

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM50299343 ((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human vasopressin V1b receptor expressed in CHO cells by whole cell binding assay | Bioorg Med Chem Lett 21: 1871-5 (2011) Article DOI: 10.1016/j.bmcl.2010.12.081 BindingDB Entry DOI: 10.7270/Q2BR8SG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50299343 ((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Curated by ChEMBL | Assay Description Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in CHO cells by whole cell binding assay | Bioorg Med Chem Lett 21: 1871-5 (2011) Article DOI: 10.1016/j.bmcl.2010.12.081 BindingDB Entry DOI: 10.7270/Q2BR8SG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50299343 ((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Curated by ChEMBL | Assay Description Displacement of [3H]oxytocin from human oxytocin receptor | Bioorg Med Chem Lett 21: 1871-5 (2011) Article DOI: 10.1016/j.bmcl.2010.12.081 BindingDB Entry DOI: 10.7270/Q2BR8SG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50299343 ((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human vasopressin V1a receptor at 5 uM | Bioorg Med Chem Lett 21: 1871-5 (2011) Article DOI: 10.1016/j.bmcl.2010.12.081 BindingDB Entry DOI: 10.7270/Q2BR8SG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50299343 ((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 325 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human vasopressin V2 receptor | Bioorg Med Chem Lett 21: 1871-5 (2011) Article DOI: 10.1016/j.bmcl.2010.12.081 BindingDB Entry DOI: 10.7270/Q2BR8SG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50311657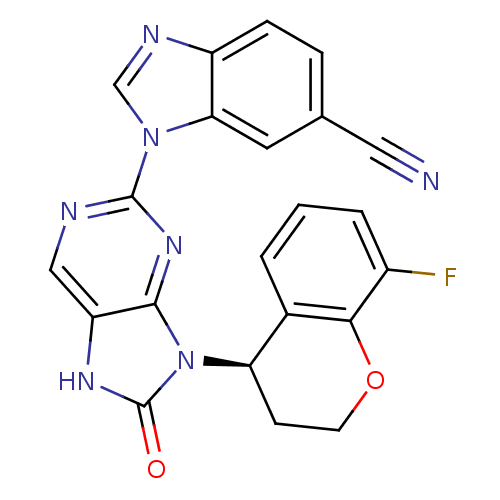 (1-(9-(8-fluorochroman-4-yl)-8-oxo-8,9-dihydro-7H-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay | Bioorg Med Chem Lett 19: 6788-92 (2009) Article DOI: 10.1016/j.bmcl.2009.09.080 BindingDB Entry DOI: 10.7270/Q24B31G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM50345056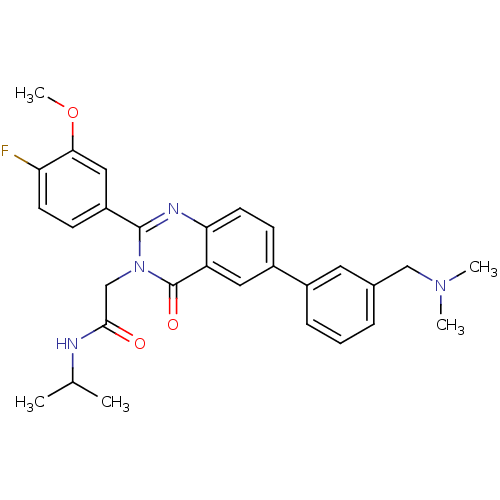 (2-(6-(3-((dimethylamino)methyl)phenyl)-2-(4-fluoro...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human vasopressin V1b receptor expressed in CHO cells co-expressing VIP-luciferase by scintillation counting-based whole... | Bioorg Med Chem Lett 21: 3813-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.022 BindingDB Entry DOI: 10.7270/Q2CV4J34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50454459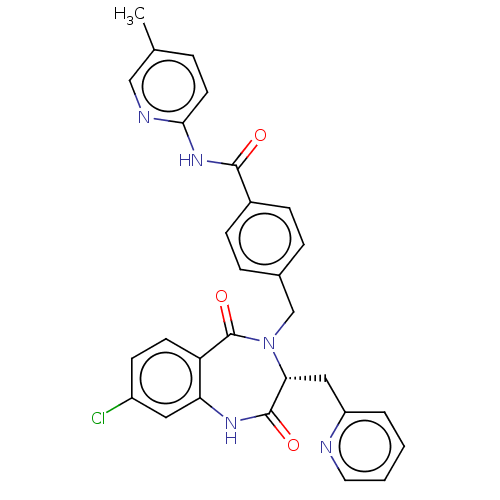 (CHEMBL4215036) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of Clostridium difficile toxin B transfected in CHO cells assessed as reduction in caspase 3/7 activation pre-incubated for 1 hr before Tc... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50454459 (CHEMBL4215036) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genesis Biotechnology Group Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B catalytic fragment (Met1 to Leu543 residues) assessed as reduction in... | Bioorg Med Chem Lett 28: 756-761 (2018) Article DOI: 10.1016/j.bmcl.2018.01.005 BindingDB Entry DOI: 10.7270/Q2QR50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V (Homo sapiens (Human)) | BDBM50277112 (3-[1-Oxo-6-(1,2,3,4-tetrahydro-benzo[4,5]imidazo[1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc. Curated by ChEMBL | Assay Description Binding affinity to human integrin alphavbeta3 receptor by TRF assay | Bioorg Med Chem Lett 19: 352-5 (2008) Article DOI: 10.1016/j.bmcl.2008.11.074 BindingDB Entry DOI: 10.7270/Q2N016C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM50345045 (CHEMBL1778950 | N-isopropyl-2-(2-(3-methoxyphenyl)...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human vasopressin V1b receptor expressed in CHO cells co-expressing VIP-luciferase by scintillation counting-based whole... | Bioorg Med Chem Lett 21: 3813-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.022 BindingDB Entry DOI: 10.7270/Q2CV4J34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50338810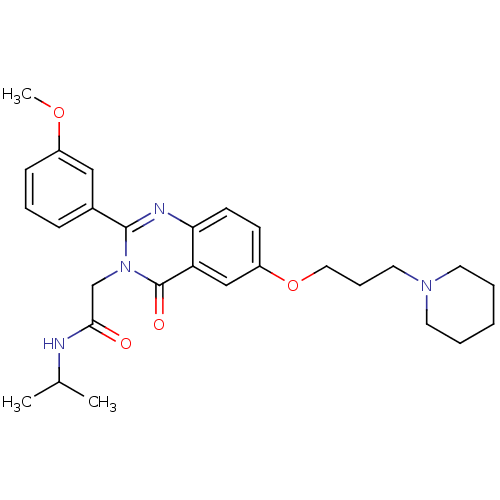 (CHEMBL1684573 | N-isopropyl-2-(2-(3-methoxyphenyl)...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Curated by ChEMBL | Assay Description Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in CHO cells by whole cell binding assay | Bioorg Med Chem Lett 21: 1871-5 (2011) Article DOI: 10.1016/j.bmcl.2010.12.081 BindingDB Entry DOI: 10.7270/Q2BR8SG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507130 (CHEMBL4452983) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V (Homo sapiens (Human)) | BDBM50277111 (3-Benzo[1,3]dioxol-5-yl-3-[1-oxo-6-(1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc. Curated by ChEMBL | Assay Description Binding affinity to human integrin alphavbeta3 receptor by TRF assay | Bioorg Med Chem Lett 19: 352-5 (2008) Article DOI: 10.1016/j.bmcl.2008.11.074 BindingDB Entry DOI: 10.7270/Q2N016C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50311656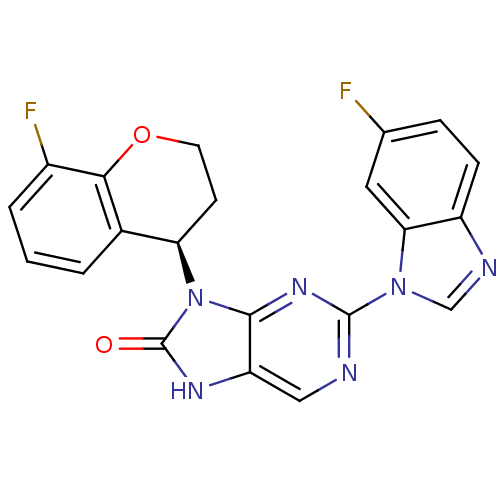 (2-(6-fluoro-1H-benzo[d]imidazol-1-yl)-9-(8-fluoroc...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay | Bioorg Med Chem Lett 19: 6788-92 (2009) Article DOI: 10.1016/j.bmcl.2009.09.080 BindingDB Entry DOI: 10.7270/Q24B31G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50454500 (CHEMBL4212258) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genesis Biotechnology Group Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B catalytic fragment (Met1 to Leu543 residues) assessed as reduction in... | Bioorg Med Chem Lett 28: 756-761 (2018) Article DOI: 10.1016/j.bmcl.2018.01.005 BindingDB Entry DOI: 10.7270/Q2QR50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50325890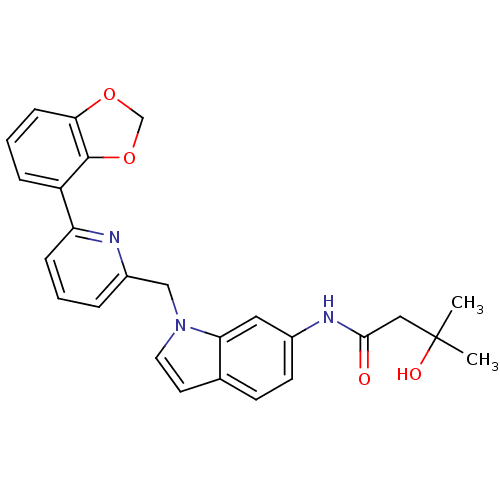 (CHEMBL1224536 | N-(1-((6-(benzo[d][1,3]dioxol-4-yl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]CC55940 human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 5449-53 (2010) Article DOI: 10.1016/j.bmcl.2010.07.091 BindingDB Entry DOI: 10.7270/Q2SN0958 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM50338810 (CHEMBL1684573 | N-isopropyl-2-(2-(3-methoxyphenyl)...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human vasopressin V1b receptor expressed in CHO cells by whole cell binding assay | Bioorg Med Chem Lett 21: 1871-5 (2011) Article DOI: 10.1016/j.bmcl.2010.12.081 BindingDB Entry DOI: 10.7270/Q2BR8SG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507108 (CHEMBL4529863) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50454498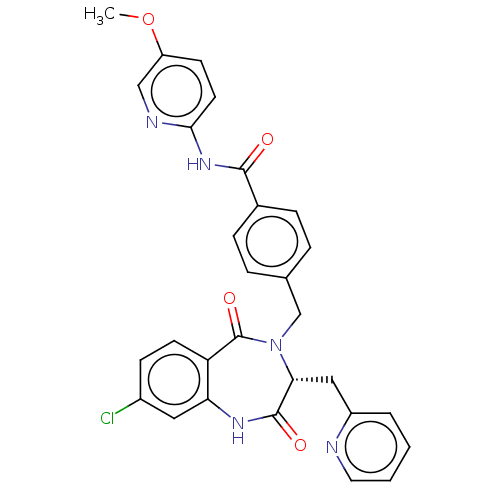 (CHEMBL4215657) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genesis Biotechnology Group Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B catalytic fragment (Met1 to Leu543 residues) assessed as reduction in... | Bioorg Med Chem Lett 28: 756-761 (2018) Article DOI: 10.1016/j.bmcl.2018.01.005 BindingDB Entry DOI: 10.7270/Q2QR50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM50338810 (CHEMBL1684573 | N-isopropyl-2-(2-(3-methoxyphenyl)...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human V1b receptor expressed in CHO cells co-expressing VIP-luciferase by whole cell binding assay | Bioorg Med Chem Lett 21: 1871-5 (2011) Article DOI: 10.1016/j.bmcl.2010.12.081 BindingDB Entry DOI: 10.7270/Q2BR8SG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50454497 (CHEMBL4214079) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of Clostridium difficile toxin B transfected in CHO cells assessed as reduction in caspase 3/7 activation pre-incubated for 1 hr before Tc... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507128 (CHEMBL4451752) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507135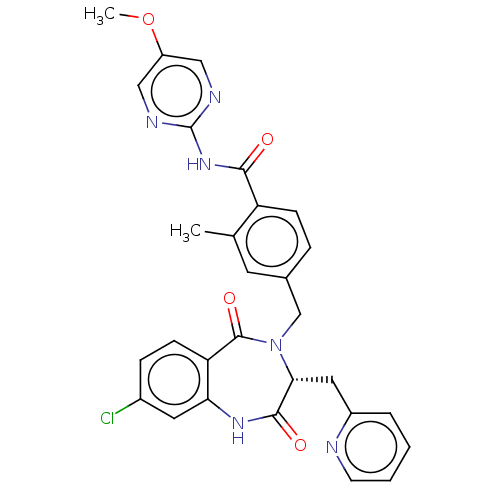 (CHEMBL4470638) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507134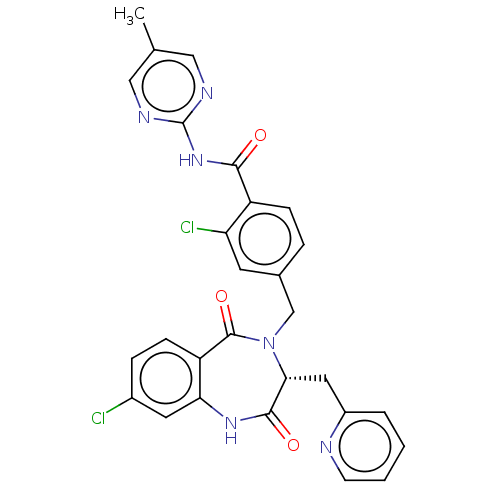 (CHEMBL4444965) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50338811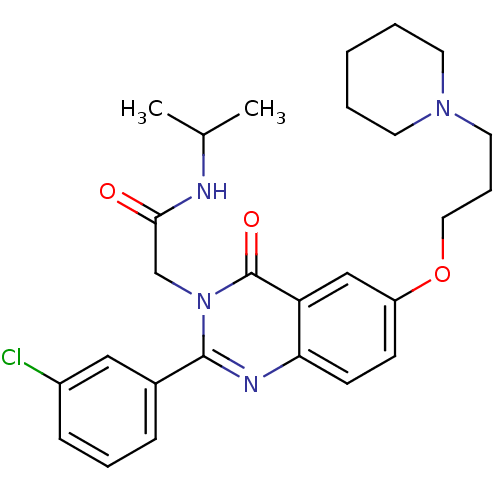 (2-(2-(3-chlorophenyl)-4-oxo-6-(3-(piperidin-1-yl)p...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Curated by ChEMBL | Assay Description Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in CHO cells by whole cell binding assay | Bioorg Med Chem Lett 21: 1871-5 (2011) Article DOI: 10.1016/j.bmcl.2010.12.081 BindingDB Entry DOI: 10.7270/Q2BR8SG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507115 (CHEMBL4520413) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM50338798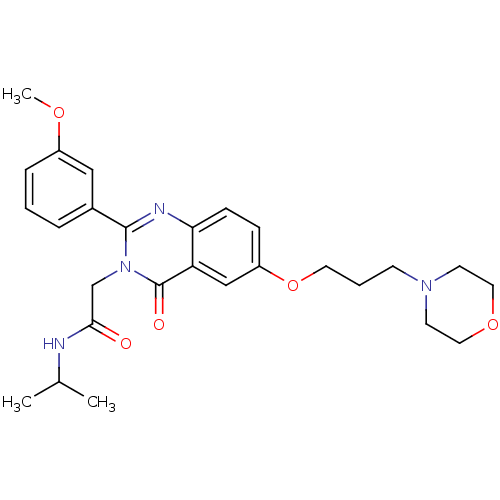 (CHEMBL1684561 | N-isopropyl-2-(2-(3-methoxyphenyl)...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human V1b receptor expressed in CHO cells co-expressing VIP-luciferase by whole cell binding assay | Bioorg Med Chem Lett 21: 1871-5 (2011) Article DOI: 10.1016/j.bmcl.2010.12.081 BindingDB Entry DOI: 10.7270/Q2BR8SG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507107 (CHEMBL4473557) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50311657 (1-(9-(8-fluorochroman-4-yl)-8-oxo-8,9-dihydro-7H-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of human JAK2 (532-1132) by time resolved fluorescence assay | Bioorg Med Chem Lett 19: 6788-92 (2009) Article DOI: 10.1016/j.bmcl.2009.09.080 BindingDB Entry DOI: 10.7270/Q24B31G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50325894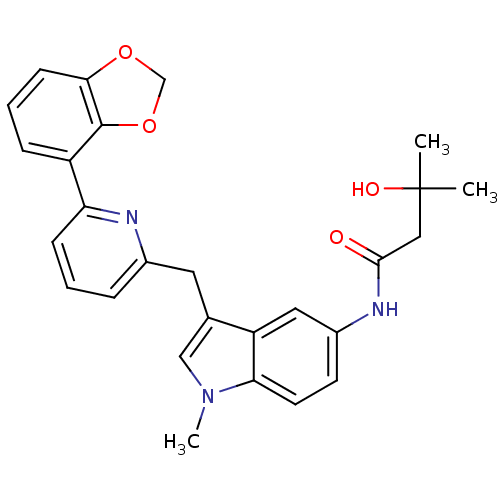 (CHEMBL1224615 | N-(3-((6-(benzo[d][1,3]dioxol-4-yl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]CC55940 human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 5449-53 (2010) Article DOI: 10.1016/j.bmcl.2010.07.091 BindingDB Entry DOI: 10.7270/Q2SN0958 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V (Homo sapiens (Human)) | BDBM50277112 (3-[1-Oxo-6-(1,2,3,4-tetrahydro-benzo[4,5]imidazo[1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc. Curated by ChEMBL | Assay Description Binding affinity to human integrin alphavbeta5 receptor by TRF assay | Bioorg Med Chem Lett 19: 352-5 (2008) Article DOI: 10.1016/j.bmcl.2008.11.074 BindingDB Entry DOI: 10.7270/Q2N016C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM50338799 (2-(2-(3-chlorophenyl)-6-(3-morpholinopropoxy)-4-ox...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human V1b receptor expressed in CHO cells co-expressing VIP-luciferase by whole cell binding assay | Bioorg Med Chem Lett 21: 1871-5 (2011) Article DOI: 10.1016/j.bmcl.2010.12.081 BindingDB Entry DOI: 10.7270/Q2BR8SG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507127 (CHEMBL4476228) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507120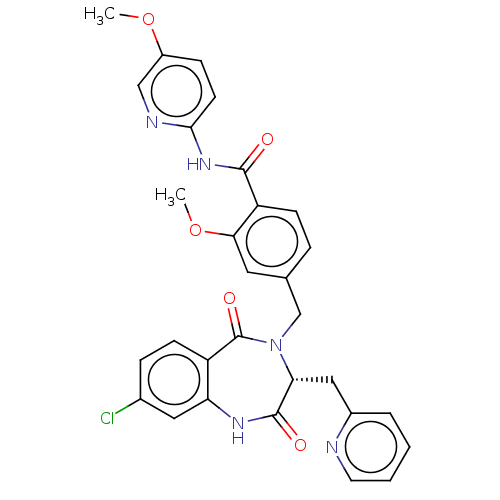 (CHEMBL4473443) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507105 (CHEMBL4557223) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50311644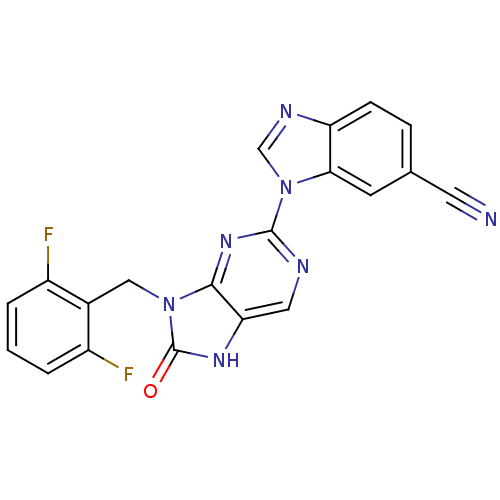 (1-(9-(2,6-difluorobenzyl)-8-oxo-8,9-dihydro-7H-pur...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay | Bioorg Med Chem Lett 19: 6788-92 (2009) Article DOI: 10.1016/j.bmcl.2009.09.080 BindingDB Entry DOI: 10.7270/Q24B31G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM50338811 (2-(2-(3-chlorophenyl)-4-oxo-6-(3-(piperidin-1-yl)p...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human vasopressin V1b receptor expressed in CHO cells by whole cell binding assay | Bioorg Med Chem Lett 21: 1871-5 (2011) Article DOI: 10.1016/j.bmcl.2010.12.081 BindingDB Entry DOI: 10.7270/Q2BR8SG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM50338811 (2-(2-(3-chlorophenyl)-4-oxo-6-(3-(piperidin-1-yl)p...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human V1b receptor expressed in CHO cells co-expressing VIP-luciferase by whole cell binding assay | Bioorg Med Chem Lett 21: 1871-5 (2011) Article DOI: 10.1016/j.bmcl.2010.12.081 BindingDB Entry DOI: 10.7270/Q2BR8SG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507133 (CHEMBL4461315) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM50345055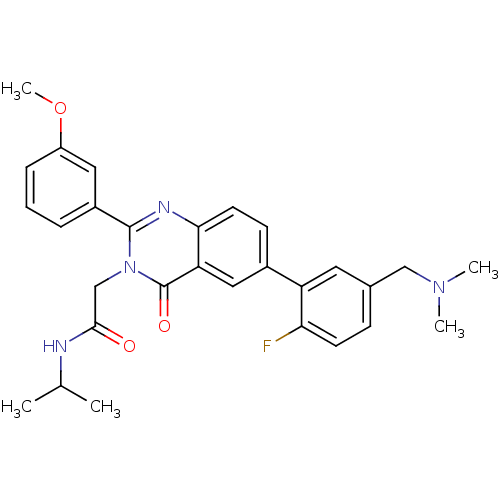 (2-(6-(5-((dimethylamino)methyl)-2-fluorophenyl)-2-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human vasopressin V1b receptor expressed in CHO cells co-expressing VIP-luciferase by scintillation counting-based whole... | Bioorg Med Chem Lett 21: 3813-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.022 BindingDB Entry DOI: 10.7270/Q2CV4J34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50454497 (CHEMBL4214079) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Genesis Biotechnology Group Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B catalytic fragment (Met1 to Leu543 residues) assessed as reduction in... | Bioorg Med Chem Lett 28: 756-761 (2018) Article DOI: 10.1016/j.bmcl.2018.01.005 BindingDB Entry DOI: 10.7270/Q2QR50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50325888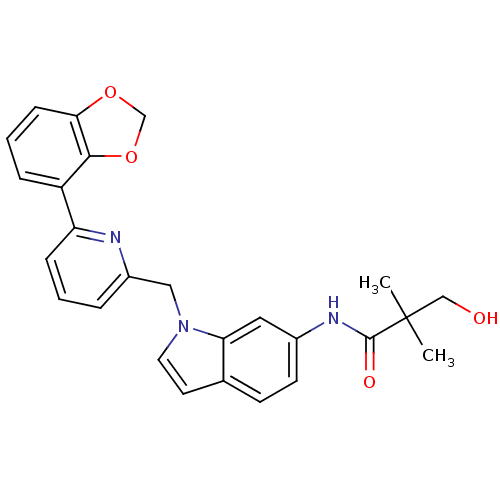 (CHEMBL1224534 | N-(1-((6-(benzo[d][1,3]dioxol-4-yl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]CC55940 human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 5449-53 (2010) Article DOI: 10.1016/j.bmcl.2010.07.091 BindingDB Entry DOI: 10.7270/Q2SN0958 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507136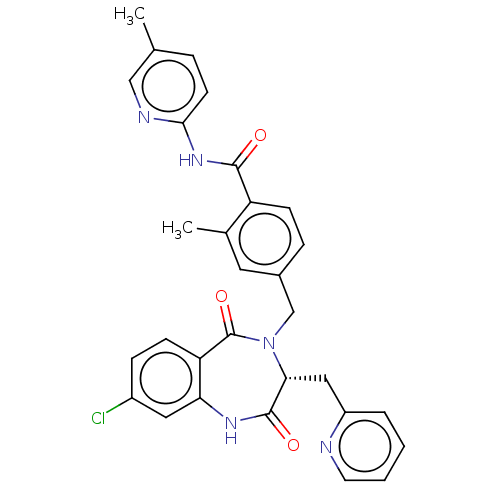 (CHEMBL4454294) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V (Homo sapiens (Human)) | BDBM50277098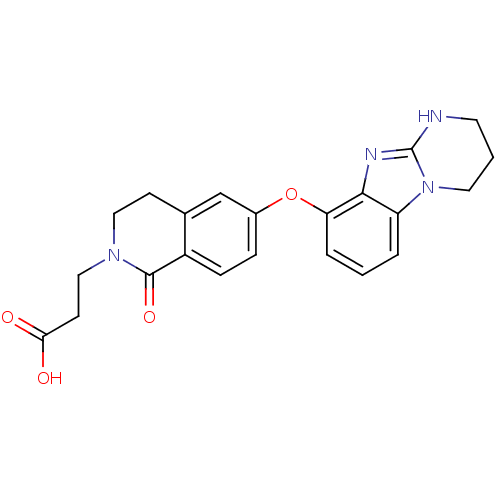 (3-[1-Oxo-6-(1,2,3,4-tetrahydro-benzo[4,5]imidazo[1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc. Curated by ChEMBL | Assay Description Binding affinity to human integrin alphavbeta3 receptor by TRF assay | Bioorg Med Chem Lett 19: 352-5 (2008) Article DOI: 10.1016/j.bmcl.2008.11.074 BindingDB Entry DOI: 10.7270/Q2N016C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50311640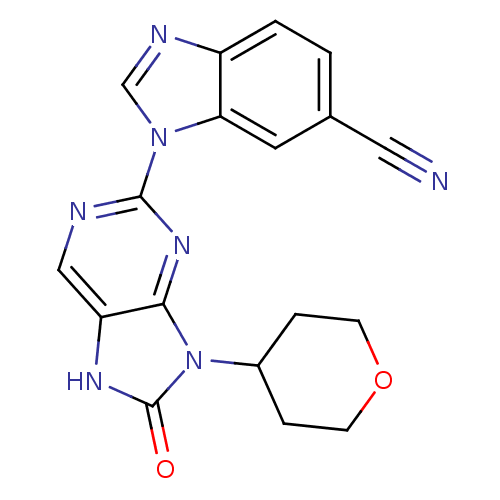 (1-(8-oxo-9-(tetrahydro-2H-pyran-4-yl)-8,9-dihydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay | Bioorg Med Chem Lett 19: 6788-92 (2009) Article DOI: 10.1016/j.bmcl.2009.09.080 BindingDB Entry DOI: 10.7270/Q24B31G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50325892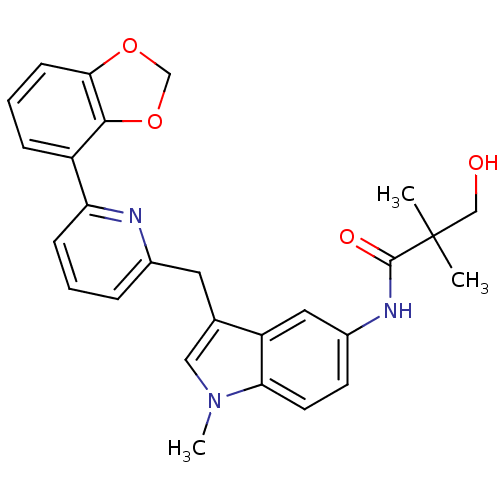 (CHEMBL1224613 | N-(3-((6-(benzo[d][1,3]dioxol-4-yl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]CC55940 human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 5449-53 (2010) Article DOI: 10.1016/j.bmcl.2010.07.091 BindingDB Entry DOI: 10.7270/Q2SN0958 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50156754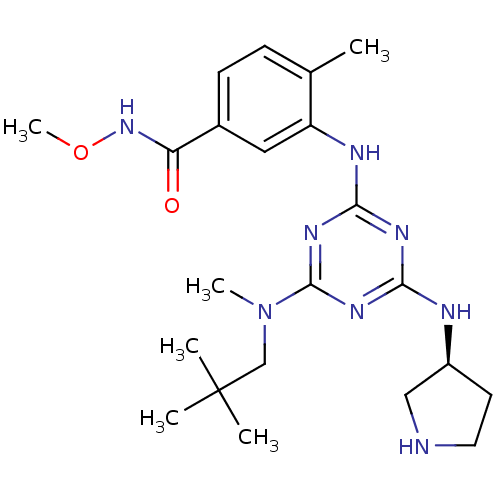 ((S)-N-methoxy-4-methyl-3-(4-(methyl(neopentyl)amin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human p38-alpha expressed in Escherichia coli | J Med Chem 47: 6283-91 (2004) Article DOI: 10.1021/jm049521d BindingDB Entry DOI: 10.7270/Q2WS8SR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507126 (CHEMBL4483028) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507114 (CHEMBL4553525) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 624 total ) | Next | Last >> |