 Found 226 hits with Last Name = 'lesnick' and Initial = 'jd'
Found 226 hits with Last Name = 'lesnick' and Initial = 'jd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149477
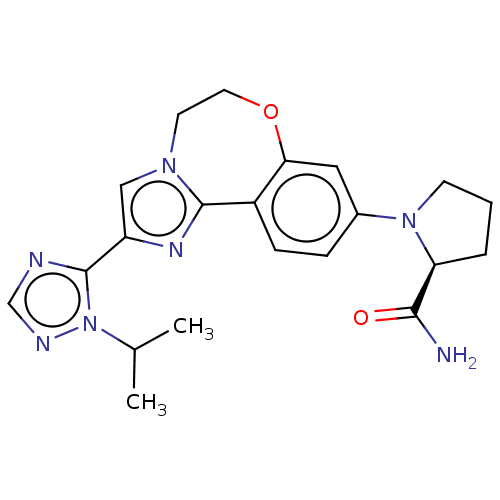
(CHEMBL3770993 | US10851091, U.S. Pat. No. 8,242,10...)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(ccc3-c2n1)N1CCC[C@H]1C(N)=O |r| Show InChI InChI=1S/C21H25N7O2/c1-13(2)28-21(23-12-24-28)16-11-26-8-9-30-18-10-14(5-6-15(18)20(26)25-16)27-7-3-4-17(27)19(22)29/h5-6,10-13,17H,3-4,7-9H2,1-2H3,(H2,22,29)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM81811
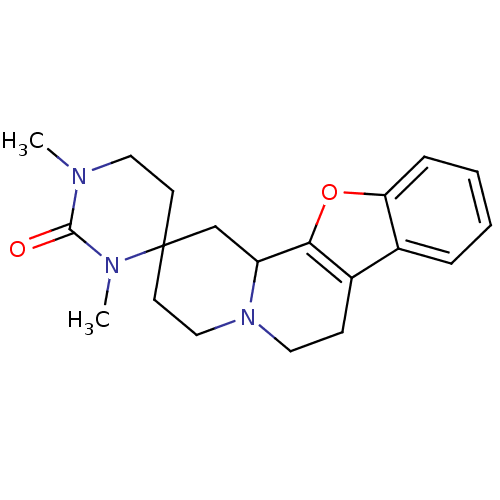
(CAS_123679 | L-657,743 | MK-912 | NSC_123679)Show InChI InChI=1S/C20H25N3O2/c1-21-11-8-20(22(2)19(21)24)9-12-23-10-7-15-14-5-3-4-6-17(14)25-18(15)16(23)13-20/h3-6,16H,7-13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Biochem Pharmacol 55: 1035-43 (1998)
Article DOI: 10.1016/s0006-2952(97)00631-x
BindingDB Entry DOI: 10.7270/Q2T72G01 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM50408198
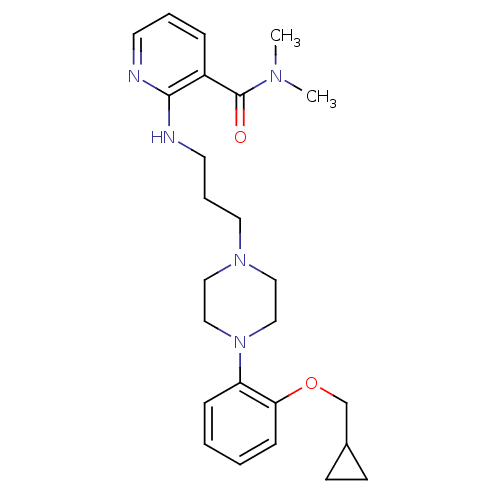
(CHEMBL91278)Show SMILES CN(C)C(=O)c1cccnc1NCCCN1CCN(CC1)c1ccccc1OCC1CC1 Show InChI InChI=1S/C25H35N5O2/c1-28(2)25(31)21-7-5-12-26-24(21)27-13-6-14-29-15-17-30(18-16-29)22-8-3-4-9-23(22)32-19-20-10-11-20/h3-5,7-9,12,20H,6,10-11,13-19H2,1-2H3,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149553

(CHEMBL3770306)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(ccc3-c2n1)N1CC[C@H]1C(N)=O |r| Show InChI InChI=1S/C20H23N7O2/c1-12(2)27-20(22-11-23-27)15-10-25-7-8-29-17-9-13(3-4-14(17)19(25)24-15)26-6-5-16(26)18(21)28/h3-4,9-12,16H,5-8H2,1-2H3,(H2,21,28)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149482
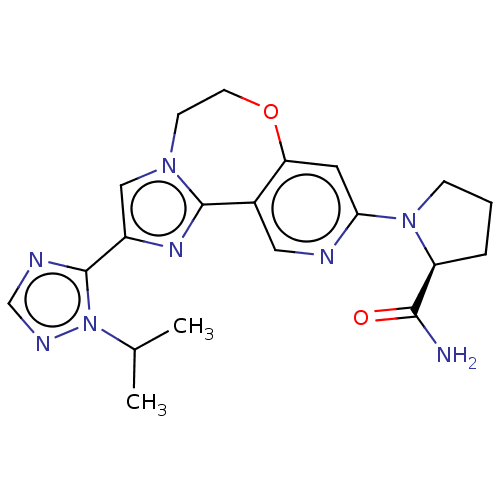
(CHEMBL3770709)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(ncc3-c2n1)N1CCC[C@H]1C(N)=O |r| Show InChI InChI=1S/C20H24N8O2/c1-12(2)28-20(23-11-24-28)14-10-26-6-7-30-16-8-17(22-9-13(16)19(26)25-14)27-5-3-4-15(27)18(21)29/h8-12,15H,3-7H2,1-2H3,(H2,21,29)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM50060964
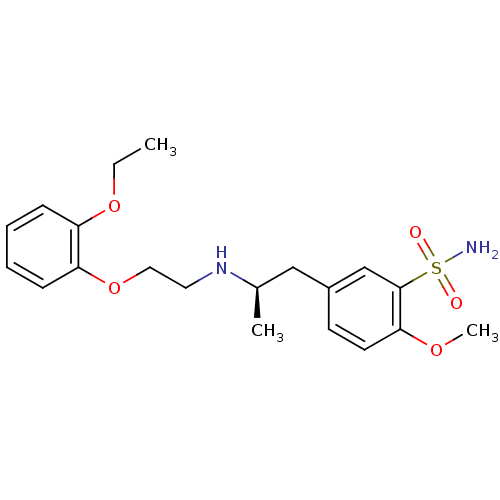
((R)-5-(2-((2-(2-ethoxyphenoxy)ethyl)amino)propyl)-...)Show SMILES CCOc1ccccc1OCCN[C@H](C)Cc1ccc(OC)c(c1)S(N)(=O)=O Show InChI InChI=1S/C20H28N2O5S/c1-4-26-17-7-5-6-8-18(17)27-12-11-22-15(2)13-16-9-10-19(25-3)20(14-16)28(21,23)24/h5-10,14-15,22H,4,11-13H2,1-3H3,(H2,21,23,24)/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM50408201
(CHEMBL88512)Show InChI InChI=1S/C20H27N5O2/c1-27-18-8-3-2-7-17(18)25-14-12-24(13-15-25)11-5-10-23-20-16(19(21)26)6-4-9-22-20/h2-4,6-9H,5,10-15H2,1H3,(H2,21,26)(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149483
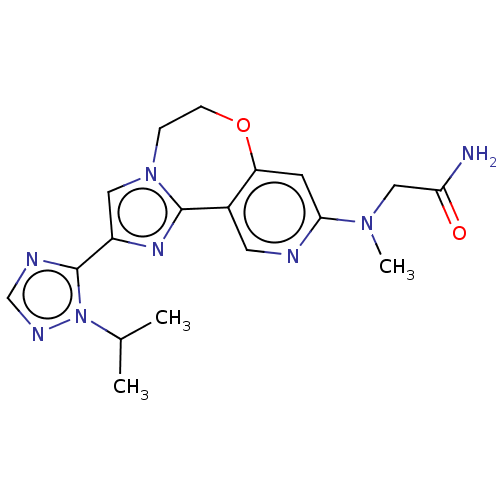
(CHEMBL3770325)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(ncc3-c2n1)N(C)CC(N)=O Show InChI InChI=1S/C18H22N8O2/c1-11(2)26-18(21-10-22-26)13-8-25-4-5-28-14-6-16(24(3)9-15(19)27)20-7-12(14)17(25)23-13/h6-8,10-11H,4-5,9H2,1-3H3,(H2,19,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149548
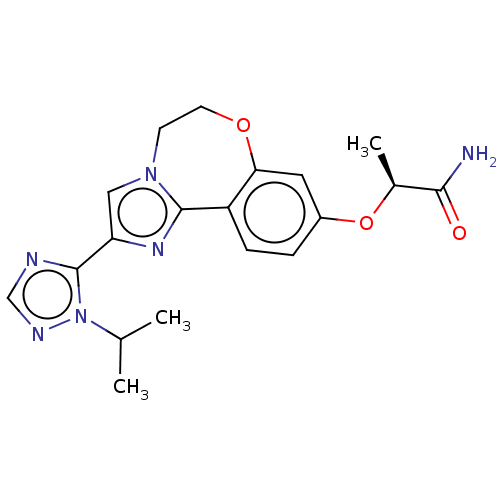
(CHEMBL3771364 | US10851091, U.S. Pat. No. 8,242,10...)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(O[C@@H](C)C(N)=O)ccc3-c2n1 |r| Show InChI InChI=1S/C19H22N6O3/c1-11(2)25-19(21-10-22-25)15-9-24-6-7-27-16-8-13(28-12(3)17(20)26)4-5-14(16)18(24)23-15/h4-5,8-12H,6-7H2,1-3H3,(H2,20,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149554
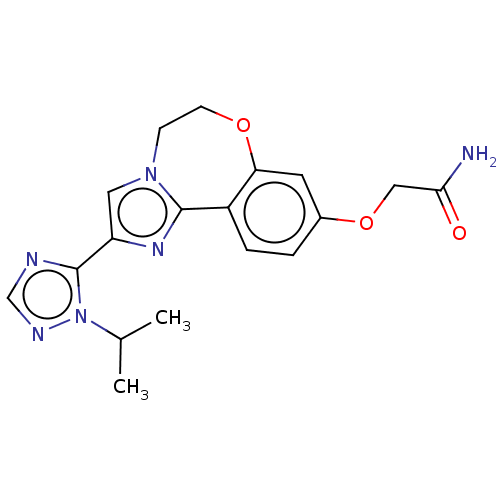
(CHEMBL3770140)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(OCC(N)=O)ccc3-c2n1 Show InChI InChI=1S/C18H20N6O3/c1-11(2)24-18(20-10-21-24)14-8-23-5-6-26-15-7-12(27-9-16(19)25)3-4-13(15)17(23)22-14/h3-4,7-8,10-11H,5-6,9H2,1-2H3,(H2,19,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149476
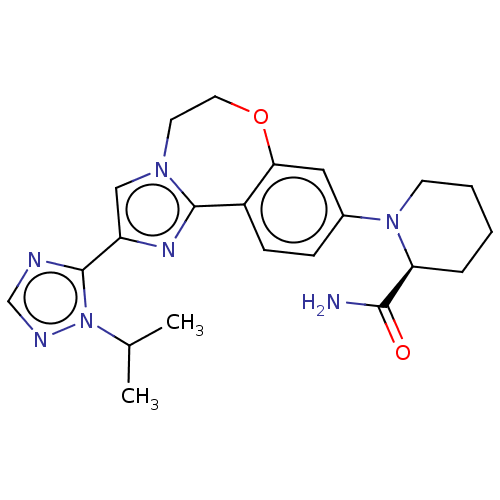
(CHEMBL3770717)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(ccc3-c2n1)N1CCCC[C@H]1C(N)=O |r| Show InChI InChI=1S/C22H27N7O2/c1-14(2)29-22(24-13-25-29)17-12-27-9-10-31-19-11-15(6-7-16(19)21(27)26-17)28-8-4-3-5-18(28)20(23)30/h6-7,11-14,18H,3-5,8-10H2,1-2H3,(H2,23,30)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434806
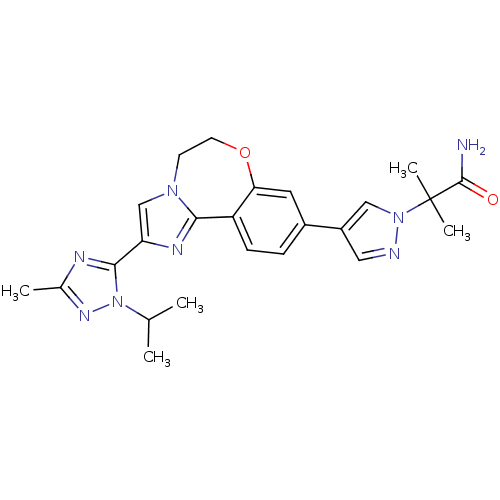
(2-(4-(2-(1-isopropyl-3-methyl-1H-1,2,4-triazol-5-y...)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(c1)C(C)(C)C(N)=O Show InChI InChI=1S/C24H28N8O2/c1-14(2)32-22(27-15(3)29-32)19-13-30-8-9-34-20-10-16(6-7-18(20)21(30)28-19)17-11-26-31(12-17)24(4,5)23(25)33/h6-7,10-14H,8-9H2,1-5H3,(H2,25,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50019492
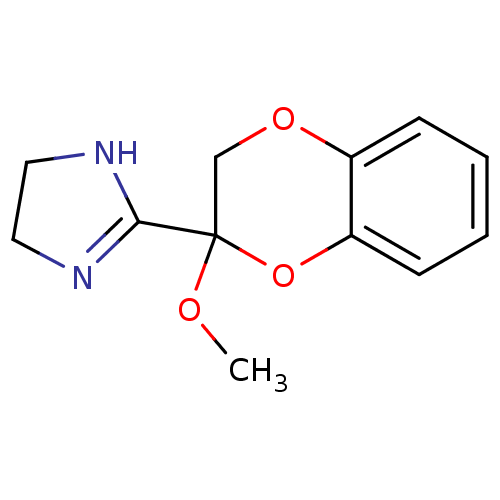
((+)2-(2-Methoxy-2,3-dihydro-benzo[1,4]dioxin-2-yl)...)Show InChI InChI=1S/C12H14N2O3/c1-15-12(11-13-6-7-14-11)8-16-9-4-2-3-5-10(9)17-12/h2-5H,6-8H2,1H3,(H,13,14) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Biochem Pharmacol 55: 1035-43 (1998)
Article DOI: 10.1016/s0006-2952(97)00631-x
BindingDB Entry DOI: 10.7270/Q2T72G01 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM50408246
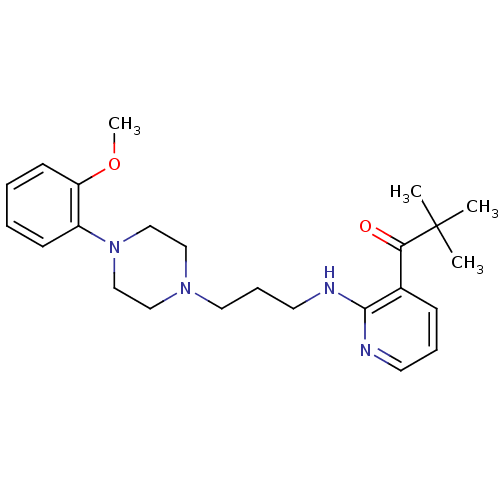
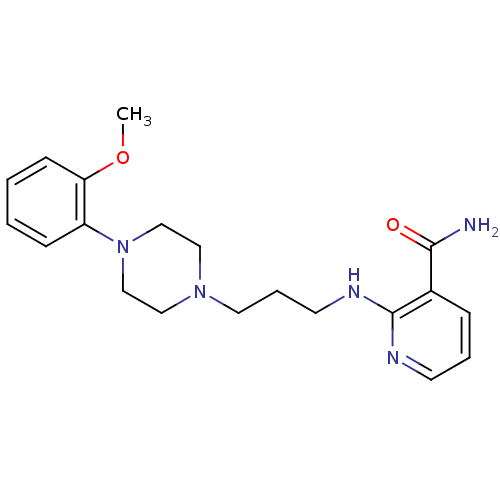
(CHEMBL92261)Show SMILES COc1ccccc1N1CCN(CCCNc2ncccc2C(=O)C(C)(C)C)CC1 Show InChI InChI=1S/C24H34N4O2/c1-24(2,3)22(29)19-9-7-12-25-23(19)26-13-8-14-27-15-17-28(18-16-27)20-10-5-6-11-21(20)30-4/h5-7,9-12H,8,13-18H2,1-4H3,(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM50408248
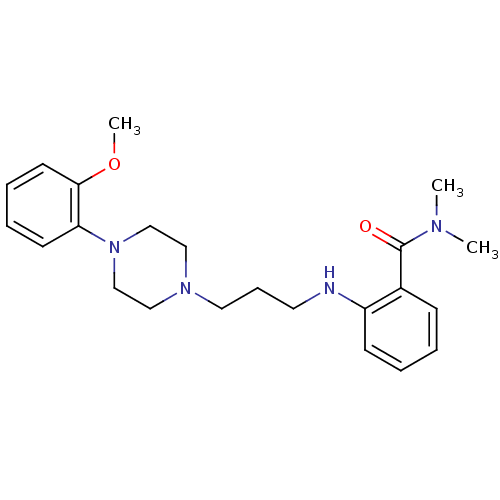
(CHEMBL330060)Show InChI InChI=1S/C23H32N4O2/c1-25(2)23(28)19-9-4-5-10-20(19)24-13-8-14-26-15-17-27(18-16-26)21-11-6-7-12-22(21)29-3/h4-7,9-12,24H,8,13-18H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149465
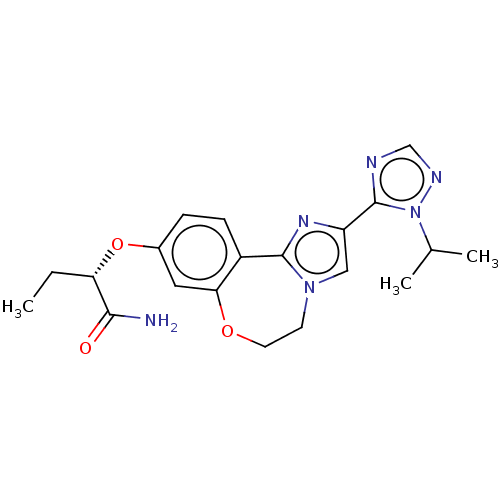
(CHEMBL3769854)Show SMILES CC[C@H](Oc1ccc2-c3nc(cn3CCOc2c1)-c1ncnn1C(C)C)C(N)=O |r| Show InChI InChI=1S/C20H24N6O3/c1-4-16(18(21)27)29-13-5-6-14-17(9-13)28-8-7-25-10-15(24-19(14)25)20-22-11-23-26(20)12(2)3/h5-6,9-12,16H,4,7-8H2,1-3H3,(H2,21,27)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM50033112
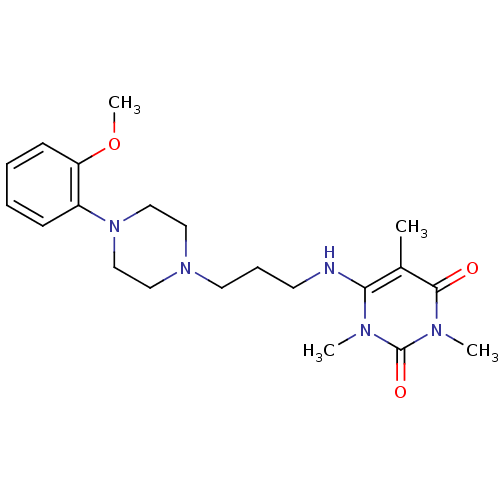
(6-{3-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-propyla...)Show SMILES COc1ccccc1N1CCN(CCCNc2c(C)c(=O)n(C)c(=O)n2C)CC1 Show InChI InChI=1S/C21H31N5O3/c1-16-19(23(2)21(28)24(3)20(16)27)22-10-7-11-25-12-14-26(15-13-25)17-8-5-6-9-18(17)29-4/h5-6,8-9,22H,7,10-15H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Biochem Pharmacol 55: 1035-43 (1998)
Article DOI: 10.1016/s0006-2952(97)00631-x
BindingDB Entry DOI: 10.7270/Q2T72G01 |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(MOUSE) | BDBM50019492

((+)2-(2-Methoxy-2,3-dihydro-benzo[1,4]dioxin-2-yl)...)Show InChI InChI=1S/C12H14N2O3/c1-15-12(11-13-6-7-14-11)8-16-9-4-2-3-5-10(9)17-12/h2-5H,6-8H2,1H3,(H,13,14) | PDB
Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Biochem Pharmacol 55: 1035-43 (1998)
Article DOI: 10.1016/s0006-2952(97)00631-x
BindingDB Entry DOI: 10.7270/Q2T72G01 |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM50019492

((+)2-(2-Methoxy-2,3-dihydro-benzo[1,4]dioxin-2-yl)...)Show InChI InChI=1S/C12H14N2O3/c1-15-12(11-13-6-7-14-11)8-16-9-4-2-3-5-10(9)17-12/h2-5H,6-8H2,1H3,(H,13,14) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Biochem Pharmacol 55: 1035-43 (1998)
Article DOI: 10.1016/s0006-2952(97)00631-x
BindingDB Entry DOI: 10.7270/Q2T72G01 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM31046
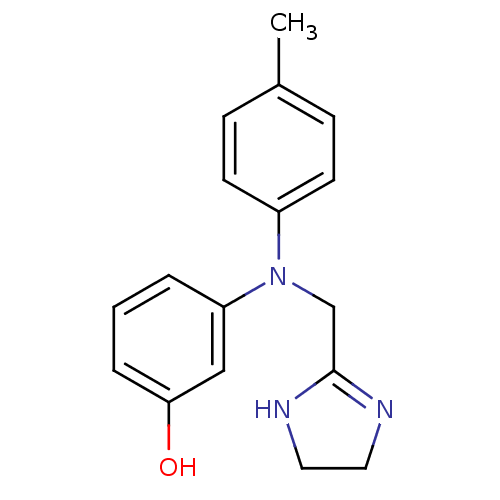
(3-[4,5-dihydro-1H-imidazol-2-ylmethyl-(4-methylphe...)Show InChI InChI=1S/C17H19N3O/c1-13-5-7-14(8-6-13)20(12-17-18-9-10-19-17)15-3-2-4-16(21)11-15/h2-8,11,21H,9-10,12H2,1H3,(H,18,19) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM50408242
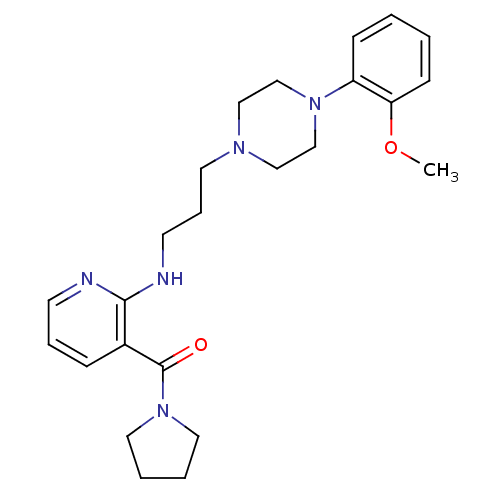
(CHEMBL91876)Show SMILES COc1ccccc1N1CCN(CCCNc2ncccc2C(=O)N2CCCC2)CC1 Show InChI InChI=1S/C24H33N5O2/c1-31-22-10-3-2-9-21(22)28-18-16-27(17-19-28)13-7-12-26-23-20(8-6-11-25-23)24(30)29-14-4-5-15-29/h2-3,6,8-11H,4-5,7,12-19H2,1H3,(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM50408205
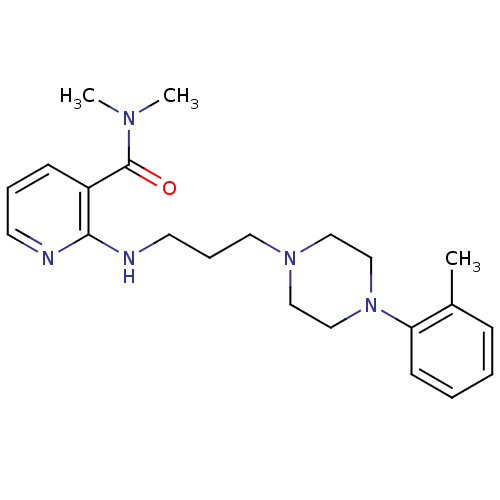
(CHEMBL88435)Show InChI InChI=1S/C22H31N5O/c1-18-8-4-5-10-20(18)27-16-14-26(15-17-27)13-7-12-24-21-19(9-6-11-23-21)22(28)25(2)3/h4-6,8-11H,7,12-17H2,1-3H3,(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM50160165
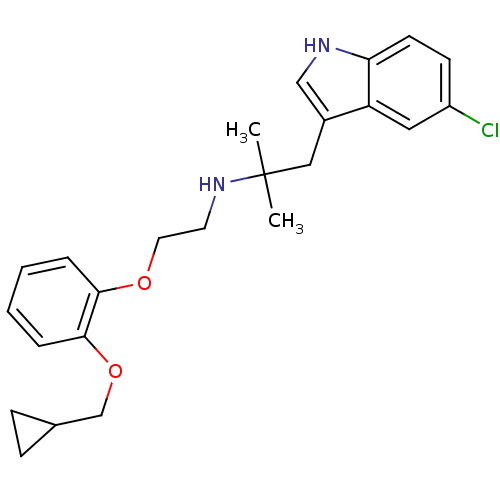
(CHEMBL88272 | RS-17053 | [2-(2-Cyclopropylmethoxy-...)Show SMILES CC(C)(Cc1c[nH]c2ccc(Cl)cc12)NCCOc1ccccc1OCC1CC1 Show InChI InChI=1S/C24H29ClN2O2/c1-24(2,14-18-15-26-21-10-9-19(25)13-20(18)21)27-11-12-28-22-5-3-4-6-23(22)29-16-17-7-8-17/h3-6,9-10,13,15,17,26-27H,7-8,11-12,14,16H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM50408244
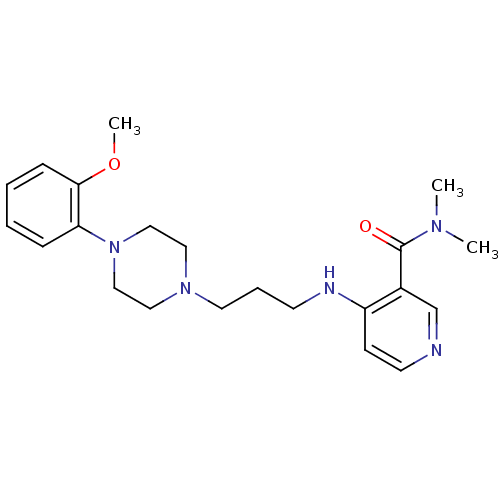
(CHEMBL93736)Show InChI InChI=1S/C22H31N5O2/c1-25(2)22(28)18-17-23-11-9-19(18)24-10-6-12-26-13-15-27(16-14-26)20-7-4-5-8-21(20)29-3/h4-5,7-9,11,17H,6,10,12-16H2,1-3H3,(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM50408232
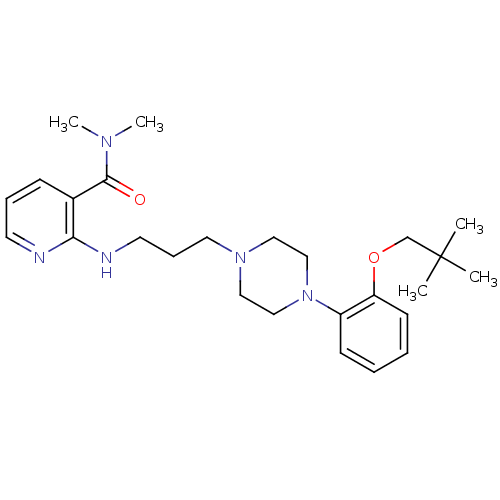
(CHEMBL91093)Show SMILES CN(C)C(=O)c1cccnc1NCCCN1CCN(CC1)c1ccccc1OCC(C)(C)C Show InChI InChI=1S/C26H39N5O2/c1-26(2,3)20-33-23-12-7-6-11-22(23)31-18-16-30(17-19-31)15-9-14-28-24-21(10-8-13-27-24)25(32)29(4)5/h6-8,10-13H,9,14-20H2,1-5H3,(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM50408229
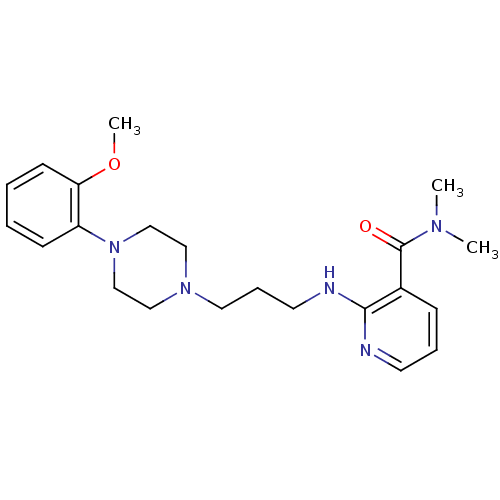
(CHEMBL329160)Show InChI InChI=1S/C22H31N5O2/c1-25(2)22(28)18-8-6-11-23-21(18)24-12-7-13-26-14-16-27(17-15-26)19-9-4-5-10-20(19)29-3/h4-6,8-11H,7,12-17H2,1-3H3,(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
The compound was screened in vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM81811

(CAS_123679 | L-657,743 | MK-912 | NSC_123679)Show InChI InChI=1S/C20H25N3O2/c1-21-11-8-20(22(2)19(21)24)9-12-23-10-7-15-14-5-3-4-6-17(14)25-18(15)16(23)13-20/h3-6,16H,7-13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Biochem Pharmacol 55: 1035-43 (1998)
Article DOI: 10.1016/s0006-2952(97)00631-x
BindingDB Entry DOI: 10.7270/Q2T72G01 |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(Homo sapiens (Human)) | BDBM81811

(CAS_123679 | L-657,743 | MK-912 | NSC_123679)Show InChI InChI=1S/C20H25N3O2/c1-21-11-8-20(22(2)19(21)24)9-12-23-10-7-15-14-5-3-4-6-17(14)25-18(15)16(23)13-20/h3-6,16H,7-13H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Biochem Pharmacol 55: 1035-43 (1998)
Article DOI: 10.1016/s0006-2952(97)00631-x
BindingDB Entry DOI: 10.7270/Q2T72G01 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149546
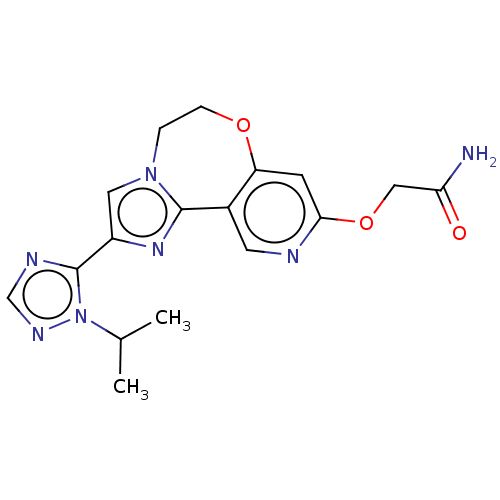
(CHEMBL3769966)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(OCC(N)=O)ncc3-c2n1 Show InChI InChI=1S/C17H19N7O3/c1-10(2)24-17(20-9-21-24)12-7-23-3-4-26-13-5-15(27-8-14(18)25)19-6-11(13)16(23)22-12/h5-7,9-10H,3-4,8H2,1-2H3,(H2,18,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149545

(CHEMBL3770824)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(O[C@@H](C)C(N)=O)ncc3-c2n1 |r| Show InChI InChI=1S/C18H21N7O3/c1-10(2)25-18(21-9-22-25)13-8-24-4-5-27-14-6-15(28-11(3)16(19)26)20-7-12(14)17(24)23-13/h6-11H,4-5H2,1-3H3,(H2,19,26)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50398400
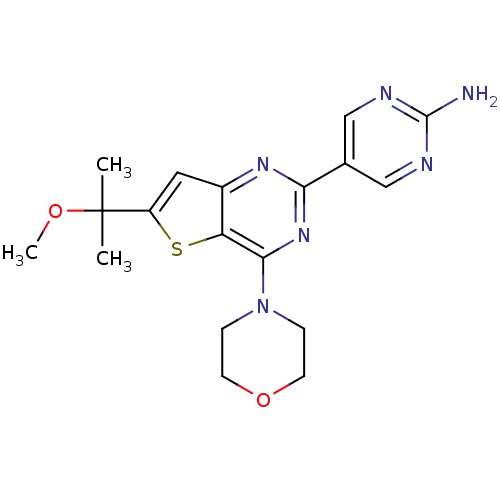
(CHEMBL2178606)Show SMILES COC(C)(C)c1cc2nc(nc(N3CCOCC3)c2s1)-c1cnc(N)nc1 Show InChI InChI=1S/C18H22N6O2S/c1-18(2,25-3)13-8-12-14(27-13)16(24-4-6-26-7-5-24)23-15(22-12)11-9-20-17(19)21-10-11/h8-10H,4-7H2,1-3H3,(H2,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha (unknown origin) using dioctanoylglycerol-PIP2 as substrate incubated for 30 mins in presence of TAMRA-PIP3 by fl... |
ACS Med Chem Lett 7: 351-6 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00005
BindingDB Entry DOI: 10.7270/Q2WQ05QD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149550
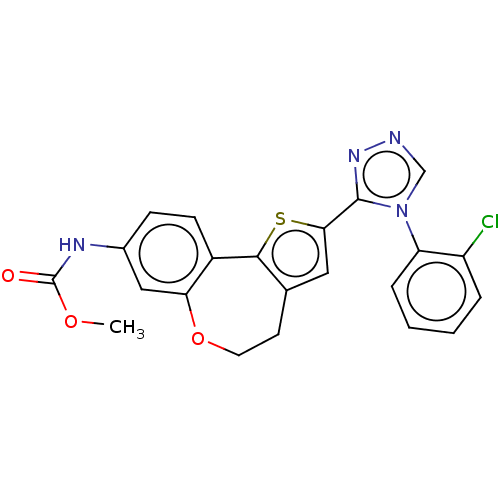
(CHEMBL3770332)Show SMILES COC(=O)Nc1ccc2-c3sc(cc3CCOc2c1)-c1nncn1-c1ccccc1Cl Show InChI InChI=1S/C22H17ClN4O3S/c1-29-22(28)25-14-6-7-15-18(11-14)30-9-8-13-10-19(31-20(13)15)21-26-24-12-27(21)17-5-3-2-4-16(17)23/h2-7,10-12H,8-9H2,1H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149543
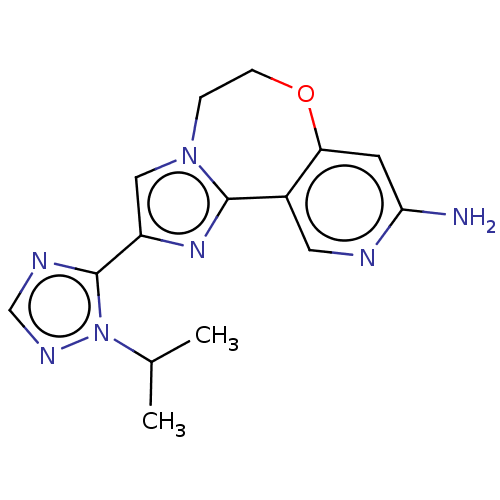
(CHEMBL3770630)Show InChI InChI=1S/C15H17N7O/c1-9(2)22-15(18-8-19-22)11-7-21-3-4-23-12-5-13(16)17-6-10(12)14(21)20-11/h5-9H,3-4H2,1-2H3,(H2,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(Homo sapiens (Human)) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Biochem Pharmacol 55: 1035-43 (1998)
Article DOI: 10.1016/s0006-2952(97)00631-x
BindingDB Entry DOI: 10.7270/Q2T72G01 |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Biochem Pharmacol 55: 1035-43 (1998)
Article DOI: 10.1016/s0006-2952(97)00631-x
BindingDB Entry DOI: 10.7270/Q2T72G01 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149479
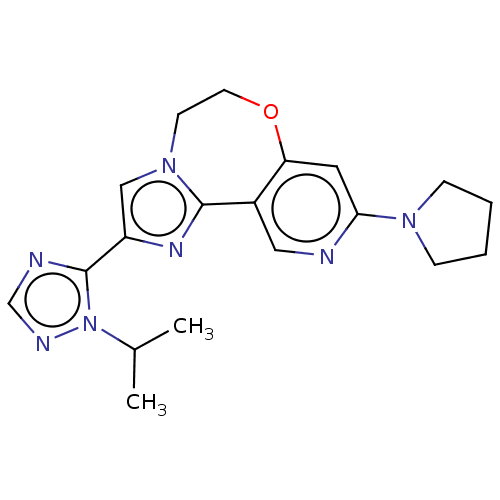
(CHEMBL3770092)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(ncc3-c2n1)N1CCCC1 Show InChI InChI=1S/C19H23N7O/c1-13(2)26-19(21-12-22-26)15-11-25-7-8-27-16-9-17(24-5-3-4-6-24)20-10-14(16)18(25)23-15/h9-13H,3-8H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM50408208
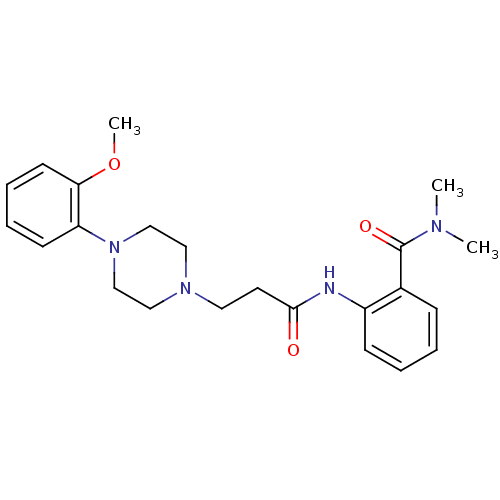
(CHEMBL88820)Show SMILES COc1ccccc1N1CCN(CCC(=O)Nc2ccccc2C(=O)N(C)C)CC1 Show InChI InChI=1S/C23H30N4O3/c1-25(2)23(29)18-8-4-5-9-19(18)24-22(28)12-13-26-14-16-27(17-15-26)20-10-6-7-11-21(20)30-3/h4-11H,12-17H2,1-3H3,(H,24,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM50408192
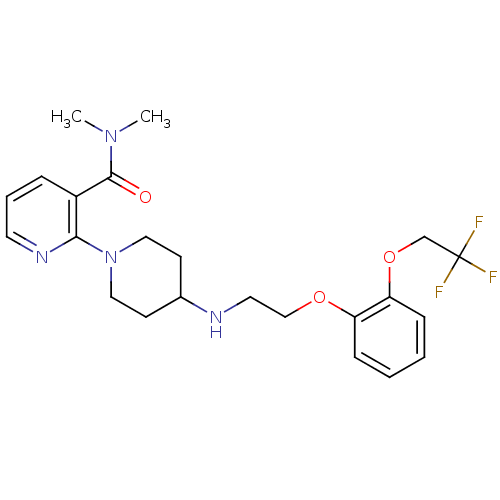
(CHEMBL90869)Show SMILES CN(C)C(=O)c1cccnc1N1CCC(CC1)NCCOc1ccccc1OCC(F)(F)F Show InChI InChI=1S/C23H29F3N4O3/c1-29(2)22(31)18-6-5-11-28-21(18)30-13-9-17(10-14-30)27-12-15-32-19-7-3-4-8-20(19)33-16-23(24,25)26/h3-8,11,17,27H,9-10,12-16H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM50408238
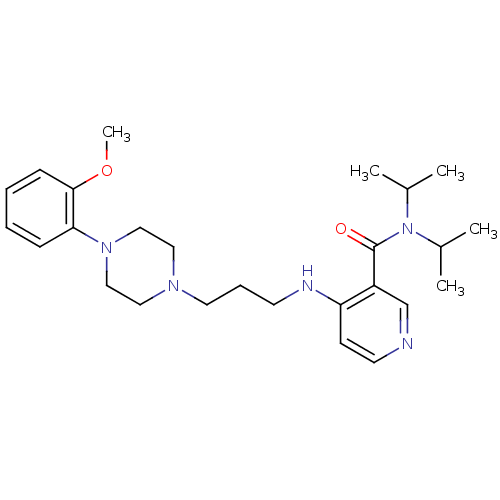
(CHEMBL89916)Show SMILES COc1ccccc1N1CCN(CCCNc2ccncc2C(=O)N(C(C)C)C(C)C)CC1 Show InChI InChI=1S/C26H39N5O2/c1-20(2)31(21(3)4)26(32)22-19-27-13-11-23(22)28-12-8-14-29-15-17-30(18-16-29)24-9-6-7-10-25(24)33-5/h6-7,9-11,13,19-21H,8,12,14-18H2,1-5H3,(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM50408202

(CHEMBL92901)Show SMILES COc1ccccc1N1CCN(CCCNc2nc(C)cc(C)c2C(N)=O)CC1 Show InChI InChI=1S/C22H31N5O2/c1-16-15-17(2)25-22(20(16)21(23)28)24-9-6-10-26-11-13-27(14-12-26)18-7-4-5-8-19(18)29-3/h4-5,7-8,15H,6,9-14H2,1-3H3,(H2,23,28)(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM50408239
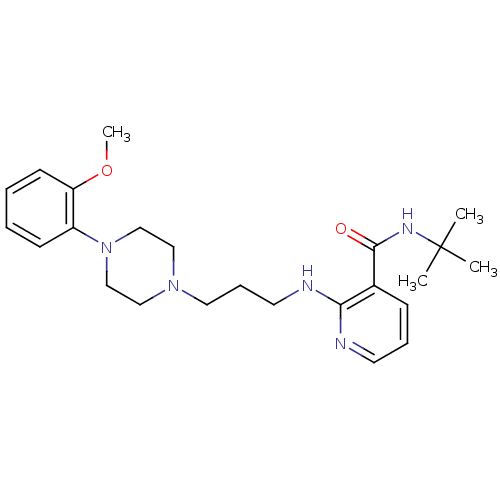
(CHEMBL90874)Show SMILES COc1ccccc1N1CCN(CCCNc2ncccc2C(=O)NC(C)(C)C)CC1 Show InChI InChI=1S/C24H35N5O2/c1-24(2,3)27-23(30)19-9-7-12-25-22(19)26-13-8-14-28-15-17-29(18-16-28)20-10-5-6-11-21(20)31-4/h5-7,9-12H,8,13-18H2,1-4H3,(H,25,26)(H,27,30) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(Homo sapiens (Human)) | BDBM50019492

((+)2-(2-Methoxy-2,3-dihydro-benzo[1,4]dioxin-2-yl)...)Show InChI InChI=1S/C12H14N2O3/c1-15-12(11-13-6-7-14-11)8-16-9-4-2-3-5-10(9)17-12/h2-5H,6-8H2,1H3,(H,13,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Biochem Pharmacol 55: 1035-43 (1998)
Article DOI: 10.1016/s0006-2952(97)00631-x
BindingDB Entry DOI: 10.7270/Q2T72G01 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM50408194
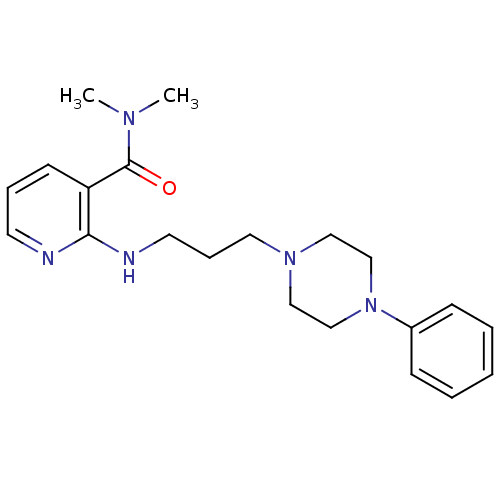
(CHEMBL90287)Show InChI InChI=1S/C21H29N5O/c1-24(2)21(27)19-10-6-11-22-20(19)23-12-7-13-25-14-16-26(17-15-25)18-8-4-3-5-9-18/h3-6,8-11H,7,12-17H2,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50177662
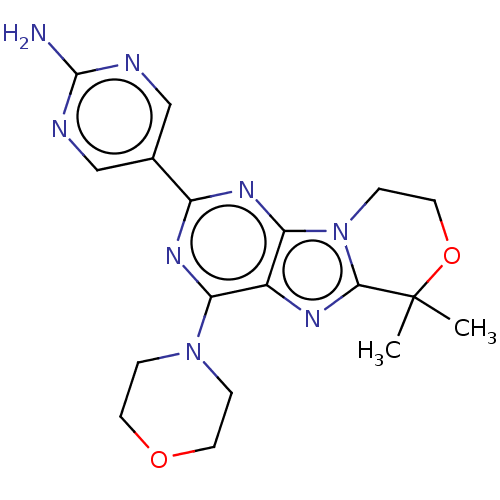
(CHEMBL3813842)Show SMILES CC1(C)OCCn2c1nc1c(nc(nc21)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C18H22N8O2/c1-18(2)16-22-12-14(25-3-6-27-7-4-25)23-13(11-9-20-17(19)21-10-11)24-15(12)26(16)5-8-28-18/h9-10H,3-8H2,1-2H3,(H2,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha (unknown origin) using dioctanoylglycerol-PIP2 as substrate incubated for 30 mins in presence of TAMRA-PIP3 by fl... |
ACS Med Chem Lett 7: 351-6 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00005
BindingDB Entry DOI: 10.7270/Q2WQ05QD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50177664
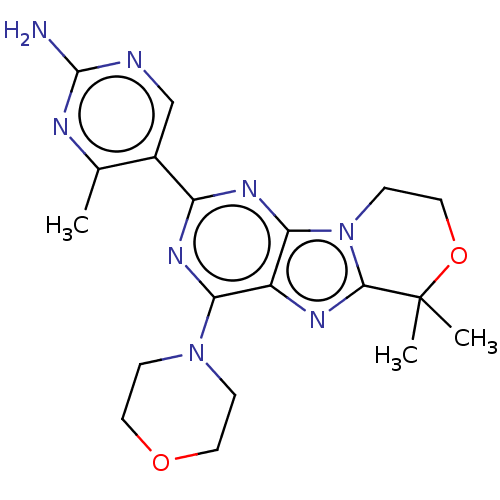
(CHEMBL3814414)Show SMILES Cc1nc(N)ncc1-c1nc(N2CCOCC2)c2nc3n(CCOC3(C)C)c2n1 Show InChI InChI=1S/C19H24N8O2/c1-11-12(10-21-18(20)22-11)14-24-15(26-4-7-28-8-5-26)13-16(25-14)27-6-9-29-19(2,3)17(27)23-13/h10H,4-9H2,1-3H3,(H2,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha (unknown origin) using dioctanoylglycerol-PIP2 as substrate incubated for 30 mins in presence of TAMRA-PIP3 by fl... |
ACS Med Chem Lett 7: 351-6 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00005
BindingDB Entry DOI: 10.7270/Q2WQ05QD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50398399
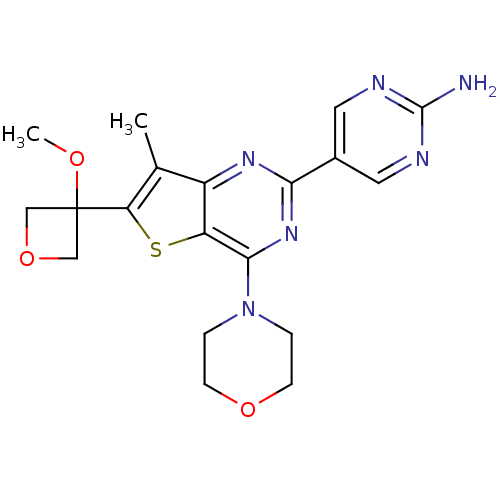
(CHEMBL2178608)Show SMILES COC1(COC1)c1sc2c(nc(nc2c1C)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C19H22N6O3S/c1-11-13-14(29-15(11)19(26-2)9-28-10-19)17(25-3-5-27-6-4-25)24-16(23-13)12-7-21-18(20)22-8-12/h7-8H,3-6,9-10H2,1-2H3,(H2,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha (unknown origin) using dioctanoylglycerol-PIP2 as substrate incubated for 30 mins in presence of TAMRA-PIP3 by fl... |
ACS Med Chem Lett 7: 351-6 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00005
BindingDB Entry DOI: 10.7270/Q2WQ05QD |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM50033111
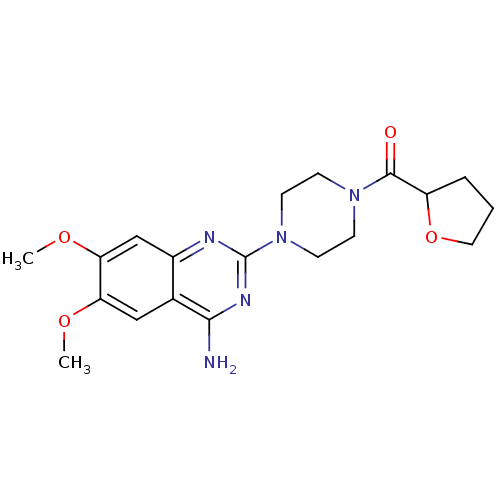
(1-(4-Amino-6,7-dimethoxy-2-quinazolinyl)-4-((tetra...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)C1CCCO1 Show InChI InChI=1S/C19H25N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h10-11,14H,3-9H2,1-2H3,(H2,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50177669

(CHEMBL3814544)Show SMILES Cn1c(nc2c(nc(nc12)-c1cnc(N)nc1)N1CCOCC1)C1CCC1 Show InChI InChI=1S/C18H22N8O/c1-25-15(11-3-2-4-11)22-13-16(25)23-14(12-9-20-18(19)21-10-12)24-17(13)26-5-7-27-8-6-26/h9-11H,2-8H2,1H3,(H2,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha (unknown origin) using dioctanoylglycerol-PIP2 as substrate incubated for 30 mins in presence of TAMRA-PIP3 by fl... |
ACS Med Chem Lett 7: 351-6 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00005
BindingDB Entry DOI: 10.7270/Q2WQ05QD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data