9-11,18,20-21H,3,5H2,(H,13,19,22)/t7-,9-,10-,11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Inhibition of bovine pancreatic RNase A |
Bioorg Med Chem 20: 7184-93 (2012)
Article DOI: 10.1016/j.bmc.2012.09.067
BindingDB Entry DOI: 10.7270/Q2M61MDG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50194701
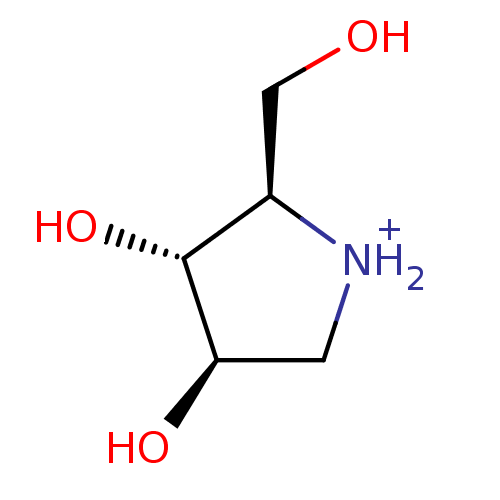
((2R,3R,4R)-2-Hydroxymethylpyrrolidin-3,4-diol hydr...)Show InChI InChI=1S/C5H11NO3/c7-2-3-5(9)4(8)1-6-3/h3-9H,1-2H2/p+1/t3-,4-,5-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 342 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The National Hellenic Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of non-phosphorylated form of rabbit muscle glycogen phosphorylase in presence of glycogen and 45 mM phosphate |
J Med Chem 49: 5687-701 (2006)
Article DOI: 10.1021/jm060496g
BindingDB Entry DOI: 10.7270/Q25Q4VQP |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50263773

(1-(2-naphthoyl)-3-((2R,3R,4S,5S,6R)-3,4,5-trihydro...)Show SMILES OC[C@H]1O[C@@H](NC(=O)NC(=O)c2ccc3ccccc3c2)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C18H20N2O7/c21-8-12-13(22)14(23)15(24)17(27-12)20-18(26)19-16(25)11-6-5-9-3-1-2-4-10(9)7-11/h1-7,12-15,17,21-24H,8H2,(H2,19,20,25,26)/t12-,13-,14+,15-,17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibition of rabbit muscle glycogen phosphorylase b assessed as inorganic phosphate release at pH 6.8 |
Bioorg Med Chem 20: 1801-16 (2012)
Article DOI: 10.1016/j.bmc.2011.12.059
BindingDB Entry DOI: 10.7270/Q2SN09DD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50263773

(1-(2-naphthoyl)-3-((2R,3R,4S,5S,6R)-3,4,5-trihydro...)Show SMILES OC[C@H]1O[C@@H](NC(=O)NC(=O)c2ccc3ccccc3c2)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C18H20N2O7/c21-8-12-13(22)14(23)15(24)17(27-12)20-18(26)19-16(25)11-6-5-9-3-1-2-4-10(9)7-11/h1-7,12-15,17,21-24H,8H2,(H2,19,20,25,26)/t12-,13-,14+,15-,17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Inhibition of rabbit skeletal muscle glycogen phosphorylase b assessed as inorganic phosphate release |
Bioorg Med Chem 22: 4810-25 (2014)
Article DOI: 10.1016/j.bmc.2014.06.058
BindingDB Entry DOI: 10.7270/Q2KS6T58 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50194701

((2R,3R,4R)-2-Hydroxymethylpyrrolidin-3,4-diol hydr...)Show InChI InChI=1S/C5H11NO3/c7-2-3-5(9)4(8)1-6-3/h3-9H,1-2H2/p+1/t3-,4-,5-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 396 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The National Hellenic Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of non-phosphorylated form of rabbit muscle glycogen phosphorylase in presence of 6.17 mM glycogen and phosphate |
J Med Chem 49: 5687-701 (2006)
Article DOI: 10.1021/jm060496g
BindingDB Entry DOI: 10.7270/Q25Q4VQP |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM34110
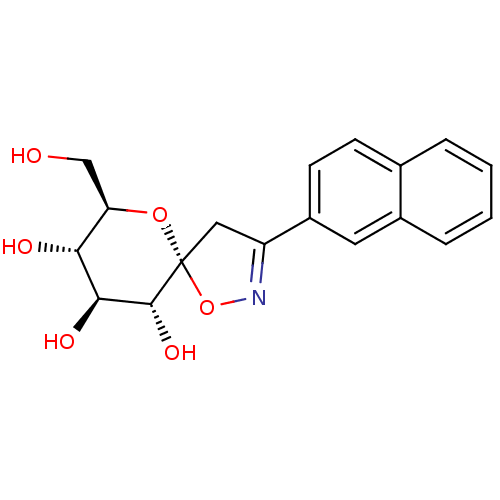
(glucopyranosylidene-spiro-isoxazoline, 4e)Show SMILES OC[C@H]1O[C@@]2(CC(=NO2)c2ccc3ccccc3c2)[C@H](O)[C@@H](O)[C@@H]1O |c:6| Show InChI InChI=1S/C18H19NO6/c20-9-14-15(21)16(22)17(23)18(24-14)8-13(19-25-18)12-6-5-10-3-1-2-4-11(10)7-12/h1-7,14-17,20-23H,8-9H2/t14-,15-,16+,17-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 630 | -36.0 | n/a | n/a | n/a | n/a | n/a | 6.8 | 30 |
Universite de Lyon
| Assay Description
Phosphorylase activity was measured in the direction of glycogen synthesis by the release of orthophosphate from Glc-1-P. The enzyme was assayed in g... |
Bioorg Med Chem 17: 7368-80 (2009)
Article DOI: 10.1016/j.bmc.2009.08.060
BindingDB Entry DOI: 10.7270/Q28P5XV8 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50056223
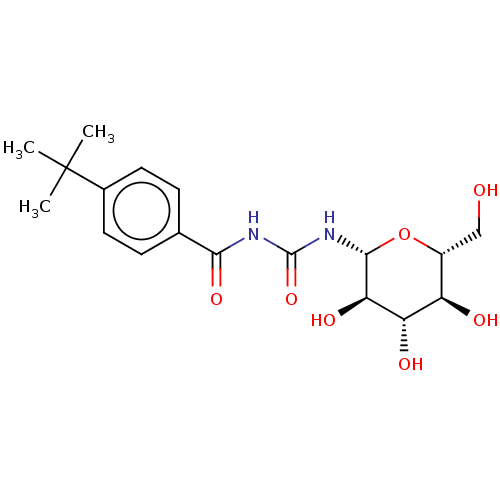
(CHEMBL3322301)Show SMILES CC(C)(C)c1ccc(cc1)C(=O)NC(=O)N[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C18H26N2O7/c1-18(2,3)10-6-4-9(5-7-10)15(25)19-17(26)20-16-14(24)13(23)12(22)11(8-21)27-16/h4-7,11-14,16,21-24H,8H2,1-3H3,(H2,19,20,25,26)/t11-,12-,13+,14-,16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Inhibition of rabbit skeletal muscle glycogen phosphorylase b assessed as inorganic phosphate release |
Bioorg Med Chem 22: 4810-25 (2014)
Article DOI: 10.1016/j.bmc.2014.06.058
BindingDB Entry DOI: 10.7270/Q2KS6T58 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50312225
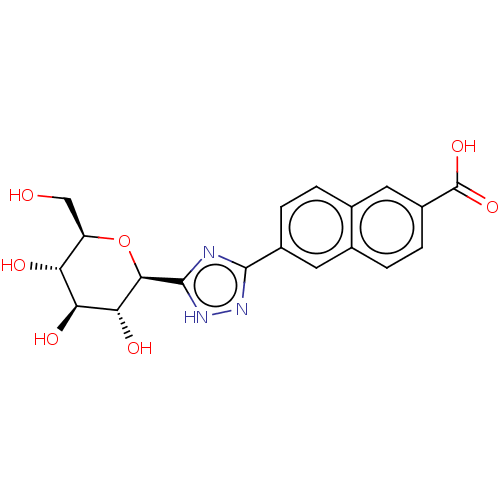
(CHEMBL4161353)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nc(n[nH]1)-c1ccc2cc(ccc2c1)C(O)=O |r| Show InChI InChI=1S/C19H19N3O7/c23-7-12-13(24)14(25)15(26)16(29-12)18-20-17(21-22-18)10-3-1-9-6-11(19(27)28)4-2-8(9)5-10/h1-6,12-16,23-26H,7H2,(H,27,28)(H,20,21,22)/t12-,13-,14+,15-,16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibition of rabbit muscle glycogen phosphorylase-a assessed as release of inorganic phosphate using glucose-1-phosphate as substrate in presence of... |
Eur J Med Chem 147: 266-278 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.095
BindingDB Entry DOI: 10.7270/Q2J67KF5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50312361

(CHEMBL4169281)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nc(n[nH]1)-c1ccc-2c(Cc3ccccc-23)c1 |r| Show InChI InChI=1S/C21H21N3O5/c25-9-15-16(26)17(27)18(28)19(29-15)21-22-20(23-24-21)11-5-6-14-12(8-11)7-10-3-1-2-4-13(10)14/h1-6,8,15-19,25-28H,7,9H2,(H,22,23,24)/t15-,16-,17+,18-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibition of rabbit muscle glycogen phosphorylase-a assessed as release of inorganic phosphate using glucose-1-phosphate as substrate in presence of... |
Eur J Med Chem 147: 266-278 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.095
BindingDB Entry DOI: 10.7270/Q2J67KF5 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50313014

(CHEMBL4172670)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nc(n[nH]1)-c1ccc(cc1)-c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C21H21N3O7/c25-9-14-15(26)16(27)17(28)18(31-14)20-22-19(23-24-20)12-5-1-10(2-6-12)11-3-7-13(8-4-11)21(29)30/h1-8,14-18,25-28H,9H2,(H,29,30)(H,22,23,24)/t14-,15-,16+,17-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibition of rabbit muscle glycogen phosphorylase-a assessed as release of inorganic phosphate using glucose-1-phosphate as substrate in presence of... |
Eur J Med Chem 147: 266-278 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.095
BindingDB Entry DOI: 10.7270/Q2J67KF5 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50386288
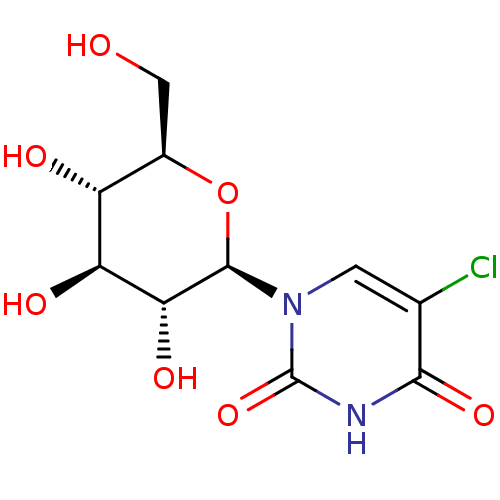
(CHEMBL2041081)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)n1cc(Cl)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C10H13ClN2O7/c11-3-1-13(10(19)12-8(3)18)9-7(17)6(16)5(15)4(2-14)20-9/h1,4-7,9,14-17H,2H2,(H,12,18,19)/t4-,5-,6+,7-,9-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Competitive inhibition of rabbit skeletal muscle glycogen phosphorylase b using Glc-1-P as substrate |
Eur J Med Chem 54: 740-9 (2012)
Article DOI: 10.1016/j.ejmech.2012.06.029
BindingDB Entry DOI: 10.7270/Q2VX0HKH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50386288

(CHEMBL2041081)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)n1cc(Cl)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C10H13ClN2O7/c11-3-1-13(10(19)12-8(3)18)9-7(17)6(16)5(15)4(2-14)20-9/h1,4-7,9,14-17H,2H2,(H,12,18,19)/t4-,5-,6+,7-,9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Inhibition of glycogen phosphorylase b |
Eur J Med Chem 54: 740-9 (2012)
Article DOI: 10.1016/j.ejmech.2012.06.029
BindingDB Entry DOI: 10.7270/Q2VX0HKH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50312361

(CHEMBL4169281)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nc(n[nH]1)-c1ccc-2c(Cc3ccccc-23)c1 |r| Show InChI InChI=1S/C21H21N3O5/c25-9-15-16(26)17(27)18(28)19(29-15)21-22-20(23-24-21)11-5-6-14-12(8-11)7-10-3-1-2-4-13(10)14/h1-6,8,15-19,25-28H,7,9H2,(H,22,23,24)/t15-,16-,17+,18-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibition of rabbit muscle glycogen phosphorylase-b assessed as release of inorganic phosphate using glucose-1-phosphate as substrate in presence of... |
Eur J Med Chem 147: 266-278 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.095
BindingDB Entry DOI: 10.7270/Q2J67KF5 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50437355
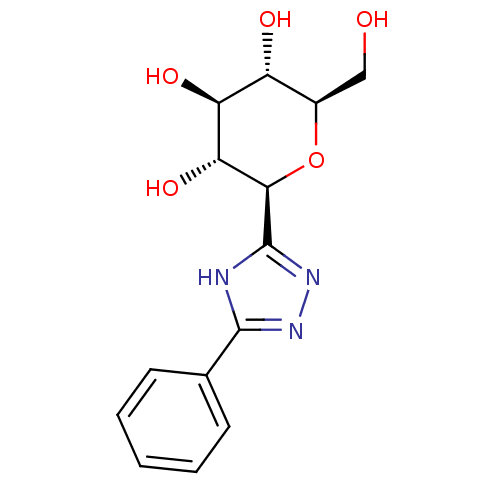
(CHEMBL2408224)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nnc([nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C14H17N3O5/c18-6-8-9(19)10(20)11(21)12(22-8)14-15-13(16-17-14)7-4-2-1-3-5-7/h1-5,8-12,18-21H,6H2,(H,15,16,17)/t8-,9-,10+,11-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition of fibrinogen receptor |
J Med Chem 60: 9251-9262 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01056
BindingDB Entry DOI: 10.7270/Q2ZC851N |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50437355

(CHEMBL2408224)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nnc([nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C14H17N3O5/c18-6-8-9(19)10(20)11(21)12(22-8)14-15-13(16-17-14)7-4-2-1-3-5-7/h1-5,8-12,18-21H,6H2,(H,15,16,17)/t8-,9-,10+,11-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Inhibition of human liver GPa using Glc-1-P as substrate incubated for 15 mins |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115196
BindingDB Entry DOI: 10.7270/Q25X2DGG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50056224

(CHEMBL3322302)Show SMILES OC[C@H]1O[C@@H](NC(=O)NC(=O)c2ccc(cc2)C(F)(F)F)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C15H17F3N2O7/c16-15(17,18)7-3-1-6(2-4-7)12(25)19-14(26)20-13-11(24)10(23)9(22)8(5-21)27-13/h1-4,8-11,13,21-24H,5H2,(H2,19,20,25,26)/t8-,9-,10+,11-,13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Inhibition of rabbit skeletal muscle glycogen phosphorylase b assessed as inorganic phosphate release |
Bioorg Med Chem 22: 4810-25 (2014)
Article DOI: 10.1016/j.bmc.2014.06.058
BindingDB Entry DOI: 10.7270/Q2KS6T58 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50386290
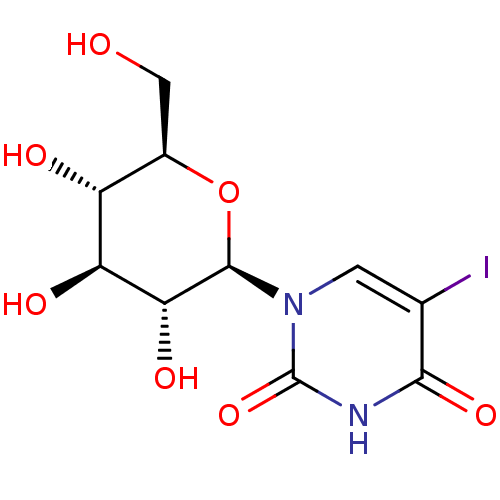
(CHEMBL2040853)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)n1cc(I)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C10H13IN2O7/c11-3-1-13(10(19)12-8(3)18)9-7(17)6(16)5(15)4(2-14)20-9/h1,4-7,9,14-17H,2H2,(H,12,18,19)/t4-,5-,6+,7-,9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Inhibition of glycogen phosphorylase b |
Eur J Med Chem 54: 740-9 (2012)
Article DOI: 10.1016/j.ejmech.2012.06.029
BindingDB Entry DOI: 10.7270/Q2VX0HKH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50312361

(CHEMBL4169281)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nc(n[nH]1)-c1ccc-2c(Cc3ccccc-23)c1 |r| Show InChI InChI=1S/C21H21N3O5/c25-9-15-16(26)17(27)18(28)19(29-15)21-22-20(23-24-21)11-5-6-14-12(8-11)7-10-3-1-2-4-13(10)14/h1-6,8,15-19,25-28H,7,9H2,(H,22,23,24)/t15-,16-,17+,18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase-a assessed as release of inorganic phosphate using glucose-1-phosphate as substrate in presence of g... |
Eur J Med Chem 147: 266-278 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.095
BindingDB Entry DOI: 10.7270/Q2J67KF5 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50312362
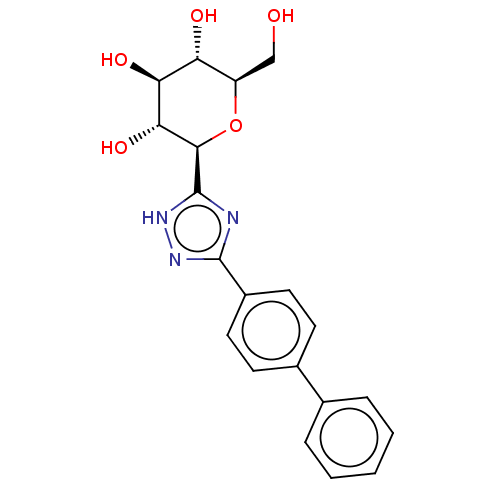
(CHEMBL4176460)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nc(n[nH]1)-c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C20H21N3O5/c24-10-14-15(25)16(26)17(27)18(28-14)20-21-19(22-23-20)13-8-6-12(7-9-13)11-4-2-1-3-5-11/h1-9,14-18,24-27H,10H2,(H,21,22,23)/t14-,15-,16+,17-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibition of rabbit muscle glycogen phosphorylase-a assessed as release of inorganic phosphate using glucose-1-phosphate as substrate in presence of... |
Eur J Med Chem 147: 266-278 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.095
BindingDB Entry DOI: 10.7270/Q2J67KF5 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50312362

(CHEMBL4176460)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nc(n[nH]1)-c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C20H21N3O5/c24-10-14-15(25)16(26)17(27)18(28-14)20-21-19(22-23-20)13-8-6-12(7-9-13)11-4-2-1-3-5-11/h1-9,14-18,24-27H,10H2,(H,21,22,23)/t14-,15-,16+,17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase-a assessed as release of inorganic phosphate using glucose-1-phosphate as substrate in presence of g... |
Eur J Med Chem 147: 266-278 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.095
BindingDB Entry DOI: 10.7270/Q2J67KF5 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50295843

(4-methyl-N-((2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(h...)Show SMILES Cc1ccc(cc1)C(=O)NC(=O)N[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C15H20N2O7/c1-7-2-4-8(5-3-7)13(22)16-15(23)17-14-12(21)11(20)10(19)9(6-18)24-14/h2-5,9-12,14,18-21H,6H2,1H3,(H2,16,17,22,23)/t9-,10-,11+,12-,14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibition of rabbit muscle glycogen phosphorylase b assessed as inorganic phosphate release at pH 6.8 |
Bioorg Med Chem 20: 1801-16 (2012)
Article DOI: 10.1016/j.bmc.2011.12.059
BindingDB Entry DOI: 10.7270/Q2SN09DD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50312362

(CHEMBL4176460)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nc(n[nH]1)-c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C20H21N3O5/c24-10-14-15(25)16(26)17(27)18(28-14)20-21-19(22-23-20)13-8-6-12(7-9-13)11-4-2-1-3-5-11/h1-9,14-18,24-27H,10H2,(H,21,22,23)/t14-,15-,16+,17-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibition of rabbit muscle glycogen phosphorylase-b assessed as release of inorganic phosphate using glucose-1-phosphate as substrate in presence of... |
Eur J Med Chem 147: 266-278 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.095
BindingDB Entry DOI: 10.7270/Q2J67KF5 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50313014

(CHEMBL4172670)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nc(n[nH]1)-c1ccc(cc1)-c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C21H21N3O7/c25-9-14-15(26)16(27)17(28)18(31-14)20-22-19(23-24-20)12-5-1-10(2-6-12)11-3-7-13(8-4-11)21(29)30/h1-8,14-18,25-28H,9H2,(H,29,30)(H,22,23,24)/t14-,15-,16+,17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase-a assessed as release of inorganic phosphate using glucose-1-phosphate as substrate in presence of g... |
Eur J Med Chem 147: 266-278 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.095
BindingDB Entry DOI: 10.7270/Q2J67KF5 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50241633

(CHEMBL4095486)Show SMILES N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1c1nc(c[nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C15H19N3O4/c16-11-13(21)12(20)10(7-19)22-14(11)15-17-6-9(18-15)8-4-2-1-3-5-8/h1-6,10-14,19-21H,7,16H2,(H,17,18)/t10-,11-,12-,13-,14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition of fibrinogen receptor |
J Med Chem 60: 9251-9262 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01056
BindingDB Entry DOI: 10.7270/Q2ZC851N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50241633

(CHEMBL4095486)Show SMILES N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1c1nc(c[nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C15H19N3O4/c16-11-13(21)12(20)10(7-19)22-14(11)15-17-6-9(18-15)8-4-2-1-3-5-8/h1-6,10-14,19-21H,7,16H2,(H,17,18)/t10-,11-,12-,13-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Competitive inhibition of 6xHis-tagged human liver glycogen phosphorylase-a expressed in Escherichia coli BL21 Gold (DE3) assessed as release of inor... |
J Med Chem 60: 9251-9262 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01056
BindingDB Entry DOI: 10.7270/Q2ZC851N |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50263768
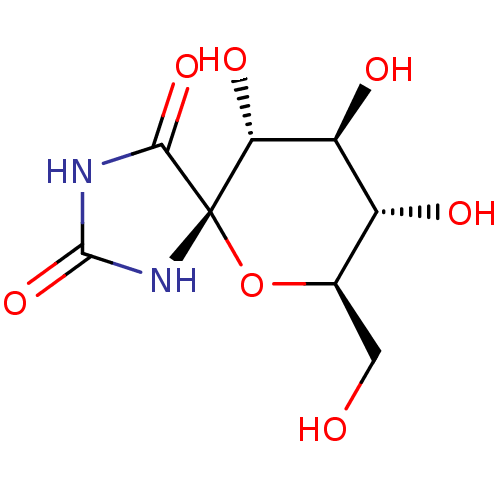
((5S,7R,8S,9S,10R)-8,9,10-Trihydroxy-7-hydroxymethy...)Show SMILES OC[C@H]1O[C@@]2(NC(=O)NC2=O)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C8H12N2O7/c11-1-2-3(12)4(13)5(14)8(17-2)6(15)9-7(16)10-8/h2-5,11-14H,1H2,(H2,9,10,15,16)/t2-,3-,4+,5-,8+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibition of rabbit muscle glycogen phosphorylase b assessed as inorganic phosphate release at pH 6.8 |
Bioorg Med Chem 20: 1801-16 (2012)
Article DOI: 10.1016/j.bmc.2011.12.059
BindingDB Entry DOI: 10.7270/Q2SN09DD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50241633

(CHEMBL4095486)Show SMILES N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1c1nc(c[nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C15H19N3O4/c16-11-13(21)12(20)10(7-19)22-14(11)15-17-6-9(18-15)8-4-2-1-3-5-8/h1-6,10-14,19-21H,7,16H2,(H,17,18)/t10-,11-,12-,13-,14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 3.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Competitive inhibition of rabbit muscle glycogen phosphorylase-a in presence of varying levels of glucose-1-phosphate and constant concentration of g... |
J Med Chem 60: 9251-9262 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01056
BindingDB Entry DOI: 10.7270/Q2ZC851N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50363878

(CHEMBL1947268)Show SMILES COc1ccc(cc1)C(=O)NC(=O)N[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C15H20N2O8/c1-24-8-4-2-7(3-5-8)13(22)16-15(23)17-14-12(21)11(20)10(19)9(6-18)25-14/h2-5,9-12,14,18-21H,6H2,1H3,(H2,16,17,22,23)/t9-,10-,11+,12-,14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibition of rabbit muscle glycogen phosphorylase b assessed as inorganic phosphate release at pH 6.8 |
Bioorg Med Chem 20: 1801-16 (2012)
Article DOI: 10.1016/j.bmc.2011.12.059
BindingDB Entry DOI: 10.7270/Q2SN09DD |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50538946
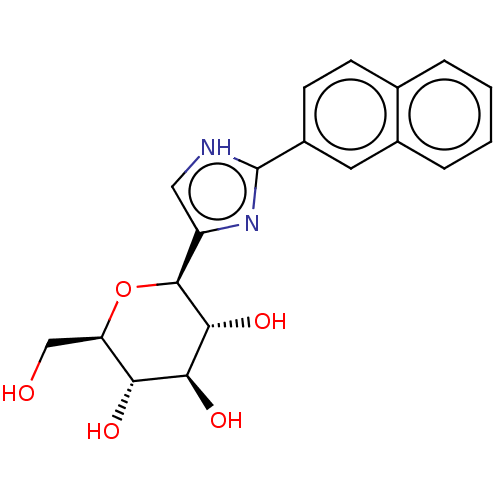
(CHEMBL4646575)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1c[nH]c(n1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C19H20N2O5/c22-9-14-15(23)16(24)17(25)18(26-14)13-8-20-19(21-13)12-6-5-10-3-1-2-4-11(10)7-12/h1-8,14-18,22-25H,9H2,(H,20,21)/t14-,15-,16+,17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Inhibition of human liver GPa using Glc-1-P as substrate incubated for 15 mins |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115196
BindingDB Entry DOI: 10.7270/Q25X2DGG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50386289
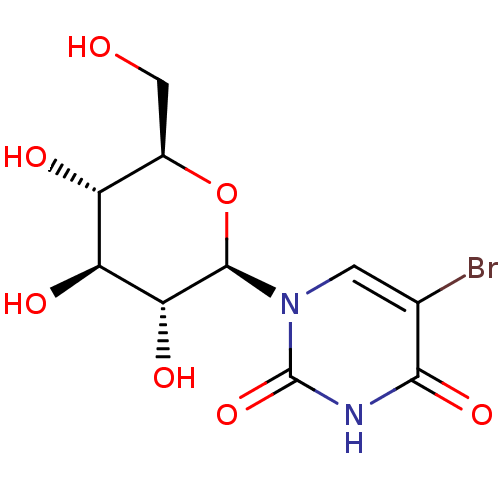
(CHEMBL2041082)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)n1cc(Br)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C10H13BrN2O7/c11-3-1-13(10(19)12-8(3)18)9-7(17)6(16)5(15)4(2-14)20-9/h1,4-7,9,14-17H,2H2,(H,12,18,19)/t4-,5-,6+,7-,9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 3.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Inhibition of glycogen phosphorylase b |
Eur J Med Chem 54: 740-9 (2012)
Article DOI: 10.1016/j.ejmech.2012.06.029
BindingDB Entry DOI: 10.7270/Q2VX0HKH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50295844
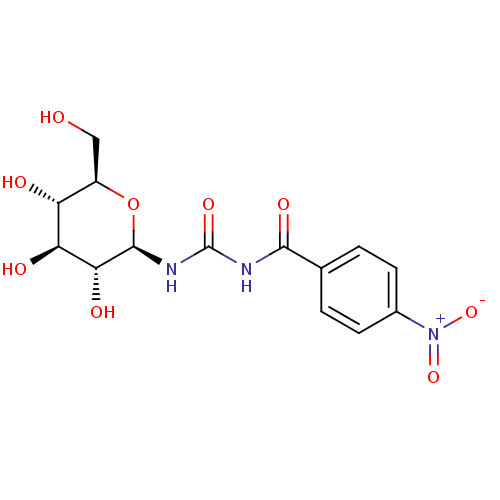
(4-nitro-N-((2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hy...)Show SMILES OC[C@H]1O[C@@H](NC(=O)NC(=O)c2ccc(cc2)[N+]([O-])=O)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C14H17N3O9/c18-5-8-9(19)10(20)11(21)13(26-8)16-14(23)15-12(22)6-1-3-7(4-2-6)17(24)25/h1-4,8-11,13,18-21H,5H2,(H2,15,16,22,23)/t8-,9-,10+,11-,13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibition of rabbit muscle glycogen phosphorylase b assessed as inorganic phosphate release at pH 6.8 |
Bioorg Med Chem 20: 1801-16 (2012)
Article DOI: 10.1016/j.bmc.2011.12.059
BindingDB Entry DOI: 10.7270/Q2SN09DD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50312632

(CHEMBL4164542)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nc(n[nH]1)-c1cccc2ccccc12 |r| Show InChI InChI=1S/C18H19N3O5/c22-8-12-13(23)14(24)15(25)16(26-12)18-19-17(20-21-18)11-7-3-5-9-4-1-2-6-10(9)11/h1-7,12-16,22-25H,8H2,(H,19,20,21)/t12-,13-,14+,15-,16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibition of rabbit muscle glycogen phosphorylase-a assessed as release of inorganic phosphate using glucose-1-phosphate as substrate in presence of... |
Eur J Med Chem 147: 266-278 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.095
BindingDB Entry DOI: 10.7270/Q2J67KF5 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50056291
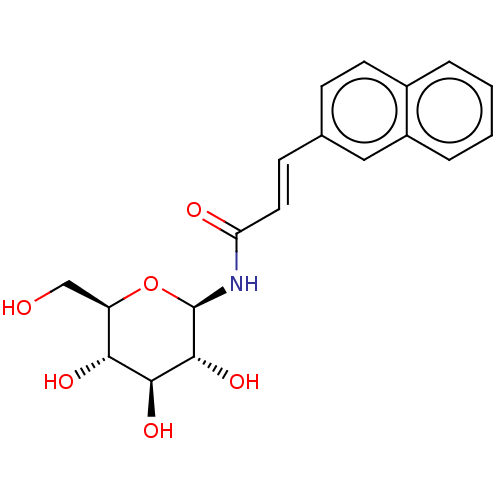
(CHEMBL3322299)Show SMILES OC[C@H]1O[C@@H](NC(=O)\C=C\c2ccc3ccccc3c2)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C19H21NO6/c21-10-14-16(23)17(24)18(25)19(26-14)20-15(22)8-6-11-5-7-12-3-1-2-4-13(12)9-11/h1-9,14,16-19,21,23-25H,10H2,(H,20,22)/b8-6+/t14-,16-,17+,18-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Inhibition of rabbit skeletal muscle glycogen phosphorylase b assessed as inorganic phosphate release |
Bioorg Med Chem 22: 4810-25 (2014)
Article DOI: 10.1016/j.bmc.2014.06.058
BindingDB Entry DOI: 10.7270/Q2KS6T58 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50363876

(CHEMBL1232637)Show SMILES OC[C@H]1O[C@@H](NC(=O)NC(=O)c2ccc(cc2)-c2ccccc2)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C20H22N2O7/c23-10-14-15(24)16(25)17(26)19(29-14)22-20(28)21-18(27)13-8-6-12(7-9-13)11-4-2-1-3-5-11/h1-9,14-17,19,23-26H,10H2,(H2,21,22,27,28)/t14-,15-,16+,17-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibition of rabbit muscle glycogen phosphorylase b assessed as inorganic phosphate release at pH 6.8 |
Bioorg Med Chem 20: 1801-16 (2012)
Article DOI: 10.1016/j.bmc.2011.12.059
BindingDB Entry DOI: 10.7270/Q2SN09DD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50312225

(CHEMBL4161353)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nc(n[nH]1)-c1ccc2cc(ccc2c1)C(O)=O |r| Show InChI InChI=1S/C19H19N3O7/c23-7-12-13(24)14(25)15(26)16(29-12)18-20-17(21-22-18)10-3-1-9-6-11(19(27)28)4-2-8(9)5-10/h1-6,12-16,23-26H,7H2,(H,27,28)(H,20,21,22)/t12-,13-,14+,15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 3.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase-a assessed as release of inorganic phosphate using glucose-1-phosphate as substrate in presence of g... |
Eur J Med Chem 147: 266-278 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.095
BindingDB Entry DOI: 10.7270/Q2J67KF5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data